European Academy of Neurology (EAN)/European Federation of Autonomic Societies (EFAS)/International Neuro-Urology Society (INUS) Guidelines for Practising Neurologists on the Assessment and Treatment of Neurogenic Urinary and Sexual Symptoms (NEUROGED Guidelines)
Funding: The project was funded by the EAN, EFAS, and INUS, and task force members received no remuneration for their work. The NEUROGED guidelines have been endorsed by the European Research Network for Rare Neurological Disorders. The American Academy of Neurology affirms the value of the EAN/EFAS/INUS guidelines for practising neurologists on the assessment and treatment of neurogenic urinary and sexual symptoms (NEUROGED guidelines) as an educational tool for neurologists.
Jalesh N Panicker, Alessandra Fanciulli, Magdalena Krbot Skoric, Tamara Kaplan, Katina Aleksovska, Ivan Adamec, Marcio Augusto Averbeck, Nicole Campese, Pietro Guaraldi, Fabian Leys, Jorge Moreno-Palacios, Sara Simeoni, Iva Stankovic, Sarah Wright, and Mario Habek were steering committee members.
Jalesh N Panicker, Alessandra Fanciulli, Mario Habek were co-chairs of the NEUROGED Task Force.
ABSTRACT
Background
Urinary and sexual symptoms are common following neurological disease, and we aimed to develop multidisciplinary inter-society evidence-based management guidelines.
Methods
The ADAPTE framework was used, and a systematic search of guidelines published in different languages was performed. Guidelines, consensus statements, and systematic reviews were included, and guideline quality was appraised using AGREE II. Patient representatives reviewed the relevance and suitability of recommendations. A modified Delphi process integrating the Evidence to Decision framework adapted from GRADE and the Oxford Centre for Evidence Based Medicine system was used to reach consensus on recommendation wording and strength.
Results
Recommendations were drafted, using guidelines/consensus statements (59 urinary, 50 sexual), systematic reviews (8 urinary, 2 sexual) and others (7 urinary,13 sexual), and wordings/strengths achieved at least 80% consensus through 2 Delphi rounds. Eleven evidence-based recommendations, 19 good practice statements, and 8 consensus-based recommendations were made. Individuals with neurological diseases should be asked about urogenital symptoms and undergo targeted physical examination when appropriate. Urinary symptom assessments include urinalysis, bladder diary completion, and post-void residual volume measurement. Treatments include fluid intake optimization, pelvic physiotherapy, tibial nerve stimulation, and oral medications. Urinary retention is managed by intermittent catheterization. Antibiotics should not be recommended to treat asymptomatic bacteriuria. Suprapubic catheterization is preferred for long-term catheterization. A comprehensive sexual history should be taken, focusing on multidimensional factors affecting sexual health. Treatments include lubricants, vibrators, and phosphodiesterase-5 inhibitors. Red flag symptoms warrant a shared-care approach with specialist colleagues.
Conclusions
The 38 NEUROGED recommendations will guide neurologists to comprehensively manage urogenital symptoms reported by individuals with neurological diseases.
1 Introduction
Lower urinary tract (bladder and urethra) and sexual symptoms are commonly reported by individuals with neurological disorders. The relationship between neurological disease, urogenital dysfunction, and quality of life has been well researched, and urinary tract-related complications are one of the commonest causes for unplanned hospital admissions in multiple sclerosis (MS) [1], Parkinson's disease (PD) [2] and spinal cord injury (SCI) [3]. Sexual health is a significant component of overall well-being and quality of life, and neurogenic sexual dysfunction significantly impacts mental health and relationships [4, 5]. Urinary dysfunction and symptoms differ according to the topographic distribution of neurological lesions: suprapontine disorders such as stroke, PD, and traumatic brain injury present predominantly with urinary storage symptoms due to detrusor overactivity (DO) and normal voiding, whereas suprasacral spinal cord disorders such as transverse myelitis, SCI, and MS present with urinary storage and voiding symptoms due to DO and detrusor-external sphincter dyssynergia (DSD). In contrast, lesions affecting the sacral spinal cord (conus medullaris) or more caudally such as the sacral nerve roots (cauda equina) or peripheral nerves, typically resulting from conditions such as disc prolapse, pelvic surgery, or peripheral neuropathy primarily present with urinary voiding symptoms due to detrusor underactivity, although storage symptoms can also occur. Co-morbid urological and medical conditions such as benign prostate enlargement and pelvic organ prolapse can additionally contribute to urinary symptoms [6].
There has been a greater understanding of the factors that can place individuals with neurological disease at greater risk for future damage to the upper urinary tract (kidneys and ureters) and potentially life-threatening complications such as urosepsis. A risk stratification system has recently been introduced, emphasizing the topographic distribution of neurological lesions and the pattern of lower urinary tract dysfunction in determining the risk for renal impairment [7]. Individuals classified as low risk typically have neurological lesions that are either suprapontine (e.g., stroke, PD) or infrasacral, are able to spontaneously void with low post-void residual (PVR) volumes and do not require a catheter to empty the bladder, have stable urinary symptoms, no history of recurrent urinary tract infections (UTIs), normal renal function, and, if investigations have been undertaken, normal upper urinary tract in ultrasound imaging and coordinated functioning of the detrusor and external urethral sphincter during voiding in urodynamic testing [7]. Individuals deemed to be at low risk can be appropriately managed by their neurologist, whereas shared care with a urologist is indispensable for those at a greater risk for future damage to the upper urinary tract or where co-morbid primary urological conditions are suspected.
Disparities in the availability of healthcare services for the management of autonomic nervous system disorders have been highlighted in a recently published survey [8], and access to specialist neuro-urology care is likewise limited. Neurologists have taken an interest in the assessment and treatment of urogenital symptoms in recent years, and guidelines have already been published by different urological societies. Developing guidelines specifically addressed to neurologists would help to establish a framework that best supports the integration of neurogenic urinary and sexual dysfunction management into neurology practice, and identify when care needs to be shared with other specialists. This would ultimately lead to an improvement in the quality of care provided to individuals living with neurological disorders. A global collaborative project was therefore initiated by the European Academy of Neurology (EAN), European Federation of Autonomic Societies (EFAS) and International Neuro-Urology Society (INUS) with the aim of developing evidence-based guidelines intended for practising NEurologists on neurogenic UROGEnital Dysfunction management (NEUROGED).
