Exploring amyotrophic lateral sclerosis through the visual system: A systematic review
Hélène Blasco and Philippe Corcia contributed equally.
Abstract
Background and Purpose
The human visual system relies on neural networks throughout the brain that are easily accessible for tests exploring eye structures and movements. Over the past two decades, investigations have been carried out on both afferent and efferent components of the visual system in people with amyotrophic lateral sclerosis (ALS). This approach might represent an innovative biomarker research strategy to better characterise the phenotypic variability of ALS. The purpose of this review was to determine whether exploring the visual system of patients with ALS (pwALS) is an effective strategy.
Methods
The Medline and Web of science databases were searched for studies with terms relating to ALS and vision. Of 1146 references identified, 43 articles were included.
Results
In this review article, both afferent and efferent components of the visual system were found to be impaired in pwALS in the absence of visual complaint, thereby contributing to the hypothesis that ALS is a multisystem disease with sensory involvement. Of note, some areas of the eye remain unexplored (i.e., tears, and retinal function using electroretinography).
Conclusions
According to the findings available in the literature, investigating the oculomotor system and exploring the ocular surface could represent two key promising strategies to identify new diagnostic biomarkers in pwALS. Further longitudinal studies are needed to identify relevant indicators of disease progression and response to therapeutic intervention.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease, characterised by degeneration of both upper and lower motor neurons in bulbar and spinal territories [1]. ALS is the most frequent motor neuron disease in adults, with a lifetime risk estimated at 1/264 in men and 1/418 in women by 85 years: a recent study performed in 28 European countries showed that every year in excess of 13,000 new cases are diagnosed and more than 35,000 patients have ALS [2]. The mean age of onset is approximately 63 years, with extreme spread from infancy in anecdotal cases [3] to old age [4]. In 10% of cases, there is a familial history of motor neuron disease and currently more than 30 genes have been linked to the disease, with four main genes explaining more than 60% of familial cases and approximately 10% of sporadic cases [5].
Currently, four molecules are available for the treatment of ALS with only one, riluzole, licensed worldwide for ALS [6]. Other medications, which can be prescribed in a few countries, are edaravone [7], the combination of phenylbutyrate and tauroursodeoxycholic acid (PB-TUDCA) [8], and tofersen [9] only for patients with a SOD1 mutation.
The ALS phenotype is extremely heterogeneous due to variety in its site of onset, the predominance of upper or lower motor neurons, or both, and variety of progression and prognosis 10]. ALS is currently considered as a disease spreading outside the motor neuron system, with a high frequency of cognitive disturbances related to frontotemporal lobar dementia [11]. Other neurological systems have been shown to be injured in ALS, with descriptions of Parkinsonism, cerebellar impairment, dysautonomia, and oculomotor system disorders [10]. Classically, ocular disturbances occur after a long duration of the disease, although a growing number of studies emphasise that the ocular system, including both the oculomotor and sensory systems (i.e., corneal small-fibre neuropathy [12, 13], retinal layer thickness modifications [14]), may be impaired in ALS. The eye is conventionally considered as the window onto the brain, owing to similar vasculature, anatomy, and physiology in the two organs, leading the eye to be considered as an expansion of the central nervous system.
Investigations of the ocular system that are non-invasive, readily accessible, and reproducible, therefore, make this organ a perfect target for large-scale and promising research to identify diagnostic and prognostic biomarkers of ALS.
Due to the large body of evidence, we performed a review to: (1) describe the ophthalmological abnormalities observed in ALS; (2) provide an up-to-date analysis of the characteristics of studies on the visual system and ALS; and (3) provide an overview of the various aspects of the ocular system that might be of interest to clinicians to facilitate diagnosis and better characterise ALS heterogeneity.
METHODS
We conducted a systematic review as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [15].
Search strategy
Our search strategy followed the PICO (Patient, Intervention, Comparison, Outcome) framework, using keywords related to ALS and the visual system (Supplemental Material S1).
A literature review was performed using Boolean operators and the following keywords, their abbreviations, and MeSH synonyms: [motor neuron disease OR amyotrophic lateral sclerosis] AND [ophthalmology OR vision OR cornea OR ocular surface OR tear OR colour vision OR visual field OR optical coherence tomography OR retina OR electroretinogram OR visual evoked potential OR oculomotor OR saccade OR pursuit OR eye-tracking].
Publications in English, retrieved using PubMed in the electronic database MEDLINE, were included from 2000 (i.e., date of publication of the revised EI Escorial criteria) [16] to February 2024. Automatic extraction from PubMed was performed using CSV files for each search and all data were merged. The search was first performed in MEDLINE and then the Web of Science database.
Eligibility criteria
After removing duplicates and reviews, original articles which were considered off-topic based on careful reading of the abstract, were excluded (e.g., nonhuman participants, in vitro cultures, brain imaging studies). Finally, the full-text articles were assessed for eligibility. All articles relating to visual exploration in ALS with ≥5 cases were included.
