A nomogram for predicting the risk of postoperative delirium in individuals undergoing cardiovascular surgery
Abstract
Background and Purpose
Delirium is a common mental disorder after adult cardiovascular surgery. Fifteen to 23% of patients undergoing cardiovascular surgery and cardiomyopathy experience delirium, and the efficacy of treatment interventions for delirium has been consistently unsatisfactory.
Methods
A total of 729 patients who underwent cardiovascular surgery were randomly allocated into a training set and a validation set. A nomogram was developed using a logistic regression model to predict the incidence of delirium following cardiovascular surgery. The validity of the model was assessed by determining the receiver operating characteristic (ROC) curve, calculating the area under the ROC curve (AUROC), performing a calibration plot, and executing a decision curve analysis. This model was internally validated using the bootstrap method.
Results
Postoperative delirium (POD) occurred in 165 cases (22.6%) among the 729 patients. Predictors included age, transient ischemic attack, length of preoperative stay, preoperative left ventricular injection fraction and N-terminal pro-B-type natriuretic peptide level, and intraoperative infusion of dexmedetomidine and human fibrinogen. The nomogram showed sufficient differentiation and calibration (AUROC = 0.754, 95% confidence interval = 0.703–0.804). The calibration graphs showed that the predictive values of the nomogram were in agreement with the actual values. The analysis of the training and validation sets suggested that the model possessed specific clinical significance.
Conclusions
In summary, the predictive model consists of seven factors that can roughly predict the occurrence of POD in patients who undergo cardiovascular surgery.
INTRODUCTION
Delirium is a condition characterized by a rapid decline in attention, cognition, and overall mental condition, with no identifiable cause from existing neurocognitive disorders. The incidence might differ based on the individuals being observed and the choice of diagnostic instruments for delirium [1, 2]. The occurrence of delirium among patients undergoing cardiac surgery is approximately 15%–23% [3-5] and even could escalate to 50%–70% in patients with critical illness [6, 7].
Prior studies have shown a distinct correlation between delirium and multiple adverse consequences. In the short term, delirium has been found to substantially heighten morbidity and mortality rates, prolong hospital stays, and exacerbate the financial burden on patients. Furthermore, over the long term, delirium has been linked to increased postdischarge mortality, cognitive impairment, readmission rates, and development of dementia [8].
The etiological factors contributing to delirium are multifaceted. Age is an independent risk factor for the occurrence of delirium [9-14], and mechanical ventilation is another risk factor for inducing delirium [15-17]. Atherosclerosis caused by peripheral vascular disease is also an independent predictive factor [6]. However, the underlying pathophysiological mechanisms remain unclear. Consequently, the efficacy of drug-based prevention and treatment measures is limited. Previous studies have shown that implementing preventive interventions can effectively reduce the occurrence of delirium and enhance patient outcomes [18-21]. Hence, the present study aims to examine preoperative and intraoperative factors that may influence the development of postoperative delirium (POD), with a focus on identifying the accurate predictors before the occurrence of delirium, so variables such as mechanical ventilation duration and intensive care unit (ICU) stay that cannot be determined prior to the onset of delirium were not in the scope of our study, then develop and validate a predictive model and generate a nomogram. Constructing a model for predicting POD and using it to immediately assess patients after surgery, screening high-risk populations for preventive measures or early detection, could potentially yield positive outcomes.
METHODS
Study population
All the recruited subjects were of the Chinese Han population and from the Department of Cardiovascular Surgery at the First Affiliated Hospital of Nanjing Medical University. The research was conducted from January 2022 to December 2022, with all participants being duly informed about the study's objectives and providing their consent by signing a written form. The ethics review board of the First Affiliated Hospital of Nanjing Medical University granted approval for this study. The inclusion criteria for this study were as follows: (i) all patients were 18 years of age or older; and (ii) patients had undergone cardiovascular surgery, which included isolated procedures such as coronary artery bypass graft surgery (CABG), valve replacement (such as aortic valve replacement, mitral valve replacement, and tricuspid valve replacement), and valve repair surgery; combined procedures such as CABG combined with valve replacement or valvuloplasty; surgery for ascending aortic aneurysm or dissecting aneurysm; repair of atrial or ventricular defects; and other procedures (e.g., myxoma resection surgery). Exclusion criteria were as follows: (i) age < 18 years; (ii) preoperative history of dementia or cognitive impairment; (iii) nondirect vision surgery (such as aortic stent implantation, transcatheter aortic valve implantation surgery); (iv) cardiovascular surgery combined with other chest surgeries (such as lobectomy, esophageal cancer surgery); (v) preoperative coma, or coma or deep sedation within 3 days after surgery preventing subject from being evaluated; (vi) a second surgery within 3 days; (vii) death within 3 days after surgery; and (viii) case data missing.