2 Methods
A task force of 38 experts was formed in consultation with the EAN, EFAS, and INUS (see Appendix S1 for Task Force members). This group included 24 neurologists, 8 urologists, 1 physiatrist, 2 methodologists, 1 librarian, 1 data organiser, and 1 patient representative. From this task force, a steering committee of 15 members was established to lead the process. Figure 1 illustrates the adopted methodology. As several high-quality guidelines have already been published by reputable professional societies and organisations and the evidence base for several of the recommendations is low, the NEUROGED guidelines were developed based on an assessment and adaptation of existing guidelines. We developed the recommendations using the systematic approach ADAPTE, endorsed by the Guidelines International Network [9]. Clinical questions were drafted in a PICO (Patient-Intervention-Comparison-Outcome) format, and the search of literature published in the last 25 years in English and other languages was performed in 11 databases using MeSH and free-text terms derived from the clinical questions (Appendix S2-Literature search and PICO questions) and duplicates were removed. The steering committee also included relevant papers published during the period that the guidelines were being prepared. Abstracts were screened for format, currency, and relevance to the PICOs, and only guidelines, consensus statements, and systematic reviews that met the search definition were included. We assessed the quality of the selected guidelines using the AGREE II (Appraisal of Guidelines, Research and Evaluation) instrument [10]. Each guideline was appraised independently by two steering committee members to derive a common score, and the guidelines were ranked according to quality, using the Domain 3 score of rigour of development in AGREE II (high (≥ 70%), moderate (40%–69%) and low quality (< 40%)), currency, and relevance to the neurological population (Appendix S3-Ranking documents based on quality). For each PICO, guidelines were reviewed for relevant recommendations in order of ranking, and a minimum of the top five guidelines were used primarily; however, recommendations from guidelines ranked further down were also reviewed. We presented levels of evidence (LOE) from the original guidelines, and the Oxford Centre for Evidence-Based Medicine system (2011) [11] was used to determine the level of evidence of the NEUROGED guideline recommendations; a conversion was performed for guidelines that used a different grading system, apart from those using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) framework (Appendix S4-LOE conversion). We developed the recommendations for the NEUROGED guidelines by adapting or adopting recommendations from existing guidelines, and they were prepared de novo using consensus if not available in the included guidelines or could be answered using additional evidence. Nine individuals with neurological disease reviewed the draft recommendations during an online meeting to assess relevance and suitability. The wording of proposed recommendations underwent a review during a hybrid meeting of the steering committee that was attended by the patient representative and was accepted only after reaching 80% consensus amongst the committee members. The levels of evidence for the recommendations varied, leading to evidence-based recommendations, good practice statements, and consensus-based recommendations (see Appendix S3-Definitions of recommendation types). The strength of the evidence-based recommendations was determined using an Evidence to Decision (EtD) framework adapted from GRADE [12] (Appendix S3-Determining the strength of recommendations). We integrated this into a modified Delphi process to achieve consensus on the wording and strength of the recommendations. Task force members were presented with summaries of the desirable and undesirable effects and the screened guidelines for each clinical question (Appendix S3-Delphi survey). Recommendations wordings and strengths that did not achieve 80% consensus were then revised based on feedback and went through a second Delphi round.
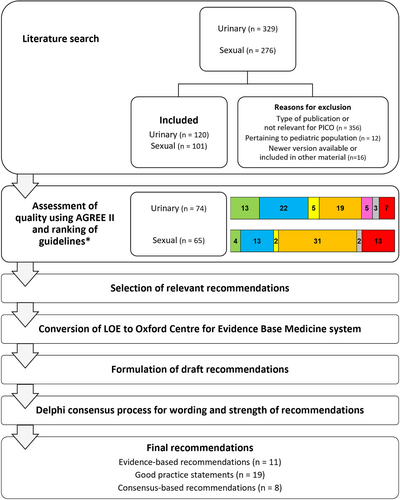
3 Results
Two rounds of Delphi voting were conducted with 100% participation from the task force, and all the recommendation wordings and strengths ultimately achieved at least 80% consensus. Eleven clinical questions had sufficient evidence to make evidence-based recommendations, and the strength of six met the criteria for being strong. Nineteen good practice statements and eight consensus-based recommendations were made for the remaining clinical questions. Results of the literature search, data synthesis, and Delphi voting are provided as Supporting Information (Appendices S5–S8). Tables 1–4 summarise the evidence supporting recommendations for the assessment and treatment of urinary and sexual symptoms. Based on the recommendations, algorithms that illustrate the assessment and treatment of urinary and sexual symptoms were developed (Figures 1 and 2). Enlarged versions of these algorithms suitable for use in clinic are also available (Appendices S9 and S10). Table 5 presents a practical checklist of urogenital symptoms that should be covered during history taking.
| Clinical question 1. History taking |
History taking should include [7, 13-19] (checklist presented in Table 5):
|
| Clinical question 2. Targeted physical examination |
Targeted physical neuro-urological examination should include [7, 13, 15, 17, 20-22]:
|
| Clinical question 3. Urinalysis |
|
| Clinical question 4. Urine culture |
|
| Clinical question 5. Bladder diary |
|
| Clinical question 6. Post-void residual volume |
|
| Clinical question 7. Renal function tests |
|
| Clinical question 8. PSA testing |
|
| Clinical question 9. Urodynamics testing |
|
| Clinical question 10. Red-flags for specialist referral |
Shared care with specialist urology input is recommended in the following situations:
|
- Abbreviations: CFU, colony forming units; GFR, glomerular filtration rate; IC, intermittent catheterisation; MS, multiple sclerosis; PSA, prostate specific antigen; PVR, post-void residual volume; UTI, urinary tract infection.
| Clinical question 11. Fluid intake |
|
| Clinical question 12. Bladder retraining |
|
| Clinical question 13. Pelvic floor exercises |
|
| Clinical question 14. Intermittent catheterisation |
|
| Clinical question 15. Indwelling catheterisation |
|
| Clinical question 16. Antibiotic prophylaxis |
|
| Clinical question 17. Treating asymptomatic bacteriuria |
|
| Clinical question 18. Treating UTIs |
|
| Clinical question 19. Tibial nerve stimulation |
|
| Clinical question 20. Appliances |
|
| Clinical question 21. Antimuscarinic agents |
|
| Clinical question 22. Beta-3 adrenoceptor agonists |
|
| Clinical question 23. Cholinergic agents |
| Clinical question 24. Desmopressin for nocturia |
|
| Clinical question 25. α 1 -adrenoceptor blockers |
α1-adrenoceptor blockers can cause hypotension, and should be avoided if orthostatic hypotension has been documented
|
- Abbreviations: IC, intermittent catheterisation; MS, multiple sclerosis; MSA, multiple system atrophy; PD, Parkinson's disease; PDE, phosphodiesterase; PTNS, percutaneous tibial nerve stimulation; PVR, post-void residual; SCI, spinal cord injury; UTI, urinary tract infection.
| Clinical question 26. History taking |
|
History taking should include the following [13, 85-90] (checklist presented in Table 5):
Medical history is crucial for identifying the cause of sexual dysfunction, and include onset and duration
|
| Clinical question 27. Targeted physical examination |
When appropriate, individuals with neurological disease reporting sexual dysfunction should undergo a targeted physical examination which includes [85, 87, 89, 93, 94]:
|
| Clinical question 28. Screening laboratory testing |
In the appropriate clinical context, individuals with neurological diseases experiencing sexual problems should undergo screening laboratory testing to assess for additional contributing factors. The following should be considered [85, 87, 89, 90, 93, 95-98]:
|
| Clinical question 29. Diagnostic examinations |
Electrodiagnostic tests evaluating the sacral somatic innervation [13, 89, 96]:
|
Electrodiagnostic tests may be helpful in the following situations:
|
| Clinical question 30. Red-flags for specialist referral |
Individuals with any of the following presentations should be referred by their neurologist for specialist assessments [89, 99]:
|
- Abbreviations: ED, erectile dysfunction; HIV, human immunodeficiency virus; MS, multiple sclerosis; PE, premature ejaculation; SCI, spinal cord injury; STI, sexually transmitted infection.
