Association of dementia with impaired kidney function and plasma biomarkers: A population-based study
Abstract
Background and Purpose
Emerging evidence has linked impaired kidney function with dementia in older adults, but the neuropathological pathways underlying their association remain poorly understood. We sought to examine the relationships of kidney function with dementia and plasma biomarkers in a Chinese rural population.
Methods
This population-based study used data from the baseline examination of the Multimodal Interventions to Delay Dementia and Disability in rural China (MIND-China) cohort (March–September 2018; n = 5715). Kidney function was assessed using estimated glomerular filtration rate (eGFR) based on serum creatinine level. Dementia, Alzheimer's disease (AD) and vascular dementia (VaD) were diagnosed according to the international criteria. Plasma biomarkers were measured using the SIMOA platform in a subsample (n = 1446). Data were analyzed using logistic, general linear, and mediation models.
Results
Of the 5715 participants, 306 were diagnosed with dementia, including 195 with AD and 100 with VaD. Impaired kidney function (eGFR <60 vs. ≥90 mL/min/1.73 m2) was associated with multivariable-adjusted odds ratios of 2.24 (95% confidence interval [CI] 1.44–3.46) for all-cause dementia, 1.85 (1.07–3.18) for AD, and 2.49 (1.16–5.22) for VaD. In the biomarker subsample, impaired kidney function was significantly associated with higher plasma amyloid-β (Aβ)40 (β-coefficient = 54.36, 95% CI 43.34–65.39), Aβ42 (β-coefficient = 3.14, 95% CI 2.42–3.86), neurofilament light chain (β-coefficient = 10.62, 95% CI 5.62–15.62), and total tau (β-coefficient = 0.68, 95% CI 0.44–0.91), and a lower Aβ42/Aβ40 ratio (β-coefficient = −4.11, 95% CI −8.08 to −0.14). The mediation analysis showed that plasma total tau significantly mediated 21.76% of the association between impaired kidney function and AD (p < 0.05).
Conclusion
Impaired kidney function is associated with dementia and plasma biomarkers among rural-dwelling older Chinese adults, and the association with AD is partly mediated by plasma biomarkers for neurodegeneration.
INTRODUCTION
Dementia is a devastating disorder that poses huge economic and societal burden. In 2019, ~58 million people worldwide were affected by dementia, with the number being projected to reach nearly 153 million by 2050 [1]. While there is currently no cure for dementia, global efforts have been made to identify modifiable risk factors that can be targeted for interventions to reduce the risk or delay the onset of dementia [2, 3].
In recent years, evidence has emerged that impaired kidney function is associated with an increased risk of dementia, including Alzheimer's disease (AD) and vascular dementia (VaD) [4-7], although several studies showed no association between impaired kidney function and dementia risk [8-11]. Notably, the majority of the previous population-based studies have been conducted in North American and European populations, in whom the prevalence of impaired kidney function and chronic kidney disease (CKD) differs considerably from that in Chinese adult populations [12]. Thus, investigating the association between impaired kidney function and dementia in the ethnically, geographically, and socio-culturally diverse population in China may help bridge the knowledge gap.
In addition, the neuropathological mechanisms underlying the association between impaired kidney function and dementia are poorly understood. It has been suggested that ~40%–60% of brain-derived amyloid-β (Aβ) is cleared through transport across the blood–brain barrier into the peripheral circulatory system and that the kidney is involved in clearance of circulating Aβ and tau proteins [13, 14]. Previous studies have shown that plasma Aβ and total tau (t-tau) concentrations are increased in patients with CKD [15-18]. However, to what extent plasma biomarkers for Alzheimer's pathologies or neurodegeneration in the brain might mediate the association of impaired kidney function with dementia or AD remains unclear.
In this population-based cross-sectional study, therefore, we sought to investigate the associations of impaired kidney function with dementia and main subtypes of dementia among rural older adults in China, and further to explore the mediation of plasma biomarkers in their associations. We hypothesized that impaired kidney function was associated with dementia in older adults and that the associations might be partly mediated by plasma biomarkers for AD and neurodegeneration.
