Stable excess mortality in a multiple sclerosis cohort diagnosed 1970–2010
Abstract
Background and purpose
Multiple sclerosis (MS) is associated with excess mortality. The use of disease-modifying treatments (DMTs) has recently been associated with survival benefits.
Methods
A regional MS database was linked with national registries. People with MS (pwMS) diagnosed in 1971–2010 were included and followed up until the end of the year 2019. Five matched controls were acquired for every person with MS. DMTs included in the analyses were interferon and glatiramer acetate.
Results
Median follow-up time of the 1795 pwMS was 20.0 years (range 0.1–48.7 years). Survival did not differ between decades of diagnosis (p = 0.20). Amongst pwMS, male sex (adjusted hazard ratio [aHR] 1.70; 95% confidence interval [CI] 1.41–2.06), higher age at diagnosis (aHR 1.83; 95% CI 1.65–2.03 per 10-year increment) and primary progressive disease course (aHR 1.29; 95% CI 1.04–1.60) were independently associated with poorer survival. DMT use was associated with better survival (p < 0.0001) and better survival during follow-up (aHR 0.56; 95% CI 0.38–0.81). Compared to matched controls, median life expectancy was 8–9 years shorter in pwMS with survival diverging from controls during the first decade after diagnosis, more clearly in men than women.
Conclusion
Despite DMT use being associated with better survival, relative life expectancy of pwMS did not change over five decades in Western Finland. Male sex was an independent risk factor for death amongst pwMS, but excess mortality was higher in women. More work and methods are needed to improve survival in pwMS.
INTRODUCTION
Multiple sclerosis (MS) is the most widespread disabling neurological disease amongst young adults in western countries and it has become more common during the last decades [1-4]. Longitudinal studies have thus far generally reported reduced life expectancy in people with MS (pwMS) and there is no association between standardized mortality rates (SMRs) and time period [5, 6], indicating that excess mortality associated with MS remains stable. However, there is substantial variability between studies [6].
Survival of pwMS starts to diverge from that of the general population early in the disease, after approximately 10 years of disease duration [7, 8]. Recent reports on favourable outcome in MS during the disease-modifying treatment (DMT) era overlap with the reduced delay from MS onset to diagnosis [8-14]. This suggests a prognostic role for early diagnostic verification and DMT initiation. On the other hand, improving prognosis could also partly result from an overall milder disease course resulting from several factors [15]. Data from the Danish MS registry also show that the prognosis began to improve before the advent of DMTs [10], suggesting that other factors are also at play. Recent data from Norway suggest the same [16]. It is also important to note that prognosis differs according to patient- and phenotype-specific factors, such as sex and type of MS [12].
Finland is situated in the northern European high MS risk zone and during recent decades considerable changes in incidence patterns have been reported [2, 4, 17, 18]. Previous survival estimates therefore need to be updated [19]. The aim of the present study was to assess the relative life expectancy and long-term survival up to the end of the year 2019 in a population-based 1971–2010 MS incidence database in Western Finland.
MATERIALS AND METHODS
Study design
This is a population-based retrospective cohort study where data on retrospectively confirmed MS cases diagnosed in 1971–2010 were linked to Statistics Finland and Population Register Centre's Population Information System for dates of deaths. The study was approved by the National Institute for Health and Welfare (THL 733/5.0500/2020) and the Ethics Committee of the Tampere University Hospital District. Since this was a retrospective study involving no contact with the patients informed consent was not required by Finnish law.
Study population
The MS database utilized in this study includes information on individually confirmed pwMS diagnosed from 1 January 1971 to 31 December 2010 in the three central hospital districts Pirkanmaa, Seinäjoki (South Ostrobothnia) and Vaasa (Ostrobothnia), belonging to Tampere University Hospital's Special Responsibility Area in 2010 (Figure S1) [18]. MS diagnostics in this area are performed only in these three hospitals and MS diagnoses are made according to international guidelines in Finland. This area includes regions with the highest MS risk in Finland and from 1972 to 2020 consistently included 16% of the population of the country [4].
Cases with codes of MS and optic neuritis (codes 340, 341, 377, G35, G37 and H46 in the International Classification of Diseases, versions 8 to 10) were identified from hospital administrative registries. Two authors (AV, MLS) scrutinized the patient records. Cases fulfilling the definite MS diagnosis according to the diagnostic criteria in use at that time were included [17, 18]. Disease course was classified as relapsing onset (ROMS) or primary progressive (PPMS) [20]. Information on year of birth, MS onset and diagnosis were included.