| Clinical question 31. Education |
Clinicians should inform individuals with neurological diseases experiencing sexual problems about the factors that can influence sexual activity and intimacy. Clinicians should [87-89, 93, 96]:
|
| Clinical question 32. Lubricants |
Consider vaginal lubricants for females with neurological diseases experiencing dyspareunia or dryness, and discuss benefits and risks [100, 101]:
|
| Clinical question 33. Vibrators |
Discuss vibrator use with individuals having neurological diseases and sexual problems, weighing benefits and risks [87, 100, 102, 103]:
|
| Clinical question 34. Vacuum devices |
Vacuum devices are a second-line treatment for ED in men with neurological diseases; benefits and risks should be discussed [13, 87, 89, 95]
|
| Clinical question 35. PDE5 Inhibitors |
PDE5 inhibitors are recommended as a first-line treatment for ED in males with neurological diseases, emphasising the need to discuss benefits and potential risks [13, 85, 95, 105]
|
| Clinical question 36. Intracavernous prostaglandin injections |
Intracavernous injections of prostaglandin are recommended as a second-line treatment for ED in males with neurological diseases, with a discussion on benefits and risks being essential [13, 85, 87, 89]:
|
| Clinical question 37. Multidimensional Factors |
|
Address multidimensional factors affecting sexual activity and intimacy in individuals with neurological diseases, including secondary factors (spasticity, fatigue, incontinence, cognitive co-morbidities, medication side effects) and tertiary factors (changes in self or body image), through regular discussions due to their dynamic nature [85, 87, 91, 106]
|
- Abbreviations: ED, erectile dysfunction; IIEF, International Index of Erectile Function; MS, Multiple sclerosis; PDE-5, phosphodiesterase-5; SCI, spinal cord injury.
| Bladder functions | ||
| □ Urinary symptoms | ||
| Storage symptoms | Voiding symptoms | |
| Daytime urinary frequency | Hesitancy | |
| Nocturia/nocturnal polyuria | Inability to void | |
| Urgency | Straining to void | |
Sensations of bladder filling
|
Slow stream | |
Incontinence
|
Intermittency | |
| Post-micturition symptoms | ||
| Feeling of incomplete emptying | ||
| Double voiding | ||
| Post-micturition dribble | ||
| □ Symptom frequency and severity | ||
| □ Variation between night-time and daytime symptoms | ||
| □ Precipitating or relieving factors | ||
| □ Prior treatments and their success | ||
| □ Strategies used by the patients to improve their symptoms | ||
| □ Pad and catheter use | ||
| □ Recent UTIs, painful urination, haematuria | ||
| □ The impact of the symptoms on quality of life and social function | ||
| □ Bowel symptoms, incontinence, constipation, urgency | ||
| □ Patient and caregiver expectations | ||
| Sexual functions | ||
| □ Sexual symptoms | ||
| □ Altered libido | ||
| □ Arousal (Males: erectile dysfunction; Females: lubrication) | ||
| □ Ejaculation (Males): anejaculation, delayed ejaculation, premature ejaculation | ||
| □ Dyspareunia or discomfort | ||
| □ Anorgasmia | ||
| □ Patient and partner expectations | ||
| □ Multidimensional contributors | ||
| Multidimensional contributors to sexual dysfunction [91] | ||
| Definition | Symptoms | |
| Primary | Result of neurologic changes that directly affect sexual feelings and/or sexual response | Impaired genital sensation, decreased libido, Males: inability to achieve or maintain an erection; Females: genital numbness, pain, burning, decreased vaginal lubrication |
| Secondary | Related physical changes that affect the sexual response indirectly | Fatigue, muscle tightness, weakness, spasms, bladder and bowel dysfunction, incoordination, cognitive difficulties, numbness, pain in non-genital areas, side effects from medications |
| Tertiary | Psychological, emotional, social, and cultural aspects that impact sexuality | Negative changes in self-image, body image, feeling less confident about one's sexuality, worries about sexually satisfying one's partner, difficulty communicating with one's partner |
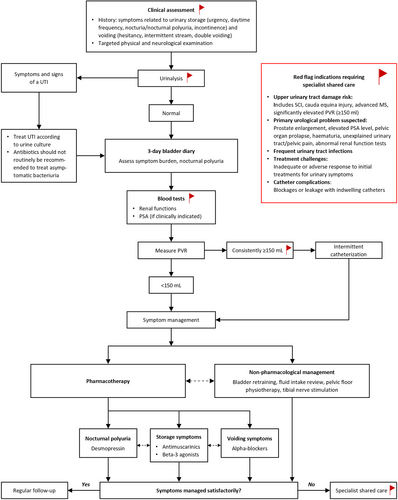
3.1 Section 1: Assessment of Urinary Symptoms
Table 1 and Appendix S5 present the evidence supporting the recommendations for assessing urinary symptoms. Figure 2 illustrates the assessment and treatment algorithm for urinary symptoms based on these recommendations.
Clinical Question 1. Should neurologists obtain a history of a patient's urinary symptoms versus not asking about urinary symptoms?
History taking forms the cornerstone of the assessment of urinary symptoms, and this is summarised as a checklist in Table 5.
Recommendation: Neurologists should actively ask about urinary symptoms in individuals with neurological diseases on a regular basis. (Good practice statement; Consensus: 100%).
Clinical Question 2. Should individuals with neurological disease reporting urinary symptoms undergo a focused physical examination versus not undergoing a physical examination?
Individuals reporting urinary symptoms should undergo a targeted physical examination at initial evaluation, which is repeated annually in moderate or high-risk individuals [7]. The examination helps to plan investigations and treatments and screen for complications.
Recommendation: Neurologists should perform a targeted physical examination in individuals with neurological diseases and urinary symptoms. (Good practice statement; Consensus: 94%).
Clinical Question 3. Should individuals with neurological disease reporting urinary symptoms undergo a urinalysis versus not undergoing a urinalysis?
Screening urinalysis, which includes physical, chemical (dipstick testing) and/or microscopic evaluation of urine, should be a part of the initial evaluation when an individual reports urinary symptoms [7, 13, 20]. The urine should be tested at follow-up in case of significant changes in urinary symptoms [14]. Urinalysis is more useful to exclude UTIs, and when a UTI is suspected, the urine should be sent for culture. Urinalysis should not be routinely performed to screen for UTIs in individuals who are using a catheter, given the high prevalence of asymptomatic bacteriuria and leukocyturia [7, 13, 15]. Urine dipstick testing can also screen for glucosuria, proteinuria, and microscopic haematuria, prompting further investigations if persistent and unexplained.
Recommendation: Urinalysis should be performed at initial evaluation and when clinically indicated at follow-up visits for individuals with neurological diseases and urinary symptoms. (Good practice statement; Consensus: 94%).
Clinical Question 4. Should urine cultures versus no testing be offered for individuals with neurological disease and urinary symptoms?