METHODS
Study population
This was a population-based cross-sectional study. The study participants were derived from the Multimodal Interventions to Delay Dementia and Disability in rural China (MIND-China) [19, 20], a participating project in the World-Wide FINGERS Network [3]. In brief, MIND-China targeted people who were aged 60 years and older and living in the 52 villages of Yanlou Town, Yanggu County, western Shandong province, China. In March–September 2018, 5765 individuals (74.9% of all eligible persons) undertook the baseline examination. Of these, 50 participants were excluded due to missing information on serum creatinine (n = 1) and insufficient information for dementia diagnosis (n = 49), yielding an analytical sample of 5715 individuals for the analysis involving kidney function and dementia (Analytical Sample 1). In addition, data on AD-related plasma biomarkers were available in a subsample of 1446 individuals (Analytical Sample 2) that was randomly selected using a cluster (village)-based sampling approach from all the 52 villages. This biomarker subsample was used to analyze the relationship of kidney function with plasma biomarkers. Figure 1 shows the flowchart of the study participants.
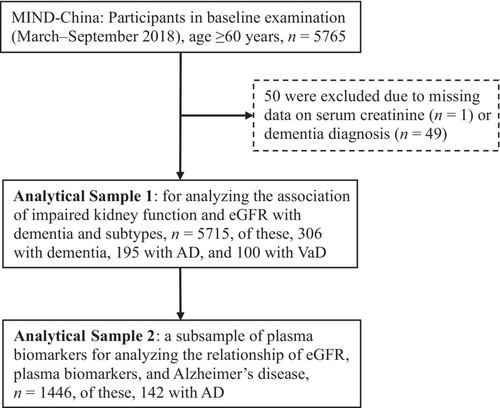
The MIND-China project was approved by the Ethics Committee at Shandong Provincial Hospital affiliated to Shandong University in Jinan, Shandong. All participants provided written informed consent prior to data collection, or in case of persons with severe cognitive impairment, their informants provided written informed consent. Research within MIND-China has been conducted in accordance with the ethical principles expressed in the Declaration of Helsinki as well as national relevant guidelines and regulations. MIND-China was registered in the Chinese Clinical Trial Registry (registration no. ChiCTR1800017758).
Data collection and assessments
The procedure of baseline data collection and definitions was fully described elsewhere [20, 21]. In brief, all data were collected via face-to-face interviews, clinical and neurological examinations, neuropsychological testing, and laboratory tests by trained research staff. We collected the data using a structured questionnaire that covered demographic features (e.g., age, sex, and education), lifestyles (e.g., smoking and alcohol drinking status), health history (e.g., hypertension, diabetes, dyslipidemia, coronary heart disease [CHD], and stroke), and cognitive function. Arterial blood pressure was measured on the right arm in a sitting position using an electronic sphygmomanometer (HEM-7127J; Omron Corporation, Kyoto, Japan) after at least a 5-min rest. Height and weight were measured in light clothes without shoes. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Educational achievement was classified as no formal school education, primary school, and middle school or above. Smoking and alcohol consumption were dichotomized as current or not current smoking or drinking alcohol. After an overnight fast, peripheral blood samples were taken and fasting blood glucose and blood lipids (e.g., total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and very low-density lipoprotein cholesterol) were measured using an automatic analyser (DIRUI CS-600B; DIRUI Corporation, Changchun, China). Hypertension, diabetes, dyslipidemia, CHD, and stroke were defined and ascertained as previously described [20]. Apolipoprotein E (APOE) genotypes were determined using multiple polymerase chain reaction through MultipSeqCustom Panel (iGeneTech, Beijing, China) according to the manufacturer's recommendations and Sanger sequencing [22]. The APOE genotype was dichotomized into carriers versus noncarriers of the ε4 allele.
Assessment of kidney function
Serum creatinine was measured using the identical method on an automatic biochemical analyzer (DIRUI CS-600B, Changchun, China). The estimated glomerular filtration rate (eGFR) was calculated using the 2021 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations based on serum creatinine [23]. Kidney function was categorized into normal function (eGFR ≥90 mL/min/1.73m2), mildly decreased function (60–90 mL/min/1.73 m2), and impaired function (<60 mL/min/1.73 m2) [24].