Assessment of delirium
We initially screened preoperative patients using the Mini-Mental State Examination (MMSE) to exclude those with dementia. After the surgery, all participants were admitted to the ICU and given either propofol or dexmedetomidine to maintain a postoperative Richmond Agitation Sedation Scale (RASS) score between −2 and 0. As soon as the patient was conscious, evaluations were conducted every 8–12 h over three consecutive days by ICU nurses and doctors who had received relevant training. To determine whether a patient was suffering from POD, we utilized the Confusion of Assessment Method for the Intensive Care Unit (CAM-ICU). Before initiating the assessment, the patient's baseline mental state was established, with the preceding 24-h RASS score serving as a reference. If the RASS score was −4 or −5, the assessment was temporarily postponed; if the RASS score was higher than −4, further screening using the CAM-ICU method was immediately performed.
Intraoperative management
A standardized anesthesia protocol was typically employed. This involved the induction of anesthesia with either propofol or etomidate, in conjunction with fentanyl or sufentanil, and midazolam or remimazolam. Muscle relaxation was achieved using nondepolarizing muscle relaxants such as rocuronium or atracurium. Anesthesia was sustained through a combination of intravenous administration (propofol or dexmedetomidine) and inhalation anesthesia (sevoflurane, desflurane, or isoflurane). Invasive arterial blood pressure and continuous central vein monitoring (with or without Swan–Ganz pulmonary catheter) were used for hemodynamic monitoring. Intraoperative urinary output was monitored with catheterization. Cephalosporins were typically used as prophylactic antibiotics; vancomycin was administered in cases of penicillin allergy. Median sternotomy was the most common surgical approach, with a minority of patients undergoing minimally invasive right thoracotomy. Most surgeries were conducted under cardiopulmonary bypass (CPB), necessitating heparinization and achieving an activated clotting time (ACT) of ≥480 s prior to start CPB. Surgeries conducted without CPB also required heparinization and an ACT of >300 s. At the end of the procedure, heparin was neutralized with sulfated protamine. Depending on clinical conditions, bleeding, and heparin neutralization, vasoactive drugs, anticoagulants, blood transfusions, and blood products were administered during surgery.
Statistical analysis
All participants were randomly divided into a training set (n = 513) and an internal validation set (n = 216) at a ratio of 7:3. The training set utilized univariate logistic regression analysis to identify potential predictors. Following this, a predictive model was developed using multivariate regression analysis. The model's predictors were evaluated via receiver operating characteristic (ROC) curves, and the model's discriminability was confirmed using calibration plots, with the bootstrap method employed for resampling validation. Subgroup analyses were also conducted. Moreover, decision curve analysis was used to assess the nomogram's practical utility in decision-making.
For data analysis, we used R software (version 4.3.0), and a statistical significance threshold of p < 0.05 (bilateral) was applied.
RESULTS
The study included a total of 729 participants with an average age of 59.6 ± 11.5 years, of whom 434 (59.5%) were male. Delirium occurred in 165 (22.6%) patients after surgery. There were statistically significant differences in age, hypertension, American Society of Anesthesiologists (ASA) physical status classification, transient ischemic attack (TIA), left ventricular injection fraction (LVEF), estimated glomerular filtration rate (eGFR), direct bilirubin, white blood cell count, N-terminal pro-B-type natriuretic peptide (NT-proBNP) level, type of surgery, duration of operation and CPB, clamp time, circulatory arrest, continuous dexmedetomidine infusion, intraoperative hemoglobin (Hb) level, red blood cell (RBC) transfusion, prothrombin complex concentrate and human fibrinogen infusion, and lactate level upon ICU admission after surgery (p < 0.05; TABLE S1).