Study selection
After carefully reading the titles and abstracts, full-text articles were evaluated and, finally, 43 articles were included in this systematic review (Figure 1; detailed information on the selected articles in Supplementary material S2). Excel software was used for the whole process.

Data extraction
A systematic approach was taken to extract the following data for the 43 selected articles in structured Excel form: authors, component of visual system, journal, year of publication, country, number of ALS patients, number of control subjects, diagnosis criteria for ALS, distribution of ALS patients according to the revised EI Escorial criteria [16], number of female patients, age of ALS patients, percentage of sporadic forms of ALS, disease duration, number of bulbar, and spinal-onset forms, ALS severity according to the ALS-Functional Rating Scale-Revised (ALS-FRS-r), study design, and patient follow-up duration (data are presented in Supplemental Material S2). Careful and thorough reading of each article was performed by R.K.K. three times for each article in three different Excel files. Discrepancies were then identified and resolved. Synthesis was performed using an anatomical strategy based on the component of the visual system. The data presented in this review are structured according to the anatomical structures of the visual system (from afferent to efferent visual pathways).
Quality assessment
The Newcastle–Ottawa Scale was used to assess study quality and risk of bias [17]. This scale is the most commonly used for non-randomised studies in health research [18]. Each study included in the current systematic review was thoroughly evaluated by R.K.K. and N.M., with scores provided in Supplemental Material S3. Disagreements between R.K.K. and N.M. were resolved through discussion. The thresholds for study quality were defined as follows: ≤1 low, 2–6 moderate, and ≥7 high.
MEANS TO INVESTIGATE THE VISUAL SYSTEM
The human visual system combines sensory and motor functions from the eye to the brain. In ALS research, focus has been on the retina and oculomotor functions, where functional abnormalities should correlate with anatomical changes. We provide information about promising methods of visual evaluation as suggested by the current body of literature on ALS.
Structural investigations
Ocular surface
At the front of the eye, the ocular surface, an accessible functional unit comprising the eyelids, lachrymal gland, tear film, conjunctiva, and cornea, can be studied via in vivo confocal microscopy [19]. This swift, painless, non-invasive tool enables visualisation and quantification of the corneal sub-basal nerve plexus originating from the ophthalmic branch of the trigeminal nerve (Figure 2).
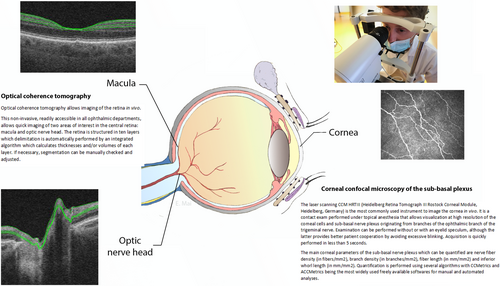
Retina
The retina, a direct extension of the brain with several shared characteristics, is of interest in neurological diseases [20]. Optical coherence tomography (OCT), a non-invasive technique, allows cross-sectional retinal imaging with 10 identifiable layers (Figure 2). These retinal changes can mirror brain changes, but a thorough ophthalmological examination should consider numerous confounding factors before establishing a link to neurological diseases.
Functional investigations
Oculomotor functions
Infrared devices record eye movements, such as fixation and saccades, and are widely used in ALS studies. Oculomotor abnormalities can pinpoint neurological disease locations and specific tasks can assess frontal lobe cognitive control in ALS, thereby contributing to understanding of the disease's neural involvement.
RESULTS
Afferent visual pathways
Ocular surface
The ocular surface has been infrequently studied in patients with ALS (pwALS) due to the postulated absence of visual disturbances. Over the past 10 years, several studies have explored the ocular surface thanks to the development of specific tools enabling the painless, non-invasive and reliable examination of this area of the human eye.
Evidence of corneal small-fibre sensory neuropathy
In 2014, Ferrari et al. [21] first conducted a prospective cross-sectional controlled study (n = 8) to evaluate corneal small-fibre sensory nerves using an automated algorithm. The number and branching of corneal nerves were significantly reduced, while corneal nerve tortuosity significantly increased in pwALS. Furthermore, corneal nerve length and branching were significantly lower.
In two other prospective cross-sectional controlled studies, Fu et al. [22] (n = 66 ALS) and Jiao et al. [23] (n = 33 ALS) evaluated corneal small fibres. Similarly to Ferrari et al. [21], Fu et al. [22] and Jiao et al. [23] found that pwALS exhibited significantly decreased corneal nerve lengths and increased corneal nerve complexity. Dendritic cell density was also found to be increased, albeit not to a level comparable to direct corneal infection, which could reflect inflammation in response to corneal cell apoptosis.