Diagnosis of dementia and subtypes of dementia
Dementia was clinically diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria [25], in which a three-step procedure was followed, as previously reported [21]. Briefly, the trained medical staff performed the first face-to-face interview, clinical examination, and cognitive and physical functional testing, and recorded all the information using the structured questionnaire. Then, neurologists specialized in dementia diagnosis and care reviewed all the records to screen people who were suspected to have dementia or who had insufficient information for a clinical judgement of dementia status for further evaluation. Finally, the neurologists conducted the second face-to-face interview with people who were selected in Step 2 or informants or both, and the diagnosis of dementia was made based on all the assessments. AD was diagnosed according to the National Institute on Aging-Alzheimer's Association criteria for probable Alzheimer's dementia [26]. The diagnosis of vascular dementia (VaD) was made according to the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l'Enseignement en Neurosciences (AIREN) criteria for probable VaD [27], which was based essentially on a history or clinical evidence of stroke and the clear temporal relationship between stroke and onset of dementia.
Measurements of plasma Aβ42, Aβ40, t-tau, and neurofilament light chain
After an overnight fast, peripheral blood samples were collected into EDTA-coated vacutainers, followed by centrifugation to obtain plasma. The plasma samples were stored at −80°C and thawed immediately before component quantification. Concentrations of plasma Aβ42, Aβ40, t-tau, and neurofilament light chain (NfL) were measured using a Human Neurology 3-Plex A assay (N3PA) Kit run on the fully automated single molecule array (SIMOA) technology (Quanterix Corp, MA, USA) according to the manufacturer's protocol, as previously reported [28]. Two quality control samples were run in duplicate on each plate for each analyte. The upper and lower detection limits for the quality control sample of each index were within the range indicated in the manual, and the inter-assay coefficient of variability and inter-plate inter-assay coefficient of variability were controlled within 13%.
Statistical analysis
We present frequency (%) for categorical variables, mean (standard deviation [SD]) for continuous variables with normal distribution, and median (interquartile range [IQR]) for continuous variables with skewed distribution. Characteristics and plasma biomarkers of study participants by eGFR levels (<60, 60–90, and ≥90 mL/min/1.73 m2) were compared using the chi-squared test for categorical variables, one-way analysis of variance for continuous variables with normal distribution, and the Kruskal–Wallis test for continuous variables with skewed distribution. We performed logistic regression analysis to estimate the odds ratio (OR) and 95% confidence interval (CI) of all-cause dementia, AD, and VaD, respectively, associated with impaired kidney function and eGFR levels. We used linear regression models to examine the associations of impaired kidney function and eGFR with plasma biomarkers for AD and neurodegeneration. We reported the main results from two models: Model 1 was adjusted for age, sex, and education and Model 2 was additionally adjusted for BMI, alcohol consumption, smoking, diabetes, hyperlipidemia, hypertension, CHD, stroke, and APOE genotype. Restricted cubic spline analysis was performed with three knots at the 10th, 50th, and 90th percentiles to flexibly model the possible non-linear patterns of association between eGFR as a continuous variable and the likelihoods of all-cause dementia, AD, and VaD. Statistical significance was set at a two-tailed p value < 0.05. Statistical analyses were performed using R version 4.1.1 (R Core Team, 2023. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org).
We employed mediation models to examine the mediation effect of plasma biomarkers in the association of eGFR with AD, controlling for age, sex, and education, BMI, smoking, alcohol consumption, diabetes, hyperlipidemia, hypertension, CHD, stroke, and APOE genotype, and the bootstrapping method (based on 5000 resamples) was used to estimate the corresponding 95% CI. The mediation effect was considered statistically significant if the bootstrap 95% CI of the β coefficient did not include zero. We used MPlus version 8.3 for mediation analysis.