Information on DMT use is available from 1995 onwards. DMTs included in the analysis of patients with and without DMTs were interferon (IFNβ) and glatiramer acetate (GLA) (the only ones generally available at the time), whereas the 10% of patients with various DMTs in randomized controlled trials were excluded from this analysis. To account for some delay in the implementation of novel drugs in routine practice, and to allow for inclusion of participants diagnosed recently leading up to DMTs becoming available, the group of pwROMS diagnosed between 1990 and 1999 were selected as the reference group when comparing time periods.
Control population
Five control persons, alive at the time of MS diagnosis and matched for age, sex and hospital district, were acquired for every person with MS from the general population database of Statistics Finland. Cases and controls were linked to the national death database, held by the same authority, until 31 December 2019. All linkages were done by using the personal identity code, given to all residents of Finland since 1 January 1967. This code is used in all main registers in Finland and allows reliable record linkages. They were followed from the time of diagnosis of the matched pwMS until death or 31 December 2019, whichever occurred first.
Statistical analysis
Overall disease course and gender-specific survival from birth (survival age) and from diagnosis were estimated by using the Kaplan–Meier estimator and log-rank test. Cox proportional hazards models were employed for studying the association of sex, age at diagnosis (10-year increments), disease course and diagnostic delay (1-year increments) with death in pwMS. The multivariable model was adjusted by region of residence (hospital district). In addition, the association of DMT with mortality was studied using a Cox model adjusted for the above-mentioned covariables. Trends were studied using Cochrane–Armitage or Jonckheere–Terpstra tests as appropriate. Results are presented as mean, median, percentage or hazard ratio (HR) with 95% confidence interval (CI) or interquartile range (IQR). Statistical significance was inferred at p < 0.05. Analyses were performed with SPSS version 27 and SAS ver 9.4. (Cary, NJ, USA).
RESULTS
Study population characteristics
Altogether 1795 pwMS were identified (Table 1). Median age was 31.9 years at MS onset and 37.0 years at diagnosis. No significant trend over four decades of diagnosis was shown for age at first symptoms. Delay from first MS symptoms to diagnosis showed a decreasing trend. Female to male ratio increased from 1.58 to 2.05 (p for trend 0.019). A significant increase was seen for the overall pwROMS/pwPPMS ratio from 3.17 to 13.13 (Table 1). Usage of DMT increased from 10.3% to 65.1%.
| 1971–1980 | 1981–1990 | 1991–2000 | 2001–2010 | All | p for trend | |
|---|---|---|---|---|---|---|
| pwMS | 175 | 357 | 599 | 664 | 1795 | |
| Male | 69 (39.4%) | 121 (33.9%) | 201 (33.6%) | 199 (30.0%) | 590 (32.9%) | |
| Female | 106 (60.6%) | 236 (66.1%) | 398 (66.4) | 465 (70.0%) | 1205 | |
| F/M ratio | 1.58 | 1.95 | 1.98 | 2.34 | 2.05 | 0.019 |
| ROMS | 133 (76.0%) | 279 (78.2%) | 527 (88.0%) | 617 (92.9%) | 1556 (86.7%) | |
| PPMS | 42 (24.0%) | 78 (21.8%) | 72 (12.0%) | 47 (7.1%) | 239 (13.3%) | |
| ROMS/PPMS ratio | 3.17 | 3.58 | 7.32 | 13.13 | 6.51 | <0.0001 |
| DMT | 18 (10.3%) | 62 (17.4%) | 288 (48.1%) | 432 (65.1%) | 800 (44.6%) | <0.0001 |
| Onset age (years)a | 30.8 (25.2–39.0) | 32.7 (24.9–40.9) | 32.8 (25.6–40.6) | 31.1 (24.8–39.1) | 31.9 (25.1–39.9) | 0.310 |
| Diagnosis age (years)a | 36.0 (29.0–43.0) | 38.0 (30.0–44.0) | 38.0 (30.0–46.0) | 36.0 (28.0–44.0) | 37.0 (29.0–44.0) | 0.078 |
| Delay to diagnosis (years)a | 3.0 (1.0–6.0) | 3.0 (1.0–6.0) | 2.0 (1.0–6.0) | 2.0 (1.0–5.0) | 2.0 (1.0–6.0) | <0.0001 |
- Abbreviations: DMT, disease-modifying treatment; F/M, female to male; MS, multiple sclerosis; PPMS, primary progressive MS; pwMS, people with MS; ROMS, relapsing onset MS.