The findings of these three studies support the hypothesis of corneal small-fibre neuropathy as a manifestation of ALS [21-23]. On the other hand, Turha et al. [24] recently reported no significant change in corneal innervation in pwALS compared with controls. Corneal confocal microscopy could emerge as a valuable diagnostic tool in pwALS, although its utility remains to be confirmed.
Corneal sensory neuropathy, without abnormal sensitivity, may reflect ALS severity
Corneal sensitivity using the Cochet-Bonnet corneal esthesiometer was not found to be significantly different between pwALS and controls [21]. Both Ferrari et al. [21] and Fu et al. [22] found a positive significant correlation between ALS-FRS-r and corneal nerve length (respectively: r = 0.908, p = 0.002 and r = 0.467, p < 0.001). Fu et al. [22] also observed a significant negative correlation between corneal nerve length and disease progression based on the fold change of ALS-FRS-r (r = −0.378; p = 0.002).
These results suggest that corneal axonopathy might represent a feature of non-motor involvement in ALS. Of note, the level of evidence for corneal axonopathy in ALS remains low in the absence of further replication. Ocular surface sensitivity finds its origin in the branches of the trigeminal system and eye lubrication is dependent on the autonomic parasympathetic system. These anatomical specificities could provide a pathophysiological basis for abnormal findings in other components of the ocular surface such as the conjunctival epithelium or tear film in pwALS; therefore, the ocular surface merits further exploration.
Retina
To date, 15 studies (Table 1) have focused on the retina of pwALS using either OCT (n = 14) [25-38] or pathological examination (n = 1) [39], in a total of 581 pwALS.
| Authors | Year of publication | Optical coherence tomography | ||||
|---|---|---|---|---|---|---|
| RNFL | Macular thickness | Ganglion cell layer | Inner nuclear layer | Outer nuclear layer | ||
| Roth et al. [25] | 2013 | NS | NA | NS | NS | NS |
| Ringelstein et al. [26] | 2014 | ↘ | ↘ | NS | ↘ | NS |
| Volpe et al. [27] | 2015 | ↘ | ↘ | NS | NS | NS |
| Simonett et al. [28] | 2016 | NA | NS | NS | NS | NS |
| Hübers et al. [29] | 2016 | ↘ | NS | NS | ↘ | NS |
| Mukherjee et al. [30] | 2017 | ↘ | NA | NA | NA | NA |
| Liu et al. [31] | 2018 | ↗ (nasal quadrant) | NS | NS | NA | NA |
| Abdelhak et al. [33] | 2018 | NS | NS | NS | NS | ↘ |
| Rohani et al. [32] | 2018 | ↘ | NA | NA | NA | NA |
| Rojas et al. [34] | 2019 | NS | ↗ | NS | NA | NA |
| Zhang et al. [35] | 2022 | ↗ (nasal quadrant) | NS | NS | NA | NA |
| Cennamo et al. [36] | 2022 | NS | NA | NS | NA | NA |
| Miscioscia et al. [38] | 2023 | ↘ (temporal quadrant) | NA | NS | NS | NS |
| Mohanty et al. [37] | 2023 | ↘ (all quadrants except temporal) | NA | NA | NA | NA |
- Abbreviations: NA, not applicable; NS, not significant (in bold); RNFL, retinal nerve fibre layer.
Contradictory findings concerning retinal architectural modifications in patients with ALS
Roth et al. [25] first investigated retinal layers using OCT in 76 pwALS. They found no significant difference in the thickness of any retinal layer between patients and controls (Table 1), nor any significant correlation between OCT parameters, ALS-FRS-r score or ALS-FRS-r rate of progression. It is important to note that several issues could be raised for consideration or discussion, relating to the lack of complete ophthalmological examination (including intraocular pressure, refractive data) and the lack of age matching between pwALS and controls in some studies. Although numerous studies have been conducted, retinal involvement in pwALS remains a matter of debate (Table 1).
Results concerning the relationship between OCT findings and clinical characteristics of pwALS are discordant. While three studies highlighted a significant correlation between the duration of the disease and OCT parameters [27, 31, 37], this was not confirmed by others [26, 28, 29, 32, 33, 38]. This was also the case with the ALS-FRS-r score, whose correlation with OCT findings was also controversial (pros [31-34, 37]; cons [25, 28-30, 38]).
To date, only two longitudinal studies using OCT have been performed in pwALS over periods of 6 months [34] and 12 months [38]. In the 6-month study by Rojas et al. [34], significant thinning was observed in the inferior areas of the inner and outer macular rings, as well as in the inferior and superior quadrants of the peripapillary retinal nerve fibre layer (RNFL) when compared to controls. However, the 12-month study by Miscioscia et al. [38] found no significant differences in any OCT parameter. Despite the contrasting findings, we believe the results of Miscioscia et al. [38] are more robust due to their larger sample size (n = 35 compared with n = 10 in the study by Rojas et al. [34]). Nevertheless, further longitudinal studies are warranted to validate these results.