Table S2 presents the listed attributes of the patients in both the training set and validation set. Generally, the two sets exhibit equilibrium and comparability (p > 0.05).
Univariate logistic regression analysis revealed that age, level of education, hypertension, ASA classification, cerebrovascular accident, TIA, length of preoperative hospital stay, LVEF, eGFR, NT-proBNP level, duration of surgery and CPB, circulatory arrest, continuous dexmedetomidine infusion, propofol and γ-aminobutyric acid administration, intraoperative Hb level, RBC transfusion, and infusion of prothrombin complex concentrate and human fibrinogen were potential predictors of POD (p < 0.1; Table 1).
| Variables | p | OR (95% CI) |
|---|---|---|
| Preoperative | ||
| Sex | ||
| Male | 1.00 (reference) | |
| Female | 0.469 | 0.86 (0.56–1.31) |
| Age, years | <0.001 | 1.04 (1.02–1.06) |
| BMI, kg/m2 | 0.356 | 1.03 (0.97–1.10) |
| Education | ||
| Illiteracy | 1.00 (reference) | |
| Primary school | 0.083 | 0.56 (0.29–1.08) |
| Junior middle school | 0.240 | 0.72 (0.41–1.25) |
| High school | 0.510 | 0.80 (0.41–1.56) |
| College | 0.694 | 1.21 (0.47–3.09) |
| University and above | 0.364 | 0.61 (0.21–1.77) |
| Smoking | ||
| Never | 1.00 (reference) | |
| Yes | 0.862 | 1.05 (0.63–1.73) |
| Ever | 0.494 | 1.23 (0.68–2.20) |
| Drinking | ||
| Never | 1.00 (reference) | |
| Yes | 0.283 | 1.30 (0.80–2.10) |
| Hypertension | ||
| No | 1.00 (reference) | |
| Yes | 0.056 | 1.56 (0.99–2.46) |
| Diabetes | ||
| No | 1.00 (reference) | |
| Yes | 0.498 | 1.19 (0.72–1.95) |
| Asthma | ||
| No | 1.00 (reference) | |
| Yes | 0.259 | 1.90 (0.62–5.78) |
| Malignancy | ||
| No | 1.00 (reference) | |
| Yes | 0.995 | 1.00 (0.27–3.71) |
| ASA | ||
| II + III | 1.00 (reference) | |
| IV | 0.511 | 1.18 (0.72–1.95) |
| IIIE + IVE | <0.001 | 3.57 (1.82–6.99) |
| Cardiac surgery | ||
| Never | 1.00 (reference) | |
| Ever | 0.733 | 1.22 (0.38–3.92) |
| PCI | ||
| Never | 1.00 (reference) | |
| Ever | 0.584 | 0.73 (0.24–2.22) |
| CVA | ||
| Never | 1.00 (reference) | |
| Ever | 0.076 | 1.63 (0.95–2.81) |
| TIA | ||
| Never | 1.00 (reference) | |
| Ever | 0.031 | 2.52 (1.09–5.83) |
| AF | ||
| Never | 1.00 (reference) | |
| Ever | 0.196 | 1.35 (0.86–2.12) |
| Days before surgery | 0.028 | 0.95 (0.90–0.99) |
| Statins | ||
| No | 1.00 (reference) | |
| Yes | 0.965 | 0.99 (0.58–1.69) |
| β-blockers | ||
| No | 1.00 (reference) | |
| Yes | 0.273 | 0.74 (0.44–1.26) |
| Calcium channel blockers | ||
| No | 1.00 (reference) | |