- a Median ± interquartile range.
Survival in the study population
Median follow-up time from diagnosis was 20.0 years (IQR 14.6–26.9, range 0.1–48.7 years). Male sex, higher age at diagnosis and primary progressive disease course were associated with increased risk of death after MS diagnosis in multivariable modelling (Table 2). Risk of death was higher in men compared to women in both pwROMS (adjusted HR [aHR] 1.72; 95% CI 1.38–2.15) and pwPPMS (aHR 1.63; 95% CI 1.13–2.34; p < 0.0001 for both).
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| p value | HR | 95% CI | p value | HR | 95% CI | |
| Male sex | <0.0001 | 1.72 | 1.43–2.08 | <0.0001 | 1.70 | 1.41–2.06 |
| Age at diagnosis (per 10-year increment) | <0.0001 | 1.83 | 1.66–2.02 | <0.0001 | 1.83 | 1.65–2.03 |
| PPMS | <0.0001 | 1.91 | 1.55–2.35 | 0.022 | 1.29 | 1.04–1.60 |
| Diagnostic delay (per 1-year increment) | 0.005 | 1.02 | 1.01–1.04 | 0.417 | 0.99 | 0.98–1.01 |
- Abbreviations: CI, confidence interval; HR, hazard ratio; PPMS, primary progressive multiple sclerosis.
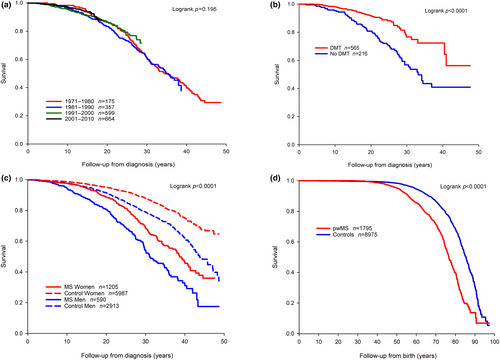
There were no differences in survival when comparing decades of diagnosis (Figures 1a and S2A). The follow-up showed 10-, 20- and 30-year survival estimates of 96.6% (95% CI 95.6%–97.3%), 86.0% (95% CI 84.1%–87.7%) and 63.7% (95% CI 60.1%–67.0%) from diagnosis, respectively. Survival of all MS patients was 85.6% (95% CI 83.6%–87.4%) at 60 years of age, 71.4% (95% CI 68.3%–74.3%) at 70 years of age and 39.9% (95% CI 34.8%–44.8%) at 80 years of age.
Disease-modifying treatments and survival
Patients treated with DMTs had better survival compared to patients with no DMT (Figures 1b and S2B). DMT was associated with lower risk of death during follow-up in univariable (HR 0.42; 95% CI 0.29–0.60; p < 0.0001) and multivariable analysis (aHR 0.56; 95% CI 0.38–0.81; p = 0.002).
Survival in the study population compared to controls
Median life expectancy was almost 10 years shorter in pwMS compared to controls but similar in pwROMS compared to pwPPMS (Table 3). Survival diverged between pwMS and controls already during the first decade after diagnosis and in both sexes, albeit more clearly in men (Figures 1c and S2C). At 65 years from birth overall survival was 78.2% in pwMS and 91.1% in controls (Figure 1d, p < 0.0001). The SMR up to the year 2019 was 2.47 (95% CI 2.20–2.78).
| Median life expectancy (95% CI) | Median survival from diagnosis (95% CI) | |
|---|---|---|
| Women with MS | 80.0 (78.5–81.5) | 38.0 (35.9–40.2) |
| Men with MS | 74.0 (72.4–75.6) | 31.0 (28.2–33.8) |
| Women without MS | 88.0 (86.8–89.1) | |
| Men without MS | 83.0 (81.9–84.1) | |
| pwROMS | 78.0 (76.5–79.5) | 38.0 (35.4–40.6) |
| pwPPMS | 76.0 (73.9–78.1) | 30.0 (28.0–32.0) |
- Abbreviations: CI, confidence interval; MS, multiple sclerosis; pwPPMS, persons with primary progressive MS; pwROMS, persons with relapsing onset MS.