There may be a molecular basis to retinal modifications in pwALS as retinal ganglion cell loss can relate to the role played by Ranbp2, a unique vertebrate nucleoporin highly expressed in motor neurons and retinal ganglion neurons involved in nucleocytoplasmic transport, whose regulation is impaired in both familial and sporadic ALS [40].
Nevertheless, in vivo findings using OCT in pwALS have yielded contradictory findings and comparisons among studies are made difficult owing to several factors: heterogeneity of ALS phenotypes (bulbar or spinal onset; overlap with frontotemporal dementia or primary lateral sclerosis); different rates of disease progression; few studies with systematic genetic screening (some genes, such as optineurin, ataxin2 and TBK1, can cause retinal and optic nerve modifications, such as glaucoma); heterogeneity of the technologies used; ocular refractive status; ocular axial length; and statistical procedures (e.g., age and gender-matched controls, assessment of inter-eye asymmetry, correlations rather than statistical models that take into account potential confounding factors). Moreover, retinal changes in ALS may not be specific to the disease and have been reported in a wide range of neurodegenerative conditions [20]. There is therefore a strong need for quality control, and caution is advised when inferring causality from correlative studies of OCT metrics with clinical metrics.
These limitations hinder the use of retinal imaging with OCT as a tool for diagnosis and disease severity assessment for ALS in routine clinical practice and further longitudinal investigations are necessary to identify sensitive prognostic retinal biomarkers.
The retina could reflect microvascular damage
Abdelhak et al. [33] developed an innovative approach for studying retinal blood vessels from OCT assessment. In a case–control study including 34 pwALS, inner wall thickness, lumen diameter, and mean wall thickness did not significantly differ, while outer wall thickness was significantly increased (p = 0.037). The authors postulated that the thickening of the outer wall might reflect the microvascular alterations observed in mouse models of ALS before motor neuron loss [41]. Microvascular impairment of the retina and the integration of such findings into the neurovascular impairment hypothesis in ALS remains to be confirmed [42]. OCT angiography, an emerging non-invasive technique for imaging the microvasculature of the retina and the choroid (first clinical study published in 2014 [43]), failed to reveal any differences in superficial and deep macular capillary plexuses between pwALS and controls [36].
The retina: A window onto spinal motor neurons
Sharma et al. [39] conducted a post-mortem case–control study in 10 pwALS (19 eyes). They conducted histopathological examinations of retinal sections to identify periodic acid Schiff-positive spheroids (PAS-S; i.e., round structures indicating axonal swelling) and used immunofluorescence to quantity both phosphorylated and non-phosphorylated forms of neurofilaments. Neurofilament light chain (NfL) is a promising biomarker found in blood and cerebrospinal fluid (CSF) that has received considerable attention and shows great potential [44]. Blood and CSF contain neurofilaments, levels of which reflect the extent of neuronal death. Studies have revealed that NfL level is a highly meaningful marker to distinguish ALS from ALS mimics, other neurodegenerative disorders and healthy individuals. This remarkable accuracy is primarily explained by the rapid degeneration of motor neurons which is characteristic of ALS [45].
In 9 out of 10 ALS patients and 5 out of 10 age-matched controls, PAS-S were identified and most commonly located in the peripheral and peripapillary RNFL. PAS-S density was higher in ALS patients than in controls (p = 0.027) due to increased PAS-S density in the peripapillary RNFL (p = 0.047) with no significant difference of PAS-S density in the central and peripheral retina. Phosphorylated neurofilament spheroids were found in 9 out of 10 ALS patients and none in controls. Phosphorylated neurofilament immunoreactivity was significantly greater in the RNFL of pwALS compared with controls (p = 0.002). They were primarily located in the peripheral and peripapillary RNFL, with none in the central RNFL. Non-phosphorylated neurofilament spheroids were found in 2 out of 10 ALS patients and were absent in controls without any significant difference in spheroid density. Non-phosphorylated neurofilament immunoreactivity was increased in the RNFL and inner plexiform layer.
No correlation was found between PAS-S and neurofilaments and clinical characteristics at age of death (i.e., sex, duration of disease, mode of disease onset, ALS-FRS-r bulbar score, and rate of progression).