| Yes | 0.977 | 0.99 (0.63–1.57) |
| ACEIs | ||
| No | 1.00 (reference) | |
| Yes | 0.349 | 0.59 (0.20–1.76) |
| ARBs | ||
| No | 1.00 (reference) | |
| Yes | 0.369 | 0.79 (0.47–1.33) |
| Diuretics | ||
| No | 1.00 (reference) | |
| Yes | 0.453 | 1.23 (0.71–2.13) |
| NSAIDs | ||
| No | 1.00 (reference) | |
| Yes | 0.615 | 1.14 (0.68–1.93) |
| LVEF, % | <0.001 | 0.96 (0.94–0.98) |
| eGFR, mL/min | 0.004 | 0.99 (0.98–0.99) |
| ALT, U/L | 0.116 | 0.99 (0.98–1.00) |
| AST, U/L | 0.999 | 1.00 (0.99–1.01) |
| TG, mmol/L | 0.436 | 0.88 (0.65–1.21) |
| CHOL, mmol/L | 0.302 | 0.90 (0.75–1.09) |
| HDL, mmol/L | 0.605 | 0.82 (0.40–1.71) |
| LDL, mmol/L | 0.631 | 0.94 (0.72–1.22) |
| TB, μmol/L | 0.340 | 1.01 (0.99–1.03) |
| DBIL, μmol/L | 0.190 | 1.03 (0.99–1.07) |
| IBIL, μmol/L | 0.687 | 1.01 (0.97–1.05) |
| WBC, 109/L | 0.116 | 1.06 (0.99–1.13) |
| NT-ProBNP, pg/mL | 0.045 | 1.01 (1.01–1.01) |
| Intraoperative | ||
| Duration of surgery, h | 0.003 | 1.19 (1.06–1.34) |
| Duration of CPB, h | 0.045 | 1.17 (1.01–1.36) |
| Duration of clamp, min | 0.192 | 1.00 (1.00–1.01) |
| Circulatory arrest | ||
| No | 1.00 (reference) | |
| Yes | 0.003 | 2.46 (1.36–4.45) |
| MAP, mmHg | 0.186 | 0.98 (0.96–1.01) |
| Hb, g/L | 0.027 | 0.86 (0.76–0.98) |
| Red blood cell transfusion | 0.052 | 1.07 (1.00–1.14) |
| Medication administration | ||
| Dexmedetomidine continuous infusion, mL/h | 0.010 | 1.28 (1.06–1.55) |
| Propofol injection, mg | 0.088 | 1.00 (1.00–1.01) |
| Aminocaproic acid injection, mg | 0.039 | 1.11 (1.01–1.22) |
| Prothrombin complex concentrate infusion, IU | <0.001 | 1.01 (1.01–1.01) |
| Fibrinogen infusion, g | <0.001 | 1.44 (1.24–1.67) |
| Postoperative | ||
| Lactate level, mmol/L | 0.126 | 1.06 (0.98–1.15) |
- Abbreviations: ACEI, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; ASA, American Society of Anesthesiologists; AST, aspartate aminotransferase; BMI, body mass index; CHOL, cholesterol; CI, confidence interval; CPB, cardiopulmonary bypass; CVA, cerebrovascular accident; DBIL, direct bilirubin; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; HDL, high-density lipoprotein; IBIL, Indirect bilirubin; LDL, low-density lipoprotein; LVEF, left ventricular injection fraction; MAP, mean arterial pressure; NSAID, nonsteroidal anti-inflammatory drug; NT-ProBNP, N-terminal pro-B-type natriuretic peptide; OR, odds ratio; PCI, percutaneous coronary intervention; TB, Total bilirubin; TG, triglyceride; TIA, transient ischemic attack; WBC, white blood cell count.