DISCUSSION
In this population-based cohort of incident MS cases a lower risk of death was observed in pwROMS treated with INFβ or GLA compared to those not treated with DMTs. In the data spanning four diagnostic decades no change in survival was observed in pwMS. Median life expectancy after the diagnosis was almost a decade shorter in pwMS compared to matched controls with an SMR of 2.5. Male sex, higher age at diagnosis and primary progressive disease course were independently associated with shorter survival whereas female sex was associated with higher excess mortality.
There are consistent reports that the use of DMTs is associated with somewhat decreased risks of relapses and disability [16, 21-23]. IFNβ has also been associated with a lower mortality risk in a cohort study reporting data from France and Canada [13]. Our results showing a decreased risk of death associated with IFNβ or GLA treatment are in line with these data. In another Canadian study exposure to any DMT was associated with a 26% lower mortality risk [14]. Norwegian registry data have shown that, after DMTs were made readily available, the mortality in pwMS was more than halved [8]. This result is similar to our finding of aHR 0.56 for death in pwMS treated with DMTs. These studies with converging results strongly suggest that DMTs reduce mortality in MS and the effect is also evident with platform therapies such as IFNβ and GLA. Considering that our and previous data have shown that excess mortality begins to cumulate already during the first decade of the disease [8], it seems that early treatment initiation might reduce this. However, considering that in the recent Canadian data the effect waned and eventually disappeared 15 years after the index date [14], it is obvious that there remains work to be done in obviating the increased risk of death in MS.
The overall significance of the association between DMT use in ROMS and improved survival in our data is unclear since survival from the time of diagnosis did not improve over time in pwMS. In other recent studies life expectancy improvements in MS have generally mirrored the improvements seen in the general population [12, 24, 25]. The SMR of 2.47 in our study is mostly comparable to that reported internationally and even slightly lower compared to the SMR of 2.8 in another Finnish cohort diagnosed in 1964–1993 [6, 19], although with overlapping confidence intervals. The survival estimates in the current study correspond to the 25-year survival rate of 78% in the Finnish cohort diagnosed in 1964–1993 and reports from other contemporary incidence cohorts for 25-year survival rate of 62% and 20-year survival rate of 75% [19, 26, 27]. Nevertheless, along with other recent reports [8-10, 12-14], the current data support early DMT initiation in MS. This is all the more important since mortality increases early in the course of MS [7, 8], as also observed in our data.
It is unclear why the overall excess mortality in our data did not decline despite the observed benefit in the use of DMTs. The subject requires further investigation where amongst other factors known mortality predictors such as smoking and socioeconomic factors are taken into account [28-30]. Interestingly, data from an area quite similar to ours in Western Norway reported declining mortality during the past seven decades in pwMS and associated this with improved diagnostics, better symptomatic treatment and access to DMTs [8]. However, whilst diagnostic delay decreased with time also in our data and DMT use was associated with better survival the life expectancy of pwMS did not improve over time. It is also important to note that data from the Danish national MS registry has revealed a declining mortality trend already before the DMT era [10]. Our earlier observations on MS mortality risk from 2004 to 2012 have shown a survival disadvantage related to comorbidities, especially circulatory diseases and diabetes mellitus type 1 [31]. It therefore appears that, in addition to striving for earlier diagnosis and DMT initiation, it is also necessary to keep investigating other ways to improve survival in pwMS. Furthermore, it seems quite possible that some factors related to survival may be population- and time-specific. In the meantime, measures to prevent and treat comorbidities, accidents and suicides should be used to decrease mortality in pwMS [6, 31, 32].