The study by Sharma et al. [39] provides an interesting anatomical basis from which to understand morphological modifications of the retina that can be imaged in vivo using OCT in pwALS. Neurofilaments are neuron-specific cytoskeletal proteins, the levels of which increase according to the intensity of axonal damage [46]. Therefore, the retina could reflect damage to spinal cord motor neurons in ALS [47]. Additionally, another study by Pediconi et al. [48] (n = 10 patients) examined retinal cells of deceased ALS patients and identified increased mislocalised TDP-43, SQSTM1/p62 aggregates, activated cleaved caspase-3 and microglia density in the ganglion cell layer. The findings by Sharma et al. [39] and Pediconi et al. [48] suggest that changes in the retina could potentially be used as an additional diagnostic tool for ALS, providing an anatomical basis for disease identification. Furthermore, modifications observed in vivo through OCT could offer a non-invasive, cost-effective method for longitudinally tracking disease progression and assessing the effectiveness of treatments over time.
Nevertheless, retinal layer thinning or thickening can be encountered in many other neurodegenerative disorders or can be related to other diseases (e.g., glaucoma). There is an unmet need for controlled cross-sectional and longitudinal studies before in vivo assessment of the retina using OCT could represent a sensitive marker of axonal loss and neurodegeneration.
Miscellaneous findings (visual acuity, contrast sensitivity, vision-related quality of life, visual field, and colour vision)
Although impairments of the sensory visual pathway have been reported, visual function is classically considered to be preserved in ALS. Visual acuity and visual field testing represent the two main functional components of vision most often assessed in ophthalmic departments. Contrast sensitivity, a more rarely tested component of visual function in routine practice, refers to the ability to discern an object from its background from light to dark conditions. Both high- and low-contrast visual acuity have been reported to be normal in pwALS [27, 34, 49], although one study by Moss et al. [50] (n = 63) showed significantly decreased visual acuity at both high and low contrast using Sloan charts in pwALS (p = 0.003 and p = 0.001). These differences, although significant, are mild (<5 letters) and do not provide evidence for a clinically significant impact. This is supported by the absence of abnormal vision-related quality of life in pwALS [27] using the NEI VFQ-25, a widespread questionnaire that was initially validated for glaucoma disease [51].
Only one study investigated visual field in ALS patients (n = 51) compared with patients with primary open-angle glaucoma and controls [31]. Visual field examination is a commonly performed test, known to involve a learning curve by the patient. It should be interpreted cautiously, taking into account the patient's level of attention, as indicated by parameters such as fixation loss and the number of false positives and negatives. Liu et al. [31] used standard automated perimetry (Octopus 900, HAAG-STREIT international) to measure mean defect (MD), mean sensitivity (MS), and square of loss variance (sLV). ALS patients had similar MD but significantly lower MS (p < 0.001) and sLV (p < 0.001) than controls. The MS and sLV in ALS patients did not differ significantly compared with primary open-angle glaucoma patients. Moreover, the authors reported unreliable visual field examination, which they attributed to motor difficulties (i.e., increased fixation loss, false positives and negatives). The functional impairment was not supported by structural modifications assessed by OCT concurrently in the study by Liu et al. [31], and therefore requires further replication as visual field findings correlate closely with patient cooperation and dexterity. Addressing the challenges posed by dexterity and upper limb motor function in pwALS when performing automated perimetry is essential. Consideration of how this may impact test results and interpretations is crucial to understanding potential issues with the visual field or test performance. Furthermore, slit-lamp and fundus examination was not carried out in the studies by Moss et al. [50] and Liu et al. [31], while it is well known that other age-related ocular conditions (e.g., cataract) can co-occur with ALS and represent confounding bias.
To date, two studies have investigated colour discrimination in ALS [27, 52]. Boven et al. [52] (n = 25), performed the L'Anthony D15 (desaturated) colour test in a cohort of 25 ALS patients and calculated the confusion index for which the pathological value suggestive of diffuse colour discrimination is ≥1.8. ALS patients had a significantly increased confusion index compared with controls (i.e., average 2.1 in ALS patients vs. 1.6 in controls; p = 0.01) suggestive of diffuse colour discrimination. An abnormal colour index was found in 16/25 (64%) ALS versus 9/21 (43%) controls. Volpe et al. [27] (n = 16) reported a mean confusion index of 1.4 in 12 ALS patients using the same L'Anthony D15 (desaturated) colour test, with 4/12 patients having a pathological colour index (≥1.8). As colour vision is related to retinal and optic nerve function, increased confusion index could reflect abnormal retinal or optic nerve function, but the underlying pathophysiology remains to be elucidated. With regard to the visual field examination, abnormal findings can be related to impaired test performance in patients with upper limb dysfunction.
Efferent visual pathways
Oculomotor muscles
It is commonly believed that oculomotor muscles are spared until late-stage disease in ALS [53]. To date, five post-mortem case–control studies have been performed on oculomotor muscles compared with limb muscles of ALS patients [54-58].