Quantitative urine culture is used to diagnose a UTI by testing for the type of organisms and antibiotic sensitivity, and should be performed only in patients with symptoms such as dysuria, cloudy and/or malodorous urine, lower abdominal pain, and fever.
Recommendation: A urine culture should be performed for individuals with neurological diseases reporting urinary symptoms only if there is a suspicion of a UTI. (Good practice statement; Consensus: 91%).
Clinical Question 5. Should individuals with neurological diseases reporting urinary symptoms complete a bladder diary versus not complete a bladder diary?
Recording fluid intake, ideally together with urine output, helps to corroborate the history, recognize beverages and drinking habits detrimental to urinary symptoms, and provide an assessment of the functional bladder capacity. Nocturnal polyuria and polydipsia can be diagnosed only by using a bladder diary.
Recommendation: A three-day bladder diary should be completed by individuals with neurological diseases having urinary problems at initial evaluation and at follow-up visits when clinically indicated to provide an objective assessment of urinary symptoms. (Good practice statement; Consensus: 94%).
Clinical Question 6. Should individuals with neurological disease reporting urinary symptoms have their post-void residual measured vs. not have their post-void residual measured?
Post-void residual volume is defined as the volume of urine left in the urinary bladder at the end of micturition and is a valuable indicator of bladder emptying [13].
Recommendation: The PVR should be measured for individuals with neurological diseases having urinary symptoms who void spontaneously, preferably using non-invasive methods, during the initial evaluation and during follow-up visits as deemed clinically appropriate. (Good practice statement; Consensus: 94%).
Clinical Question 7. Should individuals with neurological disease reporting urinary symptoms undergo blood tests (e.g., renal function test) versus not undergo blood tests?
Measuring serum creatinine and blood urea levels has utility in identifying renal disease, and no additional patient preparation is required when collecting samples.
Recommendation: Assessment of renal function, including blood urea and serum creatinine, is recommended for individuals with neurological diseases and urinary symptoms as part of their initial evaluation and repeated during follow-up if clinically indicated. For those with stable urinary symptoms but at risk of upper urinary tract damage, renal function should be tested annually. (Good practice statement; Consensus: 88%).
Clinical Question 8. Should male individuals with neurological disease reporting urinary symptoms undergo PSA testing versus not undergoing this test?
Prostate-specific antigen (PSA) is produced by the prostate and, in healthy males, levels in the blood are low. Measuring blood PSA levels is a validated screening test for prostate cancer.
Recommendation: Prostate cancer screening by measuring the PSA level may be offered to male individuals who have neurological diseases and urinary symptoms, particularly in men between the ages of 50 and 70. However, the decision to test should be shared with the patient after a discussion about possible benefits and harms. (Consensus-based recommendation; Consensus: 88%).
Clinical Question 9. Should individuals with neurological disease reporting urinary symptoms undergo urodynamics testing versus not undergo urodynamics testing?
Urodynamics testing is useful for evaluating the cause of lower urinary tract dysfunction and should be performed selectively.
Recommendation: Invasive urodynamic testing is not recommended as part of the initial evaluation for individuals with neurological diseases and urinary symptoms. However, if individuals exhibit atypical urinary symptoms, are at a high risk of upper urinary tract damage, or have not experienced improvement with conservative treatment options, it is recommended that they be referred for urodynamic testing. (Good practice statement; Consensus: 91%).
Clinical Question 10. Should there be red flags that initiate a urological referral for individuals with neurological disease reporting urinary symptoms versus place a urological referral for all individuals versus not to refer to urology services?
Individuals at low risk for developing upper urinary tract damage can generally be managed by a neurologist [7]. This would include those with a neurological lesion that is either suprapontine (e.g., stroke, Parkinson's disease (PD) or infrasacral), who are able to spontaneously void with low PVR volumes and do not require a catheter to empty the bladder, have stable urinary symptoms, no history of recurrent UTIs, normal renal functions, and, if the individual has undergone tests, synergistic voiding in urodynamics testing and normal upper tract imaging [7]. Following an acute neurological event, risk should be stratified only once the neurological condition has stabilized [7]. The management of individuals with greater risk for developing upper urinary tract damage (such as spinal cord injury, elevated PVR volumes, requiring catheterisation) or urinary symptoms refractory to first-line treatment should be shared between neurologists and urologists.
Recommendation: Individuals with neurological diseases reporting urinary symptoms should be referred to urologists if there is a risk of developing upper urinary tract damage, suspected urological pathology, or poor response or significant side effects to first-line treatments. (Good practice statement; Consensus: 100%).
3.2 Section 2: Treatment of Urinary Symptoms
Table 2 and Appendix S6 present the evidence supporting recommendations made for the treatment of urinary symptoms, and Figure 2 illustrates the assessment and treatment algorithm of urinary symptoms based on the recommendations.
Clinical Question 11. Should advice for fluid intake versus no advice be offered for individuals with neurological disease and urinary symptoms?
The volume and type of fluids consumed can affect urinary symptoms, and optimizing fluid management can help in the management of storage symptoms, reduce the risk of complications, and improve quality of life.
Recommendation: Advice on adequate fluid intake should be offered to individuals with neurological diseases and urinary symptoms. The benefits and potential risks/burdens should be discussed. (Good practice statement; Consensus: 94%).
Clinical Question 12. Should advice for bladder retraining versus no advice be offered to individuals with neurological disease and urinary symptoms?
Behavioural conservative measures that could help with managing urinary urgency include bladder retraining, timed voiding, prompted voiding, and habit retraining [41]. Bladder retraining involves scheduling a bladder routine with progressively increasing intervals between voids and could be offered to individuals at low risk for developing upper urinary tract damage, experiencing urinary urgency, and who can spontaneously void [13, 15].
Recommendation: Advice for bladder retraining could be offered to individuals with neurological diseases who experience urinary urgency and can spontaneously void. The benefits and potential risks/burdens should be discussed. (Evidence-based recommendation; Strength: weak; LoE III; Consensus: 82% for recommendation wording and 85% for recommendation strength).
Clinical Question 13. Should advice for performing pelvic floor exercises versus no advice be offered to individuals with neurological disease and urinary symptoms?
Pelvic floor muscle training has been shown to be effective for managing stress urinary incontinence and lower urinary tract dysfunction due to MS, stroke, or other neurological conditions where the potential to voluntarily contract the pelvic floor is preserved [15].
Recommendation: Advice on pelvic floor exercises should be offered to individuals with neurological diseases who experience urinary urgency and/or stress incontinence. The benefits and potential risks/burdens should be discussed. (Evidence-based recommendation; Strength: strong; LoE: II; Consensus: 97% for recommendation wording and 88% for recommendation strength).
Clinical Question 14. Should advice for intermittent catheterization versus no advice be offered for individuals with neurological disease and urinary symptoms?