There is a general population gender gap in survival and it is also evident in pwMS. In both pwMS and controls women lived longer than men but the difference was greater in pwMS. Male sex was also an independent predictor of poorer survival amongst pwMS. The gender difference in life expectancy seems to largely, but not completely, mirror that in the general population. These findings are similar to those in a recent study from Norway [8]. The lower MS incidence and increased mortality risk in men with MS suggest that there may be sex-specific factors influencing disease susceptibility and severity. Indeed, recent data have shown that, even despite less inflammatory activity, men accrue more disability with time than women [33]. However, although male sex is a mortality risk factor whether or not a person has MS, SMRs are generally higher in women [6]. In our data the divergence of survival between pwMS and controls was clearer in men and there are also previous reports of higher SMRs in men from all of Sweden, the Basque Country in Spain and the East Midlands county of England [11, 28, 34]. Population-specific factors and methodological differences therefore appear pertinent. Disease course and patient age were also independent predictors of mortality in our data. Young age at MS onset and diagnosis and primary progressive disease course are amongst the most common risk factors in MS survival studies [8, 10, 12, 25]. However, a recent study from Denmark reported higher excess mortality in patients with higher age of onset [10]. The Danish data showed longer surviving time but a worse prognosis in terms of lower age at death associated with young age at MS onset. Discrepancies in findings related to age of onset may therefore reflect methodological differences.
The strategies proposed to improve the validity of studies of the natural history of MS propose geography-based surveys, separate analyses of patients seen from onset and longitudinal evaluation with adequate duration, aspects which were also considered in this study. Survival was estimated here for cases that fulfilled the criteria of definite MS from the time of diagnosis including the important aspect of diagnostic latency. Based on the publicly funded and centralized healthcare system in Finland the case collection in this incidence-based study is expected to be representative, and the 50-year follow-up should cover even the most benign cases that later met the inclusion criteria of definite MS diagnosis. Given the methods used, stability of diagnostic criteria and the complete follow-up data, it is believed that the selection and immortal bias are controlled as well as possible with conventional methods in this population-based study.
The strength of our study lies on the Finnish healthcare system as public healthcare is accessible to all Finns. The referral centres in MS are tertiary care neurological clinics in public hospitals. Case ascertainment is reliable [18]. Data in this research register have been updated continuously from the 1960s [18]. Emigration in the study region is sparse and the cohort is ethnically homogeneous as cases are 99.95% of Finnish descent. Linkage to national vital statistics by personal identification codes ensures a complete and reliable coding of deaths [35]. It is believed that the generalizability of results study can be regarded as high in contemporal cohorts in the high risk areas of MS.
Limitations for inferences for the long-term DMT treatment benefit concern the limited follow-up time and lack of information for comorbidities. Similar temporal survival patterns over the diagnostic decades may indicate a similar burden of comorbidity, which eventually may have a stronger survival-modulating effect than DMT use in ageing MS patients. Results on survival related to DMT use are probably also affected by disease activity, which information was not available in our total data. Including information on these factors is important in future studies. The heterogeneity in studied diagnostic cohorts, considering both the changes in general survival and temporal variability in being diagnosed and treated, needs attention. The observed difference in survival trends in birth and diagnostic cohorts reflects these temporal factors, which are also difficult to control as sources of bias in long-term studies such as ours.
In conclusion, pwMS in Western Finland had a higher mortality compared to general population controls. Sex, disease course, age at diagnosis and the use of DMTs were independently associated with survival, which did not differ between pwMS diagnosed in four different decades.
AUTHOR CONTRIBUTIONS
M.-L. Sumelahti: Conceptualization; methodology; data curation; investigation; validation; supervision; project administration; resources; writing – original draft; writing – review and editing. A. Murtonen: Data curation; investigation; writing – review and editing. V. Kytö: Formal analysis; visualization; writing – review and editing; methodology. J. O. T. Sipilä: Methodology; supervision; formal analysis; visualization; project administration; writing – review and editing.
FUNDING INFORMATION
This study received no funding.
CONFLICT OF INTEREST STATEMENT
MLS reports honoraria, consultancy fees and congress sponsorship from Merck, TEVA, Novartis, Lundbeck, Abbvie, Orion and Lilly. AV reports no competing interests. VK reports no competing interests. JOTS reports honoraria (Terveystalo/Novartis), consultancy fees (Medaffcon/gmp-orphan, Sandoz, Boehringer-Ingelheim), travel grants and congress sponsorship (Lundbeck) and holds shares (Orion Corporation).
Open Research
DATA AVAILABILITY STATEMENT
According to Finnish law, the data associated with this paper cannot be made available outside Finland. Finnish researchers can apply for access from Findata.