Ahmadi et al. [54] (n = 8) performed immunohistochemistry with monoclonal antibodies against distinct myosin heavy chain isoforms, along with haematoxylin and eosin staining, and studied nicotinamide dehydrogenase-tetrazolium reductase activity. They found evidence of oculomotor muscle involvement as follows: altered cellular architecture (hypertrophic and atrophic fibres), hyperplasic connective tissue, increased fat, altered myosin heavy chain (fewer fibres containing myosin heavy chain slow-tonic, and a general absence of embryonic myosin heavy chain). The oculomotor muscles, however, were globally morphologically preserved compared with limb muscles. In another study, the same team investigated myosin heavy chain slow-twitch, slow-tonic, and laminin in medial rectus [58] and found a loss of marked myofibres in ALS that significantly correlated with age of death in bulbar-onset forms (r2 = 0.669; p < 0.019). They also reported changes in the fast-type myofibre composition of extraocular muscles that are more pronounced in patients with bulbar-onset form [59].
In 2011, Liu et al. [55] (n = 8) performed immunohistochemistry using antibodies against laminin chain isoforms in the neuromuscular junction and found a normal laminin composition in ALS patients compared with limb muscles.
In 2013, Liu et al. [56] (n = 7) studied synaptic protein composition at the neuromuscular junction in nerve fibres between the oculomotor muscles and limb muscles of pwALS. They reported altered protein composition in limb muscles (i.e., decreased neurofilament light subunit and synaptophysin), while the oculomotor muscles remained spared. S100B was significantly decreased in all muscles in both neuromuscular junction and nerve fibres and p75 decreased in limb muscles only at the neuromuscular junction. Taken together, these findings support denervation as a possible mechanism of ALS.
McLoon et al. [57] (n = 6) found a different pattern of expression for Wnt proteins (i.e.Wnt1, Wnt3a, Wnt5a, Wnt7a) and β-catenin between the nerve fibres of oculomotor muscles and limb muscles of pwALS.
Cadaveric oculomotor muscle studies reported significant differences in the molecular phenotypes between limb and oculomotor muscles, neuromuscular junctions, and nerve fibres that could be related to a distinct gene expression profile in extraocular muscles, with specific molecular expression different from limb muscles [60]. However, the five available cadaveric studies of oculomotor muscles were performed by the same team and further replication worldwide should be performed due to the heterogeneity of ALS phenotypes. Moreover, the diagnostic criteria of three out of the five studies were not provided and the remaining two studies used the European Federation of Neurological Societies consensus criteria for ALS [61].
Oculomotor functions
Normal visual function requires the oculomotor system to initiate and maintain fixation of a target on corresponding areas of the retina. Oculomotor control relies on complex pathways throughout the human brain, with circuits that coordinate the eyes along with cognitive networks. Eye-tracking has been a widely used tool to improve our understanding of the cerebral networks involved in fixation, pursuit, saccades (i.e., reflexive and volitional), anti-saccades (i.e., ability to inhibit reflexive saccades; for illustration of cortical areas involved in the eye movements of the human brain, see Donaghy et al. [62]). Historically, eye-tracking has been used in ALS to improve communication, especially in end-stage disease. Twelve studies [50, 63-73] aimed to assess oculomotor function in pwALS and one study [74] was carried out in presymptomatic C9orf72 expansion gene carriers (n = 48). These 13 studies aimed to discover clinical markers of disease severity and progression, along with biomarkers of cognitive dysfunction, that may help discriminate frontotemporal dementia and dementia from ALS. Previous studies do exist but these predate the Revised EI Escorial criteria [16]. The findings of the 13 studies selected in the current review are summarised in Table 2.
| Authors | Year of publication | Eye-tracking tasks | ||||
|---|---|---|---|---|---|---|
| Saccades | Anti-saccades | Pursuit | Fixation | Miscellaneous | ||
| Evdokimidis et al. [63] | 2002 |
Latencies for pro-saccades and error pro-saccadesa Latencies for remembered and delay saccades Accuracyc |
Latency for error anti-saccadesa | NA | NA | Distractibilityb |
| Donaghy et al. [64] | 2009 | NA | NA | NA | Size of saccadic intrusion (particularly greater in spinal-onset form)b | NA |
| Donaghy et al. [65] | 2010 | Speed of reflexive saccades in bulbar-onseta |
Latency for anti-saccadeb Anti-saccade type 1 errors (i.e., uncorrected saccade towards the stimulus)b |
Proportion of time spent in smooth pursuita Smooth pursuit velocity gaina |
NA | NA |
| Moss et al. [50] | 2012 | NA | NA | Saccadic horizontal pursuit | NA |
Gaze impersistence eyelid opening apraxia restricted voluntary upgaze |
| Burrell et al. [66] | 2013 | Incidence of early saccadesb | NA | NA | NA | NA |
| Gorges et al. [68] | 2015 |
Delayed saccades error rateb Visually guided reactive saccades (horizontal and vertical)b Number of voluntary gaze shiftsa |
Anti-saccades error rateb |
Smooth pursuit gainc |
Rate of saccadic intrusionb |
High distractibility (i.e., moving the eye towards the target) |