- Note: AGE p < 0.001 indicates a statistically significant difference, with a 4% increase in the risk of POD for each additional year of age. ASA IIIE+IVE p < 0.001 indicates a statistically significant difference in ASA III and IV emergency surgery, with a 2.57 times increase in the risk of POD in reference to ASA II and III surgery. TIA Ever p = 0.031 indicates a statistically significant difference in patients who have had TIA in the past, with a 1.52 times increase in the risk of POD in reference to patients who never have TIA. Days before surgery p = 0.028 indicates a statistically significant difference, with a 5% decrease in the risk of POD for each additional day of hospital stay before surgery. LVEF p < 0.001 indicates a statistically significant difference, with a 4% decrease in the risk of POD for each additional unit of LVEF. eGFR p = 0.004 indicates a statistically significant difference, with a 1% decrease in the risk of POD for each additional unit of eGFR. NT-ProBNP p = 0.045 indicates a statistically significant difference, with a 1% increase in the risk of POD for each additional unit of NT-ProBNP. Duration of surgery p = 0.003 indicates a statistically significant difference, with a 19% increase in the risk of POD for each additional surgery hour. Duration of CPB p = 0.045 indicates a statistically significant difference, with a 17% increase in the risk of POD for each additional CPB hour. Circulatory arrest p = 0.003 indicates a statistically significant difference, with a 1.46 times higher in the risk of POD than patients who did not have circulatory arrest. Hb p = 0.027 indicates a statistically significant difference, with a 14% decrease in the risk of POD for each additional unit of Hb. Dexmedetomidine continuous infusion p = 0.010 indicates a statistically significant difference, with a 28% increase in the risk of POD for each additional unit usage of Dexmedetomidine. Aminocaproic acid injection p = 0.039 indicates a statistically significant difference, with a 11% increase in the risk of POD for each additional unit usage of Aminocaproic acid. Prothrombin complex concentrate infusion p < 0.001 indicates a statistically significant difference, with a 1% increase in the risk of POD for each additional unit usage of Prothrombin complex concentrate. Fibrinogen infusion p < 0.001 indicates a statistically significant difference, with a 44% increase in the risk of POD for each additional unit usage of human Fibrinogen.
Furthermore, a stepwise multivariate logistic regression analysis was conducted. The results indicated that with each additional year of age, the risk of POD increased by 0.06 times (p < 0.001, odds ratio [OR] = 1.06, 95% confidence interval [CI] = 1.04–1.09); having a TIA increased the risk of POD by 2.56 times (p = 0.006, OR = 3.56, 95% CI = 1.40–8.80); for each additional day of preoperative hospital stay, the risk of POD decreased by 0.06 times (p = 0.039, OR = 0.94, 95% CI = 0.89–1.00); for each unit increase in LVEF, the risk of POD decreased by 0.04 times (p = 0.002, OR = 0.96, 95% CI = 0.94–0.99); for each unit increase in NT-proBNP level, the risk of POD increased by 0.00 times (p = 0.014, OR = 1.00, 95% CI = 1.00–1.00); for each unit increase in dexmedetomidine continuous infusion during surgery, the risk of POD increased by 0.39 times (p = 0.002, OR = 1.39, 95% CI = 1.12–1.72); and for each unit increase in human fibrinogen infusion, the risk of POD increased by 0.50 times (p < 0.001, OR = 1.50, 95% CI = 1.26–1.81; Table 2).
| Variables | β | p | OR (95% CI) |
|---|---|---|---|
| Age, years | 0.06 | <0.001 | 1.06 (1.04–1.09) |
| TIA | |||
| No | 1.00 (reference) | ||
| Yes | 1.27 | 0.006 | 3.56 (1.40–8.80) |
| Days in hospital before surgerya | −0.06 | 0.039 | 0.94 (0.89–1.00) |
| LVEF, % | −0.04 | 0.002 | 0.96 (0.94–0.99) |
| NT-proBNP, pg/mLa | 0.00 | 0.014 | 1.00 (1.00–1.00) |
| Dexmedetomidine continuous infusion, mL/h | 0.33 | 0.002 | 1.39 (1.12–1.72) |
| Fibrinogen infusion, g | 0.41 | <0.001 | 1.50 (1.26–1.81) |
- Abbreviations: CI, confidence interval; LVEF, left ventricular injection fraction; NT-ProBNP, N-terminal pro-B-type natriuretic peptide; OR, odds ratio; TIA, transient ischemic attack.