Intermittent catheterisation (IC) enables the bladder to be emptied in individuals with urinary retention [7, 13, 14, 20, 29, 30, 33, 34, 36, 37, 49-51, 55, 56, 58, 59, 107, 108] and is preferred over an indwelling catheter because of fewer complications [55]. A PVR volume consistently above 150 mL is considered a cut-off for commencing IC; however, this decision should take into account individual preferences and the prognosis of the underlying neurological disease. Neurological abilities such as cognition, vision, dexterity, truncal balance, and sensations impact the ability to perform self-catheterisation, and the suitability of carers to perform catheterisation may need to be considered in these situations [14, 33, 55].
Recommendation: Intermittent catheterisation should be offered as first-line therapy in individuals with neurological disease with an elevated postvoid residual urine (> 150 mL) or urinary retention (an inability to void) after considering the associated risks, benefits, and resulting burden. (Evidence-based recommendation; Strength: strong; LoE: III; Consensus: 97% for recommendation wording and 97% for recommendation strength in Delphi round 2).
Clinical Question 15. Should advice for indwelling catheterization versus no advice be offered for individuals with neurological disease and urinary symptoms?
Indwelling catheterisation may need to be considered for emptying the bladder when IC is not feasible or for managing urinary incontinence. A suprapubic catheter is preferred over urethral in view of less risk for complications such as urethral injury (false passages, strictures, sphincter injury and stretch, tears, traumatic hypospadias) and ease of catheter management when sitting or when engaging sexually. However, this requires shared decision-making, and complication risks should be discussed with the individual and, if appropriate, their family [13, 14, 20, 29, 33, 41, 50, 51, 55, 57].
Recommendation: When long-term indwelling urinary bladder drainage is unavoidable, individuals with neurological diseases should be advised that suprapubic catheter drainage is preferred over urethral catheterization. (Consensus-based recommendation; Consensus: 100%).
Clinical Question 16. Should prophylactic antibiotic therapy versus no advice be offered for individuals with neurological disease and urinary symptoms using a catheter?
Recurrent urinary tract infections should prompt an assessment for an underlying urological cause (e.g., bladder stones) or suboptimal catheterization technique if performing IC [13-15, 54, 57, 58]. Antibiotic prophylaxis should not be routinely used; however, if no modifiable causes are identified, low-dose antibiotic prophylaxis may need to be considered on an individual basis [13-15, 20, 33, 36, 41, 50, 54, 55, 57-59].
Recommendation: Antibiotic prophylaxis should not be routinely used in individuals with neurological diseases who catheterize. The benefits and potential risks/burdens should be discussed. (Good practice statement; Consensus: 100%).
Clinical Question 17. Should advice for antibiotic therapy versus no advice be offered for individuals with neurological disease and urinary symptoms having asymptomatic bacteriuria?
Asymptomatic bacteriuria should be treated with antibiotics only in exceptional circumstances [13, 20, 26, 29, 50, 54, 57-59]. The choice of antibiotics should take into account urine culture and sensitivity results, previous antibiotic use, and recommendations in local antibiotic formularies as part of antimicrobial stewardship.
Recommendation: Antibiotics should not be routinely recommended to treat asymptomatic bacteriuria in individuals with neurological diseases having urinary problems*. The benefits and potential risks/burdens should be discussed. *Exceptions where antibiotic treatment for asymptomatic bacteriuria may be considered are pregnancy, planned urological procedures, or immunomodulatory treatments. (Evidence-based recommendation; Strength: strong; LoE: I; Consensus: 100% for recommendation wording and 97% for recommendation strength).
Clinical Question 18. Should antibiotic treatment be guided by urine culture sensitivity versus given empirically for individuals with neurological disease and urinary symptoms who use catheters having UTI?
Antibiotics should be prescribed for individuals using catheters reporting symptoms of a UTI ideally only once the results of urine culture and sensitivity tests are available, and the choice of antibiotics should take into account recommendations in local antibiotic formularies as part of antimicrobial stewardship. However, in certain instances, antibiotics may need to be started empirically beforehand depending upon the severity of symptoms and the risk for developing complications if antibiotics are delayed.
Recommendation: Antibiotic treatment of urinary tract infections in individuals with neurological diseases who use catheters should be guided by the results of urine culture and antibiotic sensitivity. (Good practice statement; Consensus: 100%).
Clinical Question 19. Should advice for tibial nerve stimulation versus no advice be offered to individuals with neurological disease and urinary symptoms?
Tibial nerve stimulation is a safe and effective treatment for managing urinary storage symptoms in individuals with neurological disease [13, 50]. Both the percutaneous (PTNS) and the transcutaneous techniques (TTNS) can be considered.
Recommendation: Tibial nerve stimulation may be offered to individuals with neurological diseases having urinary symptoms who do not respond well to or cannot tolerate other treatments. Patient preference should be considered. The benefits and potential risks/burdens should be discussed. (Evidence-based recommendation; Strength: weak; LoE: II; Consensus: 82% for recommendation wording and 88% for recommendation strength).
Clinical question 20. Should advice for appliances versus no advice be offered to individuals with neurological disease and urinary symptoms?
Appliances can be used for urinary containment (absorbent products (continence pads, pants) and draining aids (condom catheter)) or to facilitate bladder emptying (flasks or jugs).
Recommendation: Appliances (i.e., urine flasks, pads, diapers, condom catheters) should be offered to alleviate the social impact of urinary incontinence in selected individuals with neurological diseases having urinary symptoms. The benefits and potential risks/burdens should be discussed. (Consensus-based recommendation; Consensus: 97%).
Clinical question 21. What is the clinical effectiveness of antimuscarinic agents versus placebo or non-pharmacological measures, or comparison with different pharmacological interventions or no treatment for individuals with neurological disease and urinary symptoms?
Antimuscarinic agents improve clinical symptoms such as urinary urgency, voided volumes, and urinary incontinence, as well as urodynamic parameters during filling cystometry, including maximum cystometric capacity (volume when voiding can no longer be delayed) and bladder compliance (measure for the distensibility of the bladder) [13, 50]. Their adverse effects should be considered before prescribing, including potential impact on neurological symptoms.
Recommendation: Antimuscarinic drugs should be offered to individuals with neurological diseases and urinary storage (overactive bladder) symptoms. The benefits and potential risks/burdens should be discussed. (Evidence-based recommendation; Strength: strong; LoE: I, Consensus: 100% for recommendation wording and 94% for recommendation strength).
Clinical question 22. What is the clinical effectiveness of beta-3 adrenoceptor agonists versus placebo or non-pharmacological measures, or comparison with different pharmacological interventions or no treatment for individuals with neurological disease and neurogenic urinary symptoms?
Beta-3 adrenoceptor agonists offer comparable patient-reported outcomes and a superior safety profile to antimuscarinic agents [13, 20, 29, 50].
Recommendation: Beta-3 adrenoceptor agonists should be offered to individuals with neurological diseases and urinary storage (overactive bladder) symptoms. The benefits and potential risks/burdens should be discussed. (Evidence-based recommendation; Strength: strong; LoE: II; Consensus: 94% for recommendation wording and 88% for recommendation strength).
Clinical question 23. What is the clinical effectiveness of cholinergic drugs versus placebo or non-pharmacological measures, or comparison with different pharmacological interventions or no treatment be offered for individuals with neurological disease and neurogenic urinary symptoms?