| Proudfoot et al. [67] | 2015 | Pro-saccadec | Error rate and latency of anti-saccade performanceb | NA | NA | Executive and visual search tasksa |
| Kang et al. [69] | 2018 | Saccadic inaccuracy (dysmetria)b | NA | Abnormal cogwheeling smooth pursuit |
Square-wave jerks positional nystagmus of central originb |
Head shakingb |
| Becker et al. [70] | 2019 | NA | NA | NA |
Size of saccadic intrusionb Rate of saccadic intrusionc |
NA |
| Behler et al. [74]d | 2021 |
Delayed saccades error rateb Visually guided reactive saccades (up)b |
Anti-saccades error rateb | Smooth pursuit gainc | Rate of saccadic intrusionb | NA |
| Rekik et al. [71] | 2022 | Latency of voluntary saccadeb | Anti-saccades error rateb | Frequency of altered smooth pursuitb | NA | NA |
| Guo et al. [72] | 2022 | Reflexive saccadec | NA | Abnormal cogwheeling smooth pursuit |
Square-wave jerksb |
NA |
| Zaino et al. [73] | 2023 |
Amplitude and multisteps saccades in the spinal-onset forma Speed of saccades in the bulbar-onset forma Error rate of memory-guided saccadeb Error rate and latency of saccadesb |
Anti-saccades error rateb | NA | NA | NA |
- Abbreviation: NA, not applicable.
- a Significantly decreased compared with controls.
- b Significantly increased compared with controls.
- c Similar with control subjects.
- d Only study carried out in presymptomatic C9orf72 expansion gene carriers.
A range of oculomotor dysfunction has been reported in pwALS. Executive control of saccades has also been found to be impaired [63, 65, 66, 68, 69, 71, 73], with only two studies reporting no significant differences between ALS and controls for pro-saccade [67] (i.e., volitional saccade) and reflexive saccade [72]. Interestingly, a recent study by Zaino et al. [73] (n = 18) found that the amplitude of volitional saccades was decreased in spinal-onset ALS, while speed was decreased in bulbar-onset ALS, suggesting the involvement of different phenotypes and brain networks. There is increasing evidence that frontal lobe cognitive control might be impaired, as shown by the anti-saccades task [63, 65, 67, 68, 71, 73]. Anti-saccades and volitional saccades were also found to be impaired in presymptomatic C9orf72 expansion gene carriers [74]. Testing specific saccadic paradigms could help to better understand the neural substrate involved in ALS. Reflexive visually guided saccades test the ability of the parieto-collicular network to localise the target and the ability of the superior colliculi to react to disengage fixation towards the target. Anti-saccade and memory-guided saccades test the voluntary fronto-basal ganglia-collicular pathway, visual working memory system, and the inhibition of reflexive movements by the dorso-lateral prefrontal cortex [73]. Contradictory findings have been reported for smooth pursuit. While two studies found no significant differences in ALS patients [68] (n = 68) and presymptomatic C9orf72 expansion gene carriers [74] (n = 48) compared with controls, five studies [50, 65, 69, 71, 72] reported abnormal pursuit, such as cogwheeling movements [69, 72], and saccadic intrusion [50], both of which represent features of extrapyramidal dysfunction also reported in Parkinson's disease.
The route towards the identification of quantitative biomarkers using eye-tracking seems promising and might help distinguish oculomotor nucleus involvement from cortical and sub-cortical involvement in ALS patients. The usefulness of such findings as prognostic biomarkers remains to be confirmed since Proudfoot et al. [67] (n = 61) reported no significant progression of eye-tracking measures (i.e., anti-saccades, trail making, visual search tasks) over a 2-year follow-up period in ALS patients. Oculomotor findings could represent a diagnostic biomarker of ALS plus disease rather than a prognostic biomarker related to the evolution of the disease, with damage spreading to the cortical and subcortical networks involved in oculomotor functions.
Miscellaneous findings
One study by Byrne et al. [75] investigated blink rate in 53 ALS patients. They reported decreased blinking in familial ALS: 6/min versus 13/min in controls (p = 0.008; Mann–Whitney U) versus 10.7/min in sporadic ALS (p = 0.004). No difference was found in bulbar versus spinal forms. As the brain pathways involved in spontaneous blink production have not yet been elucidated, the current findings remain preliminary to further studies.
QUALITY OF EVIDENCE
The Newcastle-Ottawa Scale, the most widely used tool for assessing the risk of bias in observational studies, revealed inconsistencies in the quality of the 43 studies included in this systematic review. Among the risk of bias of the 35 studies evaluated with the Newcastle-Ottawa Scale (eight post-mortem investigations were excluded from analysis due to methodological differences), one was rated as having high quality, 31 had moderate quality, and three were of low quality (median score: 5; range: 0–7). The primary drawbacks were related to the representativeness of cases and outcome measures, as only a minority of studies reported consecutive inclusion of ALS patients and masked assessment of outcomes. In summary, the quality of evidence in this systematic review varied, with most studies rated as of moderate quality. This highlights the need for higher quality observational studies in future research. Improved study design, including better representativeness and outcome measures, will be essential for providing more robust evidence.