RESULTS
Characteristics of study participants
The mean (SD; range) age of the 5715 participants was 70.9 (5.9; 60–99) years, 57.2% were women, and 40.5% had no formal schooling. Of these, only 393 (6.9%) were defined as having impaired kidney function (eGFR<60 mL/min/1.73 m2). Participants with impaired kidney function (vs. normal kidney function) were older, were more likely to be women, and less educated, and were less likely to smoke and drink alcohol, and had a higher prevalence of obesity, diabetes, hypertension, dyslipidemia, and CHD (Table 1). In addition, 306 participants (5.4%) were diagnosed with dementia, including 195 (3.4%) with AD and 100 (1.7%) with VaD.
| Characteristics | Total sample | eGFR | |||
|---|---|---|---|---|---|
| (n = 5715) | ≥90 mL/min/1.73 m2 (n = 2027) | 60–90 mL/min/1.73 m2 (n = 3295) | <60 mL/min/1.73 m2 (n = 393) | p value | |
| eGFR, mL/min/1.73 m2, median (IQR) | 84.6 (19.4) | 94.9 (4.4) | 78.6 (12.9) | 53.8 (10.3) | <0.001 |
| Age, years | 70.9 (5.9) | 68.9 (3.9) | 71.8 (6.4) | 73.8 (7.6) | <0.001 |
| Women, n (%) | 3266 (57.2) | 980 (48.3) | 2016 (61.2) | 270 (68.7) | <0.001 |
| Education, n (%) | |||||
| Illiterate | 2314 (40.5) | 700 (34.6) | 1400 (42.5) | 214 (54.5) | |
| Primary school | 2398 (42.0) | 895 (44.2) | 1377 (41.8) | 126 (32.1) | <0.001 |
| Middle school or above | 1003 (17.6) | 432 (21.3) | 518 (15.7) | 53 (13.5) | |
| BMI, kg/m2 | 24.9 (3.8) | 24.5 (3.7) | 25.0 (3.8) | 25.4 (4.1) | <0.001 |
| Current smoking, n (%) | 1200 (21.0) | 525 (25.9) | 628 (19.1) | 47 (12.0) | <0.001 |
| Current drinking, n (%) | 1663 (29.1) | 721 (35.6) | 861 (26.1) | 81 (20.6) | <0.001 |
| Diabetes, n (%) | 819 (14.3) | 287 (14.2) | 450 (13.7) | 82 (20.9) | 0.001 |
| Hyperlipidemia, n (%) | 1388 (24.3) | 402 (19.8) | 862 (26.2) | 124 (31.6) | <0.001 |
| Hypertension, n (%) | 3806 (67.3) | 1232 (60.8) | 2301 (69.8) | 273 (69.5) | <0.001 |
| CHD, n (%) | 1219 (21.3) | 344 (17.0) | 757 (23.0) | 118 (30.0) | <0.001 |
| Stroke, n (%) | 895 (15.7) | 309 (15.2) | 511 (15.5) | 75 (19.1) | 0.151 |
| APOE ε4 allele, n (%) | 873 (16.0) | 315 (15.5) | 504 (15.3) | 54 (13.7) | 0.712 |
- Note: Data are mean (standard deviation), unless otherwise specified. Missing values were as follows: alcohol consumption, n = 5; current smoking, n = 6; BMI, n = 34; hypertension, n = 52; APOE genotype n = 249; stroke, n = 7. In subsequent analyses, categorical variables with missing values were replaced with a dummy variable, and continuous variables with missing values were replaced with the mean value.
- Abbreviations: APOE, apolipoprotein E gene; BMI, body mass index; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate.
Associations of dementia with impaired kidney function and eGFR (n = 5715)
Impaired kidney function (eGFR<60 vs. ≥90 mL/min/1.73 m2) was significantly associated with an approximately 2-fold increased likelihood of all-cause dementia and AD, and a 2.5-fold increased likelihood of VaD after controlling for age, sex, and education; the associations remained statistically significant after further controlling for additional potential confounding factors (Table 2). Overall, a low eGFR level was significantly associated with increased likelihoods of all-cause dementia, AD, and VaD (p for linear trend <0.05). When kidney function was dichotomized into impaired versus non-impaired kidney function (eGFR <60 vs. ≥60 mL/min/1.73 m2), impaired kidney function was associated with the demographic-adjusted OR of 1.95 (95% CI 1.38–2.72) for all-cause dementia, 1.84 (95% CI 1.21–2.75) for AD, and 2.15 (95% CI 1.18–3.68) for VaD; the corresponding figures in the multivariable-adjusted model (Model 2) were 1.91 (1.34–2.68), 1.90 (1.25–2.88), and 1.81 (0.95–3.25), respectively.