Cholinergic drugs are expected to improve voiding function in individuals with detrusor underactivity by activating muscarinic receptors. However, the evidence supporting their use is limited due to the low quality of studies [13, 29, 37].
Recommendation: There is insufficient evidence to recommend the use of cholinergic drugs to promote bladder emptying in individuals with neurological diseases having urinary retention due to detrusor underactivity. (Consensus-based recommendation; Consensus: 94%).
Clinical question 24. What is the clinical effectiveness of desmopressin versus placebo or non-pharmacological measures, or comparison with different pharmacological interventions or no treatment for individuals with neurological disease and neurogenic urinary symptoms?
Desmopressin, a synthetic arginine-vasopressin analogue, reduces urine volume by promoting water reabsorption in the renal collecting ducts and the ascending limb of the loop of Henle. Taken at bedtime, desmopressin has been shown to reduce nocturnal urine production and nocturia.
Recommendation: Desmopressin may be offered to selected individuals with neurological diseases who experience nocturia or nocturnal polyuria that affects their quality of life. The benefits and potential risks/burdens should be discussed. (Consensus-based recommendation; Consensus: 91%).
Clinical question 25. What is the clinical effectiveness of α1-adrenoceptor blockers versus placebo or non-pharmacological measures, or comparison with different pharmacological interventions or no treatment for individuals with neurological disease and neurogenic urinary symptoms?
α1-adrenoceptor blockers reduce bladder outlet resistance by decreasing urethral resistance and are recommended for use in individuals with neurological disease [13, 50]. They have been shown to improve urinary storage symptoms and emptying in individuals with SCI, PD, and MS.
Recommendation: α1-adrenoceptor blockers could be offered to select individuals with neurological diseases who experience voiding symptoms. The benefits and potential risks/burdens should be discussed. (Evidence-based recommendation; Strength: weak; LoE III; Consensus: 100% recommendation for wording and 82% for recommendation strength in Delphi round 2).
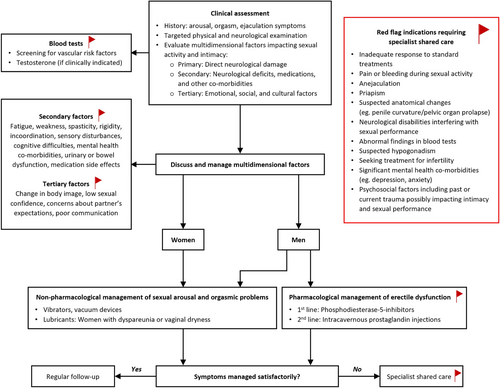
3.3 Section 3: Assessment of Sexual Symptoms
Table 3 and Appendix S7 present the evidence supporting recommendations made for the assessment of sexual symptoms, and Figure 3 illustrates the assessment and treatment algorithm of sexual symptoms based on the recommendations.
Clinical question 26. Should neurologists obtain a history of a patient's sexual symptoms versus not asking about sexual symptoms? Should there be a multidimensional assessment about primary, secondary, and tertiary factors versus no multidimensional assessment?
History taking forms the cornerstone of the assessment, and this is summarised as a checklist in Table 5. High-quality guidelines for individuals with neurological disease highlight the importance of a comprehensive medical and sexual history in evaluating sexual dysfunction, emphasising primary, secondary, and tertiary sexual dysfunctions. A detailed history should explore sexual dysfunction's nature, onset, and impact, including specific challenges faced by those with SCI or post-stroke, considering both physical and psychosocial factors. Sexual orientation, relationship history, emotional well-being, substance use, and previous treatments should also be assessed [13, 85-90, 95, 109].
Recommendation: Neurologists should actively ask individuals with neurological diseases about sexual problems regularly and explore multidimensional contributing factors. (Good practice statement; Consensus: 97%).
Clinical Question 27. Should Individuals With Neurological Disease Reporting Sexual Problems Undergo a Focused Physical Assessment Versus no Physical Assessment?
Identifying the physical contributors to sexual dysfunction in individuals with neurological disorders is crucial for effective management and treatment planning. These individuals should undergo a targeted physical examination when necessary.
Recommendation: Neurologists should perform a targeted physical examination when appropriate in individuals with neurological diseases who experience sexual problems. (Good practice statement; Consensus: 85%).
Clinical Question 28. Should Individuals With Neurological Disease Reporting Sexual Dysfunction Undergo Further Laboratory Diagnostic Evaluations (Vascular Risk Factors) Versus no Diagnostic Evaluation?
Evidence suggests that men with erectile dysfunction (ED) should undergo vascular risk screening, including fasting glucose, HbA1c, and lipid profiles, based on guidelines not aimed at individuals with neurological disease. ED is now seen as a standalone risk for cardiovascular disease and a potential early sign of diabetes, necessitating baseline measurements of serum lipids and glucose. If no recent tests are available, a comprehensive lipid and glucose profile is recommended. Although not all tests will diagnose ED directly, they offer a chance to uncover important co-morbidities. Serum testosterone should be measured in those showing signs of hypogonadism, with morning blood samples being preferred for accuracy [85, 87, 89, 90, 93, 95-98]. Screening for urogenital cancers and sexually transmitted infections (STI) may be required on an individualized basis.
Recommendation: Individuals with neurological diseases who have sexual problems should undergo screening laboratory testing for additional contributing factors in the appropriate clinical context. (Good practice statement; Consensus: 97% in Delphi round 2).
Clinical Question 29. Should Individuals With Neurological Disease Reporting Sexual Problems Undergo Further Instrumental Diagnostic Evaluations (Ex: MRI, Neurophysiology) Versus no Diagnostic Evaluation?
Routine instrumental diagnostic tests, such as pelvic neurophysiology and MRI, are not typically necessary for individuals with neurological diseases experiencing sexual dysfunction, as these tests often do not provide additional information beyond a thorough history and examination. These tests include the assessment of pelvic somatic sensory and motor functions, reflexes, and autonomic innervation, and may be useful in specific situations such as the assessment of unexplained urogenital dysfunction, evaluating incidental spinal MRI findings, cases of pelvic trauma, or for medico-legal reasons. The tests should be reserved for specialist settings where they can be accurately performed and interpreted [13, 89, 96].
Recommendation: Diagnostic evaluations such as pelvic neurophysiology and MRI are not recommended for individuals with neurological diseases and sexual problems, except in specific clinical situations. (Consensus-based recommendation; Consensus: 100%).
Clinical Question 30. Should There Be Red Flags That Initiate a Specialist Referral for Individuals With Neurological Disease Reporting Sexual Problems Versus Not Initiating a Specialist Referral?
Individuals with neurological diseases facing sexual issues should seek specialist consultation under specific circumstances.
Recommendation: Neurologists should refer individuals with neurological diseases with complex sexual dysfunction for specialist care to avoid missing potentially treatable conditions. (Good practice statement; Consensus: 97%).
3.4 Section 4: Treatment of Sexual Symptoms
Table 4 and Appendix S8 present the evidence supporting recommendations made for the treatment of sexual symptoms, and Figure 3 illustrates the assessment and treatment algorithm of sexual symptoms based on the recommendations.