RELEVANCE OF THE VISUAL SYSTEM IN ALS
The search for biomarkers in ALS has promoted investigations of the visual system during the past two decades. Impairments in both afferent and efferent visual systems seem to be features of ALS and could represent diagnostic biomarkers of ALS-plus disease. These findings suggest that ALS is a multisystem disorder with involvement of the visual system. While some studies have identified features in the visual system that may serve as biomarkers for disease severity, there is limited evidence of biomarkers for disease progression.
The eye-tracking approach to identify specific diagnostic biomarkers of ALS appears to be on the rise, but there is still insufficient evidence for retinal biomarkers. Studies investigating biomarkers of disease severity are still scarce and none have used ophthalmological findings to assess patient response to pharmacological intervention in controlled trials.
Some limitations must be addressed and generalisation of the results is still limited by methodological issues related to: (1) study design (e.g., few multicentre studies, small sample size, few longitudinal investigations); (2) population characterisation (e.g., sporadic or familial form, no systematic genotyping, unspecified diagnostic criteria, type of onset and severity of ALS, overlap with frontotemporal dementia or dementia); (3) data collection (e.g., lack of measurement of best-corrected visual acuity, intraocular pressure, corneal thickness, and refraction, along with slit-lamp and fundus examination; for OCT studies lack of data concerning ocular biometrics and for eye-tracking studies lack of cognitive and frontal lobe assessments); (4) therapeutics (e.g., riluzole status and impact of treatment on ophthalmological features); and (5) statistics (e.g., multiplication of statistical tests increasing the alpha risk in small cohorts, use of correlations rather than regressions to assess prognostic biomarkers of disease severity, in general lack of inter-eye comparisons).
There remain some unexplored areas of the visual system in ALS, such as the ocular surface (e.g., human tears) and the retina (i.e., electroretinography for functional assessment). In the presence of OCT findings suggestive of retinal ganglion cell dysfunction, electroretinography (i.e., global, multifocal, and pattern types), a diagnostic test that measures the electrical activity of the retina in response to a light stimulus, might be relevant to provide an electrophysiological basis for abnormal retinal function and to identify new biomarkers of disease severity and progression. Few studies have investigated the autonomous system, which can be indirectly assessed by studying pupillary responses to light stimulation, and represents another poorly explored area of research in ALS. Furthermore, with regard to the identification of factors that trigger the onset of disease, further investigations into presymptomatic carriers of gene mutation and family relatives of sporadic ALS patients are required.
Moreover, uncorrected refractive errors related to difficulties in examining pwALS can hinder the use of oculomotor devices and contribute to communication impairment. Careful refractive screening should be carried out to minimise visual disability. The identification of visual problems in ALS is crucial for enhancing the individual's well-being and quality of life as long as possible. Technological advancements can be particularly useful in this regard.
CONCLUSIONS AND FUTURE PERSPECTIVES
Over the past 24 years, the visual system has been explored in ALS patients and holds the promise of identifying new readily accessible, non-invasive biomarkers for the diagnosis of ALS. According to the available findings in the literature, investigating the oculomotor system and exploring the ocular surface could represent two key promising strategies to identify new diagnostic biomarkers in pwALS. This approach may contribute to better characterisation of the phenotypic variability of pwALS that could be considered as a multisystem disorder with sensory involvement. There is still a long way to go to address the current methodological challenges to increase the chances of transferring ophthalmological findings into routine daily practice.
AUTHOR CONTRIBUTIONS
Raoul K. Khanna: Conceptualization; methodology; data curation; investigation; formal analysis; writing – original draft; writing – review and editing. Sophie Catanese: Writing – review and editing; conceptualization; methodology; validation. Geoffroy Mortemousque: Writing – review and editing; conceptualization; methodology; visualization. Nicolas Mureau: Investigation; validation; methodology. Patrick Emond: Visualization. Pierre-Jean Pisella: Visualization. Hélène Blasco: Conceptualization; methodology; writing – review and editing; validation; supervision. Philippe Corcia: Conceptualization; methodology; writing – review and editing; validation; supervision.
ACKNOWLEDGEMENTS
We would like to thank Elodie Mai, who helped create Figure 2, and the University Hospital of Tours for providing the services of a native English speaker who reviewed the whole manuscript and the related documents (A.D.T. International–L'Agence de Traduction).
FUNDING INFORMATION
This study was not funded.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest to declare in relation to this review.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.