| eGFR | N/na | Odds ratio (95% CI), dementia or subtypes | |
|---|---|---|---|
| Model 1b | Model 2b | ||
| All-cause dementia (n = 5715) | |||
| ≥90 mL/min/1.73 m2 | 2027/59 | 1.00 (reference) | 1.00 (reference) |
| 60–90 mL/min/1.73 m2 | 3295/192 | 1.18 (0.87–1.65) | 1.24 (0.90–1.72) |
| <60 mL/min/1.73 m2 | 393/55 | 2.25 (1.46–3.44)** | 2.24 (1.44–3.46)** |
| p for trend | <0.001 | <0.001 | |
| Alzheimer disease (n = 5604) | |||
| ≥90 mL/min/1.73 m2 | 2005/37 | 1.00 (reference) | 1.00 (reference) |
| 60–90 mL/min/1.73 m2 | 3251/121 | 0.94 (0.63–1.42) | 0.99 (0.65–1.49) |
| <60 mL/min/1.73 m2 | 375/37 | 1.75 (1.02–2.99)* | 1.85 (1.07–3.18)* |
| p for trend | 0.061 | 0.040 | |
| Vascular dementia (n = 5509) | |||
| ≥90 mL/min/1.73 m2 | 1989/21 | 1.00 (reference) | 1.00 (reference) |
| 60–90 mL/min/1.73 m2 | 3166/63 | 1.50 (0.91–2.57) | 1.53 (0.90–2.70) |
| <60 mL/min/1.73 m2 | 354/16 | 2.95 (1.44–5.91)** | 2.49 (1.16–5.22)* |
| p for trend | 0.004 | 0.017 | |
- Abbreviations: CI, confidence interval; eGFR, estimate glomerular filtration rate.
- a N/n indicates the number of participants/number of cases.
- b Model 1 was adjusted for age, sex, and education and Model 2 was additionally adjusted for body mass index, drinking status, smoking status, diabetes, hyperlipidemia, hypertension, coronary heart disease, stroke, and APOE genotype.
- * p <0.05.
- ** p < 0.01.
The restricted cubic spline analysis suggested a linear relationship of eGFR with the likelihood of all-cause dementia (p for nonlinearity = 0.33), but a non-linear association with AD (p for nonlinearity<0.05), and there was no significant association between eGFR and VaD (p for overall = 0.11; p for nonlinearity = 0.28 [Figure S1]).
There was no statistical interaction of impaired kidney function with age, sex or APOE genotype on the likelihood of dementia or AD (all p for interaction >0.05; data not shown).
Associations of impaired kidney function and eGFR with plasma biomarkers (n = 1446)
In the biomarker subsample (n = 1446), plasma Aβ, t-tau, and NfL concentrations were significantly higher in people with impaired kidney function (eGFR<60 mL/min/1.73 m2) than those with normal kidney function or mildly reduced kidney function (p < 0.01), but there was no significant group difference in the Aβ42/Aβ40 ratio (Figure 2). Linear regression analysis suggested that impaired kidney function (eGFR <60 vs. ≥90 mL/min/1.73 m2) was significantly associated with increased plasma Aβ42, Aβ40, NfL, and t-tau concentrations and a reduced Aβ42/Aβ40 ratio, even in the fully adjusted model (Table 3). As a categorical variable, after adjusting for multiple potential confounding factors, reduced eGFR was linearly associated with increased plasma Aβ42, Aβ40, and NfL concentrations and a decreased Aβ42/Aβ40 ratio (all p for linear trend <0.05 [Table 3]). When kidney function was dichotomized into impaired versus non-impaired kidney function (eGFR<60 vs. ≥60 mL/min/1.73 m2), impaired kidney function remained significantly associated with all the examined plasma biomarkers except Aβ42/Aβ40 ratio (Table S1).