- Note: AGE p < 0.001 indicates a statistically significant difference, with a 6% increase in the risk of POD for each additional year of age. IA Ever p = 0.006 indicates a statistically significant difference in patients who have had TIA in the past, with a 2.56 times increase in the risk of POD in reference to patients who never have TIA. Days before surgery p = 0.039 indicates a statistically significant difference, with a 6% decrease in the risk of POD for each additional day of hospital stay before surgery. LVEF p < 0.002 indicates a statistically significant difference, with a 4% decrease in the risk of POD for each additional unit of LVEF. NT-ProBNP p = 0.014 indicates a statistically significant difference. Dexmedetomidine continuous infusion p = 0.002 indicates a statistically significant difference, with a 39% increase in the risk of POD for each additional unit usage of Dexmedetomidine. Fibrinogen infusion p < 0.001 indicates a statistically significant difference, with a 50% increase in the risk of POD for each additional unit usage of human Fibrinogen.
- a The OR values are close to 1, but the p-values are significant, and the CIs do not actually include 1. Therefore, both predictive factors still have statistical significance.
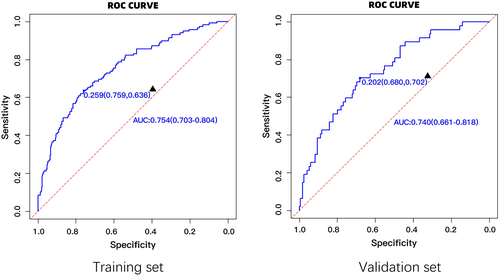
The area under the ROC curve (AUROC) for the training set was found to be 0.754 (95% CI = 0.703–0.804). Similarly, the area under the curve for the internal validation set was found to be 0.740 (95% CI = 0.661–0.818), indicating that the model exhibits promising predictive capabilities (Figure 1).
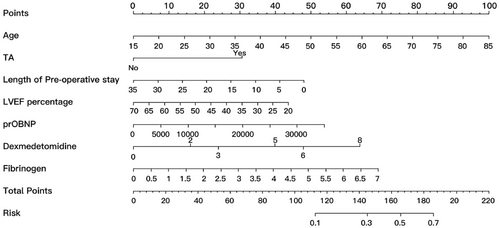
Our study identified seven predictive factors for POD: age, TIA, length of preoperative stays, preoperative LVEF and NT-proBNP level, and intraoperative dexmedetomidine and human fibrinogen infusion. Each factor was assigned a numerical value, and the total score was calculated by adding up these individual scores. This total score served as an indicator of the predicted risk of POD (Figure 2).
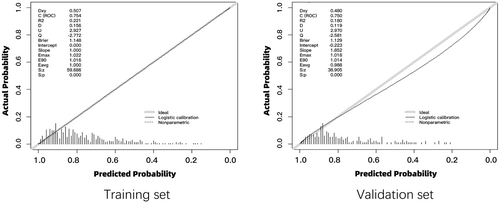
The calibration curves for both the training and validation sets demonstrated a strong concordance between the predicted values of the nomogram and the observed ones, thereby affirming the high accuracy of the predictive model (Figure 3).
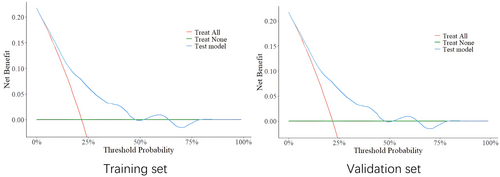
The analysis of the training and validation sets implied that the model possessed specific clinical significance (Figure 4).
A subgroup analysis was conducted, categorizing the subgroups based on circulatory arrest status and type of surgery. The AUROC for the subgroup without circulatory arrest was 0.79 (95% CI = 0.51–1.00); for the subgroup with circulatory arrest, it was 0.73 (95% CI = 0.59–0.87). In the group of patients who underwent valve replacement or repair, the AUROC was found to be 0.91 (95% CI = 0.75–1.00). In the off-pump CABG group, the AUROC was 0.58 (95% CI = 0.31–0.86). The on-pump CABG group had an AUROC of 0.80 (95% CI = 0.60–1.00). For the subgroup of CABG combined with valve replacement or repair, the AUROC was 0.75, with a 95% CI of 0.70–0.80. Finally, patients who underwent surgery for ascending aortic aneurysm or aortic dissection had an AUROC of 0.74 (95% CI = 0.66–0.82; Table 3).