Clinical Question 31. Should Education Be Offered to Individuals With Neurological Disease Reporting Sexual Problems vs. Should Not Be Offered?
Healthcare professionals should discuss issues related to sexual function, sexual activity, and sexuality with their patients while respecting professional boundaries and considering the individual's interest [89, 93, 95, 96, 110, 111].
Recommendation: Individuals with neurological diseases having sexual problems should be informed about factors that can impact sexual activity and intimacy. (Good practice statement; Consensus: 94%).
Clinical Question 32. Should Lubricants Be Used for Individuals With Neurological Disease Reporting Sexual Problems vs. no Treatment?
When suggesting the use of lubricants, it is important to consider compatibility with condoms, individual sensitivity, and the presence of possible skin irritants. There are several available products on the market, and there is insufficient evidence to recommend using one product type over another [100, 101].
Recommendation: Vaginal lubricants may be considered for female individuals with neurological diseases who experience dyspareunia or vaginal dryness. The benefits and potential risks/burdens should be discussed. (Consensus-based recommendation; Consensus: 97%).
Clinical Question 33. Should Vibrators Be Used for Individuals With Neurological Disease Reporting Sexual Problems vs. no Treatment?
Evidence on the use of vibrators for individuals with neurological disease is limited, primarily based on expert opinion without randomised controlled trials. Individuals should consult trained healthcare professionals to select suitable vibrators and consider contraindications and risks [102, 103].
Recommendation: The use of vibrators may be discussed with individuals with neurological diseases experiencing sexual problems. The benefits and potential risks/burdens should be discussed. (Good practice statement; Consensus: 85% in Delphi round 2).
Clinical Question 34. Should Vacuum Devices Be Used for Individuals With Neurological Disease Reporting Sexual Dysfunction vs. Placebo or no Treatment?
Individuals should consult healthcare professionals to select and learn to integrate appropriate vacuum devices into sexual relationships according to their preferences. While no guidelines address vacuum device use for female sexual issues, the FDA has approved a device to enhance female sexual function, addressing sensation, lubrication, and orgasmic ability [13, 87].
Recommendations: Vacuum devices may be offered as a second-line treatment to male individuals with neurological diseases who experience ED. The benefits and potential risks/burdens should be discussed. (Evidence-based recommendation; Strength: weak; LoE III; Consensus: 88% for recommendation wording and 88% for recommendation strength).
To make an evidence-based recommendation for female individuals, more research is required. However, the panel agrees that vacuum devices may be discussed with female individuals with neurological diseases having sexual arousal problems. (Consensus-based recommendation; Consensus: 82% in Delphi round 2).
Clinical Question 35. Should Phosphodiesterase-5 (PDE5) Inhibitors Be Used for Individuals With Neurological Disease Reporting Sexual Problems vs. Placebo or no Treatment?
Evidence supporting PDE5 inhibitors stems from two high-quality guidelines and five randomized controlled trials across various neurological conditions, including SCI, MS, and PD. Given their potential side effects, PDE5 inhibitors should be prescribed by and discussed with a qualified healthcare professional [13, 85].
Recommendation: PDE5 inhibitors should be offered as a first-line treatment to male individuals with neurological diseases who experience ED. The benefits and potential risks/burdens should be discussed. (Evidence-based recommendation; Strength: strong; LoE II; Consensus: 100% for recommendation wording, 97% for recommendation strength).
Clinical Question 36. Should Prostaglandins Be Used for Individuals With Neurological Disease Reporting Sexual Problems vs. Placebo or no Treatment?
Intracavernous prostaglandin injections can be a treatment especially when oral PDE5 inhibitors fail or are not advisable due to severe cardiovascular conditions (like unstable angina, recent stroke or heart attack, or significant liver impairment), following high-quality guidelines for individuals with neurological disease [13]. This treatment should be offered by a trained professional [13, 85, 87, 89, 93, 95, 112].
Recommendation: Intracavernous injections of prostaglandin should be offered as a second-line treatment to male individuals with neurological diseases who experience ED. The benefits and potential risks/burdens should be discussed. (Evidence-based recommendation; Strength: weak; LoE: III; Consensus: 91% for recommendation wording, 82% for recommendation strength).
Clinical question 37. Should treatment of secondary causes (e.g., spasticity/fatigue/incontinence/pain/depression) and tertiary causes (e.g., loss of self-esteem/poor body image) be offered to individuals with neurological disease reporting sexual problems vs no treatment?
Secondary and tertiary factors contributing to sexual dysfunction (listed in Table 5) should be addressed and managed [85, 87].
Recommendation: Multidimensional factors interfering with sexual activity and intimacy (including secondary factors such as spasticity, fatigue, incontinence, cognitive co-morbidities, medication side effects and tertiary factors such as changes in self or body image) should be addressed in individuals with neurological disorders experiencing sexual problems. Given their dynamic nature, these factors should be discussed on a regular basis. (Good practice statement; Consensus: 100%).
4 Discussion
Several high-quality guidelines on the assessment and management of neurogenic urinary and sexual dysfunction have already been published. However, these guidelines have not been specifically tailored toward neurologists, making it challenging to integrate many of the recommendations into neurology practice. The development of NEUROGED guidelines uniquely involved neurologists, urologists, and patient representatives from the onset. This collaborative effort aimed to ensure that the recommendations would be relevant and practical for neurological practice, addressing the specific needs and challenges faced by neurologists in managing neurogenic urogenital dysfunction.
Anticipating low levels of evidence for several of the PICO-structured clinical questions, the steering committee received methodological advice to develop recommendations using existing guidelines and adopted the ADAPTE framework.
Guidelines of the highest quality, as determined by the AGREE II tool, were given preference. However, lower quality guidelines were also reviewed for PICOs where there were few or no existing recommendations. This approach allowed the committee to address questions that were clinically relevant to individuals with neurological disorders where there were low levels of evidence. Consequently, there were only 11 evidence-based recommendations, and just 6 received a strong strength of recommendation. There is a need for further research to address gaps in the evidence, particularly the PICOs that received consensus-based recommendations.
The recommendations were developed collaboratively between neurologists and urologists across a wide spectrum of healthcare settings and were prepared with an international audience of practising neurologists in mind. The guidelines empower neurologists to assess and manage urinary and sexual symptoms reported by their neurological patients; however, they importantly define limits to practise through red flag symptoms and test findings that would warrant a sharing of care with their urology colleagues. A limitation of adapting the guideline using the ADAPTE framework was that the primary evidence base was reviewed only for PICOs where recommendations were not available in existing guidelines and had to be developed de novo. Despite the focus on developing practical recommendations, challenges in their implementation will be expected due to limited expertise and time and resource constraints, and this will be addressed separately. The task force intends for the NEUROGED guidelines to also serve as a framework for training neurologists in the assessment and treatment of urogenital symptoms.
In conclusion, guidelines for the assessment and management of urogenital symptoms specifically intended for practising neurologists have been prepared for the first time. They have been approved on 6th December 2024 and will be formally updated after 5 years in 2029.