| eGFR | No. of subjects | β-coefficient (95% CI), plasma biomarkers | |
|---|---|---|---|
| Model 1a | Model 2a | ||
| Aβ42 (pg/mL) | |||
| ≥90 mL/min/1.73 m2 | 539 | 0.00 (reference) | 0.00 (reference) |
| 60–90 mL/min/1.73 m2 | 832 | 0.54 (0.21–0.87)** | 0.55 (0.22–0.88)** |
| <60 mL/min/1.73 m2 | 75 | 3.23 (2.51–3.95)** | 3.14 (2.42–3.86)** |
| p for trend | <0.001 | <0.001 | |
| Aβ40 (pg/mL) | |||
| ≥90 mL/min/1.73 m2 | 539 | 0.00 (reference) | 0.00 (reference) |
| 60–90 mL/min/1.73 m2 | 832 | 9.07 (4.07–14.09)** | 9.67 (4.63–14.71)** |
| <60 mL/min/1.73 m2 | 75 | 53.43 (42.4–64.46)** | 54.36 (43.34–65.39)** |
| p for trend | <0.001 | <0.001 | |
| Aβ42/Aβ40 ratio × 1000 | |||
| ≥90 mL/min/1.73 m2 | 539 | 0.00 (reference) | 0.00 (reference) |
| 60–90 | 832 | −1.04 (−2.85–0.77) | −1.22 (−3.03–0.60) |
| <60 mL/min/1.73 m2 | 75 | −3.24 (−7.22–0.75) | −4.11 (−8.08 to −0.14)* |
| p for trend | 0.099 | 0.043 | |
| Total tau (pg/mL) | |||
| ≥90 mL/min/1.73 m2 | 539 | 0.00 (reference) | 0.00 (reference) |
| 60–90 mL/min/1.73 m2 | 832 | -0.14 (−0.25 to −0.03)* | −0.12 (−0.23 to −0.01)* |
| <60 mL/min/1.73 m2 | 75 | 0.65 (0.41–0.88)** | 0.68 (0.44–0.91)** |
| p for trend | 0.193 | 0.098 | |
| NfL (pg/mL)b | |||
| ≥90 mL/min/1.73 m2 | 539 | 0.00 (reference) | 0.00 (reference) |
| 60–90 mL/min/1.73 m2 | 831 | −0.72 (−2.98–1.55) | −0.38 (−2.67–1.90) |
| <60 mL/min/1.73 m2 | 75 | 10.44 (5.45–15.44)** | 10.62 (5.62–15.62)** |
| p for trend | 0.061 | 0.034 | |
- Abbreviations: Aβ, amyloid beta; CI, confidence interval; eGFR, estimate glomerular filtration rate; NfL, neurofilament light chain.
- a Model 1 was adjusted for age, sex, and education and Model 2 was additionally adjusted for body mass index, drinking alcohol, smoking, diabetes, hyperlipidemia, hypertension, coronary heart disease, stroke, and APOE genotype.
- b One participant with outlier in plasma NfL was excluded.
- * p <0.05.
- ** p < 0.01.
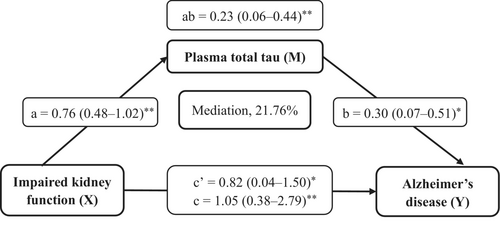
The mediation analysis showed that the cross-sectional association of impaired kidney function with AD was significantly mediated by plasma t-tau (mediation effect, ab = 0.23; 95% CI 0.06–0.44), with the proportion of mediation being 21.76% (Figure 3). We found no statistically significant mediation effects of plasma Aβ42 or NfL in the cross-sectional association of impaired kidney function with AD.