| Data | AUROC (95% CI) | Accuracy (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|
| Train | 0.75 (0.70–0.80) | 0.73 (0.69–0.77) | 0.76 (0.72–0.80) | 0.64 (0.55–0.72) | 0.44 (0.37–0.52) | 0.88 (0.84–0.91) |
| Test | 0.74 (0.66–0.82) | 0.73 (0.66–0.79) | 0.78 (0.72–0.84) | 0.53 (0.39–0.68) | 0.40 (0.28–0.53) | 0.86 (0.80–0.91) |
| Circulatory arrest | ||||||
| No | 0.79 (0.51–1.00) | 0.79 (0.54–0.94) | 0.57 (0.21–0.94) | 0.92 (0.76–1.00) | 0.79 (0.57–1.00) | 0.80 (0.45–1.00) |
| Yes | 0.73 (0.59–0.87) | 0.75 (0.65–0.83) | 0.78 (0.69–0.87) | 0.53 (0.28–0.79) | 0.31 (0.13–0.49) | 0.90 (0.83–0.97) |
| Procedure | ||||||
| Isolated | ||||||
| Valve replacement | 0.91 (0.75–1.00) | 0.80 (0.52–0.96) | 0.91 (0.74–1.00) | 0.50 (0.01–0.99) | 0.67 (0.13–1.00) | 0.83 (0.62–1.00) |
| CABG, off-pump | 0.58 (0.31–0.86) | 0.60 (0.36–0.81) | 0.62 (0.35–0.88) | 0.57 (0.21–0.94) | 0.44 (0.12–0.77) | 0.73 (0.46–0.99) |
| CABG, on-pump | 0.80 (0.60–0.99) | 0.65 (0.43–0.84) | 0.42 (0.14–0.70) | 0.91 (0.74–1.00) | 0.59 (0.35–0.82) | 0.83 (0.54–1.00) |
| Combined | 0.75 (0.70–0.80) | 0.73 (0.69–0.77) | 0.76 (0.72–0.80) | 0.64 (0.55–0.72) | 0.44 (0.37–0.52) | 0.88 (0.84–0.91) |
| Ascending aortic aneurysm or dissection surgery | 0.74 (0.66–0.82) | 0.73 (0.66–0.79) | 0.78 (0.72–0.84) | 0.53 (0.39–0.68) | 0.40 (0.28–0.53) | 0.86 (0.80–0.91) |
- Abbreviations: AUROC, area under the receiver operating characteristic curve; CABG, coronary artery bypass graft surgery; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
DISCUSSION
Various negative consequences have been linked to delirium, encompassing immediate impacts like falls, aspiration pneumonia, heightened susceptibility to surgical infections, cerebral vascular accidents, pressure ulcers, and additional complications [22, 23]. It also has been associated with extended durations in the ICU and medical facility [24], elevated rates of short-term death, and escalated expenses for hospitalization [9, 25-27]. Moreover, delirium has been shown to have negative long-term consequences, such as a decline in cognitive function over time in patients [28-31] as well as increased 1-year mortality rate and disability [32, 33].
The etiologies of POD can be classified as predisposing and precipitating factors. Predisposing risk factors include advanced age (≥65 years old), cognitive impairment, frailty, comorbidities (e.g., cardiovascular and kidney diseases), depression or other mental illnesses [34, 35], alcoholism, malnutrition [36, 37], visual and hearing impairment [9, 38], and peripheral vascular diseases [6] among others. The precipitating factors include sudden illnesses (like sepsis, hypoglycemia, stroke, and liver failure), trauma (such as fractures, head injuries), surgery, anesthesia, CPB duration, intraoperative massive blood loss [39], dehydration, psychological stress [9, 40], blood poverty, abnormal plasma protein levels, hypoxia, pain [41, 42], long-term immobilization, sleep disorders [43], et cetera. The overall risk was contingent upon the quantity and intensity of individual risk factors. Generally, multiple precipitating factors could affect patients at the same time. Furthermore, the occurrence of delirium was also linked to the utilization, withdraw, and modifications of medication.
Age is an independent risk factor for the occurrence of delirium [9-14], and our research showed that for each additional year of age, the risk of POD increased by 6%.