Author Contributions
Jalesh N. Panicker: conceptualization, methodology, funding acquisition, data curation, supervision, resources, project administration, formal analysis, software, validation, visualization, writing – review and editing, writing – original draft, investigation. Alessandra Fanciulli: conceptualization, investigation, funding acquisition, writing – original draft, methodology, validation, visualization, writing – review and editing, software, formal analysis, project administration, data curation, supervision, resources. Magdalena Krbot Skoric: conceptualization, investigation, writing – original draft, methodology, validation, visualization, writing – review and editing, software, formal analysis, project administration, data curation, supervision, resources. Tamara Kaplan: conceptualization, writing – original draft, methodology, validation, visualization, writing – review and editing, software, formal analysis, project administration, resources. Katina Aleksovska: conceptualization, investigation, writing – original draft, methodology, validation, writing – review and editing, software. Ivan Adamec: methodology, writing – review and editing, formal analysis, validation, writing – original draft, conceptualization. Marcio Augusto Averbeck: methodology, writing – review and editing, validation, formal analysis, writing – original draft, conceptualization. Nicole Campese: writing – original draft, methodology, validation, writing – review and editing, formal analysis, conceptualization. Pietro Guaraldi: conceptualization, methodology, writing – original draft, writing – review and editing, formal analysis. Fabian Leys: conceptualization, writing – original draft, methodology, writing – review and editing, formal analysis. Jorge Moreno-Palacios: conceptualization, writing – original draft, methodology, writing – review and editing, formal analysis. Sara Simeoni: conceptualization, writing – original draft, methodology, writing – review and editing, formal analysis. Iva Stankovic: conceptualization, writing – original draft, methodology, writing – review and editing, formal analysis. Sarah Wright: conceptualization, writing – original draft, methodology, writing – review and editing, formal analysis. Amit Batla: validation, writing – review and editing, formal analysis. Bertil Blok: validation, writing – review and editing, formal analysis. Claire Hentzen: validation, writing – review and editing, formal analysis. Max Josef Hilz: validation, writing – review and editing, formal analysis. Thomas M. Kessler: validation, writing – review and editing, formal analysis. Helmut Madersbacher: validation, writing – review and editing, formal analysis. Kannan Rajasekharan Nair: validation, writing – review and editing, formal analysis. Krishnan Padmakumari Sivaraman Nair: validation, writing – review and editing, formal analysis. Mahreen Pakzad: validation, writing – review and editing, formal analysis. Anne Pavy-Le Traon: validation, writing – review and editing, formal analysis. Guy Peryer: validation, writing – review and editing, formal analysis. Mikolaj Przydacz: validation, writing – review and editing, formal analysis. Ryuji Sakakibara: validation, writing – review and editing, formal analysis. Udit Saraf: validation, writing – review and editing, formal analysis. Matthew Smith: validation, writing – review and editing, formal analysis. Walter Struhal: validation, writing – review and editing, formal analysis. Roland D. Thijs: validation, writing – review and editing, formal analysis. Katarina Ivana Tudor: validation, writing – review and editing, formal analysis. Marcin Tutaj: validation, writing – review and editing, formal analysis. David B. Vodušek: validation, writing – review and editing, conceptualization, formal analysis. Gregor Wenning: validation, writing – review and editing, formal analysis. Mario Habek: conceptualization, investigation, funding acquisition, writing – original draft, methodology, validation, visualization, writing – review and editing, software, formal analysis, project administration, data curation, supervision, resources.
Acknowledgements
The authors would like to acknowledge the contributions of Ms. Toni Tan for methodological support and Ms. Helena Markulin for conducting the literature search. J.N.P. is supported in part by funding from the United Kingdom's Department of Health NIHR University College London Hospitals Biomedical Research Centres funding scheme. K.P.S.N. is partly supported by the National Institute for Health and Care Research (NIHR) Sheffield Biomedical Research Centre (BRC) and NIHR Sheffield Clinical Research Facility (CRF). G.P. is supported by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration East of England (NIHR ARC EoE) at Cambridgeshire and Peterborough NHS Foundation Trust. The views expressed are those of the author[s] and not necessarily those of the NIHR or the Department of Health and Social Care. M.H. acknowledges support from the Croatian Science Foundation. The project was funded by the EAN, EFAS, and INUS, and task force members received no remuneration for their work. The NEUROGED guidelines have been endorsed by the European Research Network for Rare Neurological Disorders. The American Academy of Neurology affirms the value of the EAN/EFAS/INUS guidelines for practising neurologists on the assessment and treatment of neurogenic urinary and sexual symptoms (NEUROGED guidelines) as an educational tool for neurologists. Open Access funding provided by Medizinische Universitat Innsbruck/KEMÖ. [Correction added on 8 May 2025 after first online publication: KEMÖ-DEAL funding statement has been added.]
Conflicts of Interest
J.N.P.: Consultant (Idorsia, Coloplast, Medice), Speaker Honorarium (Coloplast, Wellspect), Royalties (Cambridge University Press). A.F.: Royalties from Springer Verlag, speaker fees, and honoraria from Theravance Biopharma, GE Health Care, Bial, CNSystems, Broadview Ventures, KABEG, Austrian Autonomic Society, Elsevier, International Parkinson Disease and Movement Disorders Society, Austrian Neurology Society, Austrian Autonomic Society, and research grants from the FWF-Austrian Science Fund, Medical University of Innsbruck, US MSA Coalition, Dr. Johannes and Hertha Tuba Foundation, and Austrian Exchange Program, outside of the present work. M.K.S.: Participated as a clinical investigator and/or received speaker fees fromSanofi Genzyme, Merck, Novartis, and Roche. I.A.: Participated as a clinical investigator and/or received consultation and/or speaker fees from Biogen, Sanofi Genzyme, Merck, Novartis, Roche, and AstraZeneca. M.A.A.: Consultant (Coloplast), speaker honorarium (Medtronic, Coloplast, GSK, Boston Scientific). J.M.P.: Speaker Honorarium (Asofarma, Convatec). A.B.: Has received a speaker honorarium from Ipsen Pharma and receives royalties from the book “Understanding Parkinsonism” (Jaypee brothers 2017). B.B.: Consultant (Coloplast), Speaker Honorarium (Coloplast), Research Grant (Axonics). C.H.: Consultant: Convatec, BBraun. Speaker: IPSEN, Abbvie, Hollister Inc., Convatec. K.P.S.N.: Has led clinical trials on spasticity in M.S. for Celgene and GWS pharma and received royalties from Cambridge University Press. M.S.: Honoraria from Abbvie. R.D.T.: Lecture and consultancy fees from Medtronic, UCB, Theravarance, LivAssured, Zogenix, Novartis, and Arvelle, and grants from Medtronic and NewLife Wearables. M.T.: Works as a DBS (deep brain stimulation) consultant for Medtronic. M.H.: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Novartis, Roche, Astra Zeneca, and received funding from the Croatian Science Foundation. T.K., K.A., P.G., N.C., F.L., S.S., I.S., S.W., M.J.H., T.M.K., H.M., K.R.N., A.P.L.T., M.P., G.P., M.P., R.S., U.S., W.S., K.I.T., D.B.V., and G.W.: None declared.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the Supporting Information of this article.