DISCUSSION
The main findings from this large-scale population-based study of rural-dwelling older adults in China were summarized as follows: (1) impaired kidney function assessed by eGFR based on serum creatinine was notably associated with an increased likelihood of all-cause dementia, AD, and VaD; (2) impaired kidney function was correlated with increased plasma amyloid (Aβ40 and Aβ42), a reduced Aβ42/Aβ40 ratio, and increased plasma biomarkers for neurodegeneration (t-tau and NfL); and (3) plasma t-tau could partly mediate the association of impaired kidney function with AD. These results indicate that impaired kidney function is linked with dementia and AD in older adults, partly via neurodegeneration.
Previous studies have yielded mixed results with regard to the association of impaired kidney function with dementia or AD [10, 11]. For instance, two population-based cohort studies of middle-aged and older adults from the United States and Germany did not show associations between eGFR levels and risk of dementia [8, 9]. The French Three-City study of people aged ≥65 years suggested that low eGFR values were not associated with incident dementia, although a decline in eGFR over time was associated with an increased risk of dementia with vascular components [6]. In contrast, the Shanghai Aging Study of community-dwelling older adults found that a lower GFR level was independently associated with a higher risk of incident dementia and AD [4]. A register-based study in Sweden showed that a lower eGFR was associated with all-cause dementia, VaD, and AD [5]. Similarly, we also found that impaired kidney function or a lower eGFR was associated with an increased likelihood of dementia, AD, and VaD. The discrepant results could be partly due to variations in sociodemographic characteristics of the study populations, methods of eGFR measurements (e.g., serum creatinine or cystatin C), or control of potential confounders (e.g., vascular disorders).
The mechanisms underlying the association of impaired kidney function with dementia and AD are not fully understood. Several mechanisms are supposed to play a role in their association. First, impaired kidney function and dementia may share common vascular risk factors such as hypertension, diabetes, and hypercholesterolemia [29]. However, the observed association was present independent of major cardiovascular risk factors, suggesting that additional pathways are involved in their association. Second, some pathophysiological conditions induced by impaired kidney function, such as chronic inflammation, oxygen-free radicals, disorders of the immune system, and retention of uremic toxins, may affect cognitive function [30, 31]. Furthermore, AD is characterized by excessive accumulation of Aβ and phosphorylated tau proteins and neurodegeneration in the brain [32], and cerebral Aβ and tau proteins can be transported across the blood–brain barrier into blood and further cleared by peripheral organs [33], in which the kidney plays a vital role [14, 15].
Previous studies have linked impaired kidney function with plasma biomarkers for AD and neurodegeneration. For instance, the H70 study from Sweden showed an association of higher plasma NfL with lower kidney function measured with eGFR [34]. Similarly, a multiethnic community-based study of people aged ≥50 years from the United States indicated that CKD was associated with higher concentrations of plasma Aβ40, Aβ42, t-tau, and NfL [35]. In line with these previous reports, our population-based study also showed that impaired kidney function or a lower eGFR was associated with elevated plasma Aβ and a decreased Aβ42/40 ratio. We further found that a low eGFR was associated with increased plasma t-tau and NfL concentrations, biomarkers for non-specific neurodegeneration that have been associated with AD dementia [36, 37]. These studies suggest that neurodegeneration associated with kidney function impairment may be involved in its association with AD, which was supported in our mediation analysis. Additionally, evidence has suggested that impaired kidney function is related to a higher burden of cerebral amyloid angiopathy, but not to Aβ or tau protein load in the brain [38]. Taken together, these studies support the potential that impaired kidney function may affect peripheral clearance of Aβ and tau proteins.
We revealed that plasma t-tau could partly mediate the association of impaired kidney function with AD, which represents an important contribution to the current literature. Although tau protein is highly abundant in the central nervous system, 80% of the circulating tau protein originates from peripheral tissues or organs [37], and tau protein is highly expressed in the kidney [39]. However, it remains unclear to what extent reduced kidney function could affect expression of tau protein in the kidney.
The major strength of our study is the large-scale community-based sample of rural-dwelling older adults in China who had low socioeconomic position and received no or very limited formal education, a demographic group that has been largely underrepresented in dementia research [40]. Furthermore, we had the interdisciplinary database (MIND-China) in which comprehensive epidemiological, clinical, and neuropsychological data were integrated with plasma biomarkers for amyloid and neurodegeneration. This provides a unique opportunity to investigate the associations of kidney function and cognitive phenotypes as well as the potential neuropathological mechanisms underlying their associations.