On the one hand, patients exhibiting cognitive decline or dementia were at a heightened risk of developing POD [44-46]. Rudolph et al. [42] identified four independent predictors for POD: prior stroke or transient ischemic attack (TIA), MMSE score, abnormal serum albumin, and the Geriatric Depression Scale. TIA was also recognized in our model. Another predictive model for POD, devised by Varga-Martínez et al. [47], similarly incorporated the MMSE score. However, the predictors in both these models were confined to preoperative variables and did not consider the influence of intraoperative variables such as surgical procedures and anesthesia on POD. Our model has precisely addressed this gap. On the other hand, episodes of POD could also accelerate the progression of symptoms in patients suffering from dementia or cognitive dysfunction [48]. We preoperatively screened out patients with dementia (severe cognitive impairment) using the MMSE. Yet, we neither categorized cognitive dysfunction nor excluded mild cognitive decline, which are areas that require enhancement in our future research.
Our study discovered that intraoperative administration of human fibrinogen could significantly increase the risk of POD, which may be attributed to intraoperative blood loss. Notably, existing literature does not support our findings, prompting us to further investigate the correlation between intraoperative human fibrinogen usage and POD. Additionally, we observed a 39% increase in POD risk in patients administered with dexmedetomidine intraoperatively. This aligns with a randomized placebo-controlled trial demonstrating a 47% increase in POD in patients who received dexmedetomidine during cardiac surgery with CPB and within 24 h postoperation, potentially linked to hypotension that the drug provoked [49]. However, a contrasting systematic review and meta-analysis revealed that dexmedetomidine could reduce the risk of POD in patients aged >65 years undergoing noncardiac surgery. For those younger than 65 years, no correlation was found between dexmedetomidine use and POD risk [50]. However, almost all patients were administered postoperative analgesics such as fentanyl, sufentanil, or morphine in these included study, which could potentially impact the final outcomes.
Numerous prior studies have affirmed the effectiveness of preventive intervention in reducing the incidence of delirium and improving prognosis [18-21]. Consequently, it is imperative to develop a delirium prediction model that can swiftly identify high-risk groups and facilitate the prevention or prompt detection of delirium. Despite the significant attention dedicated to predictive models for delirium following cardiac surgery in existing literature, certain constraints remain, such as limited predictive power, insufficient sample size, potential bias, and lack of visual predictive models [14, 46, 51, 52]. Our study pinpointed seven predictors for POD: age, TIA, preoperative hospital stays, preoperative LVEF and NT-proBNP level, and intraoperative dexmedetomidine and human fibrinogen infusion. All these factors could be identified prior to the onset of POD. A predictive model was developed with these variables, followed by the creation of a nomogram to visually represent the model and evaluate the probability of POD. Upon internal validation, the model demonstrated remarkable discrimination, calibration, and clinical utility.
LIMITATIONS
First, all the subjects recruited for this study were from a single center, resulting in the predictive model not being externally validated and potentially introducing selection bias. Second, we only screened out patients with dementia and severe cognitive impairment using the MMSE, overlooking the potential subtle effects of cognitive decline. In future studies, these significant confounding factors should be taken into account. Moreover, we plan to investigate the correlation between intraoperative administration of human fibrinogen and the onset of POD, aiming to unravel the possible underlying mechanisms.
AUTHOR CONTRIBUTIONS
Chao Liu: Conceptualization; investigation; writing – original draft; formal analysis; data curation; methodology. Linfei Zhang: Data curation. Weifeng Tang: Writing – original draft. Sheng Zhao: Writing – original draft. Mingke Li: Data curation. Jinghang Li: Data curation. Yongfeng Shao: Supervision; validation; resources.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
All procedures performed in this study involving human participants were in accordance with ethical standards and approved by the ethics committee of the First Affiliated Hospital with Nanjing Medical University.
CONSENT STATEMENT
All participants were duly informed about the objectives of the study and provided their consent by signing a written form.
Open Research
DATA AVAILABILITY STATEMENT
The research data supporting this publication are available in the online supplementary material.