This study also has some limitations. First, as this was a cross-sectional study, we cannot infer a causal relationship for any of the observed associations of impaired kidney function and low eGFR with cognitive outcomes and plasma biomarkers, and the observed cross-sectional associations might be subject to selective survival bias. Second, eGFR, which was estimated using serum creatinine, may be affected by reduced muscle mass or chronic illnesses [41], which are common in older adults. Also, a single serum creatinine test as a screening approach may not be the best way to define impaired kidney function in the absence of albuminuria. Third, data on reliable plasma biomarkers for tau pathology in the brain (e.g., plasma phosphorylated tau and tau positron emission tomography imaging biomarkers) were not available, which prevents us from investigating the roles of tau pathology load in the central nervous system in the association of impaired kidney function and cognitive outcomes. Finally, our study sample was selected from only one rural region in western Shandong province, therefore, caution is needed when generalizing our study findings to other populations.
In conclusion, this population-based study demonstrated that impaired kidney function was associated with dementia, AD, VaD, and plasma biomarkers for AD and neurodegeneration among rural-dwelling Chinese older adults. The association of impaired kidney function with AD could be partly mediated by plasma biomarkers for neurodegeneration. These findings contribute to our understanding of the relationship between impaired kidney function and cognitive phenotypes in old age as well as the underlying neuropathological mechanisms. Future prospective cohort studies that integrate epidemiological and clinical data with reliable biomarkers for brain pathologies are warranted to clarify the potential causal relationship of impaired kidney function with dementia and AD, and to better understand the neuropathological mechanisms underlying the relationship.
AUTHOR CONTRIBUTIONS
Nan Wang: Conceptualization; investigation; writing – original draft; software; methodology; formal analysis. Yixun Ma: Investigation; methodology. Xiaoyan Liang: Investigation; methodology. Wenxin Fa: Investigation; methodology. Xunyao Tian: Investigation; methodology. Cuicui Liu: Investigation; methodology. Min Zhu: Investigation; methodology. Na Tian: Investigation; methodology. Keke Liu: Investigation; methodology. Shi Tang: Investigation; methodology. Lin Song: Investigation; methodology. Lin Cong: Investigation; methodology. Lu Dai: Investigation; writing – review and editing; methodology. Hong Xu: Investigation; writing – review and editing; methodology. Yongxiang Wang: Investigation; methodology; project administration. Tingting Hou: Conceptualization; investigation; writing – review and editing; methodology; project administration; data curation. Yifeng Du: Conceptualization; investigation; writing – review and editing; methodology; project administration; funding acquisition; data curation. Chengxuan Qiu: Conceptualization; investigation; writing – review and editing; methodology; supervision; funding acquisition; data curation.
FUNDING INFORMATION
This work was supported in part by grants from the Brain Science and Brain-Like Intelligence Technology Research Projects of China (grants no. 2021ZD0201801 and 2021ZD0201808), the National Key R&D Program of China Ministry of Science and Technology (grants no. 2017YFC1310100 and 2022YFC3501404), the National Natural Science Foundation of China (grants no. 81861138008, 81,772,448, 82,011,530,139, 82,171,175, and 82,200,980), the Academic Promotion Program of Shandong First Medical University (grant no. 2019QL020), the Integrated Traditional Chinese and Western Medicine Program in Shandong Province (grant no. YXH2019ZXY008), the Natural Science Foundation of Shandong Province (grants no. ZR2021MH392 and ZR2021QH240), the Postdoctoral Innovation Project of Shandong Province (grant no. SDCX-ZG-202203048), the Technology Development Plan Project of Jinan City (grant no. 202134028), the Shandong Provincial Key Research and Development Program (grant no. 2021LCZX03), and the Taishan Scholar Program of Shandong Province, China. C Qiu received grants from the Swedish Research Council (grants no. 2017–05819 and 2020–01574) and the Swedish Foundation for International Cooperation in Research and Higher Education (grant no. CH2019-8320). The funding agency had no role in the study design, data collection and analysis, the writing of this article, or in the decision to submit the work for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




