Stepwise dual-target magnetic resonance-guided focused ultrasound in tremor-dominant Parkinson disease: One-year follow-up
Abstract
Background and Purpose
Magnetic resonance-guided focused ultrasound (MRgFUS) is a nonsurgical treatment for Parkinson disease (PD). Some selected anatomical structures can be targeted by MRgFUS in PD. However, there is no uniform target yet. We have reported that stepwise dual-target MRgFUS was successfully applied to treat refractory tremors with akinetic–rigid features in PD. It generated two precise thermal ablations in the ventral intermediate nucleus (VIM) and pallidothalamic tract (PTT). Here, we report more PD patients to verify the safety and efficacy of stepwise dual-target MRgFUS.
Methods
Ten tremor-dominant PD patients (mean age = 66.7 ± 3.2 years, eight men) received the stepwise dual-target MRgFUS treatment with a series of primary and secondary outcome measures. The VIM and PTT were navigated based on brain magnetic resonance images. Outcome measures were categorized into primary and secondary assessments. The primary outcome measures consisted of resting tremor, action/kinetic tremor, rigidity, and bradykinesia. Secondary outcome measures encompassed non-motor symptoms scale of PD. Data collected at follow-up time points, including 1 day, 3 months, 6 months, and 1 year posttreatment, were compared with baseline data.
Results
The severity of tremor and motor deficits represented by Clinical Rating Scale for Tremor parts A and B during off-medication status and Unified Parkinson's Disease Rating Scale III on the treated side were significantly improved (p < 0.05 by paired t-test) at 1-year follow-up. At the 1-year follow-up, significant improvement was observed in the non-motor symptoms scale. Additionally, no severe adverse effects were reported, except temporary treatment-related discomfort during the procedure.
Conclusions
In conclusion, stepwise dual-target MRgFUS emerges as a safe and effective therapeutic modality for PD patients, particularly in addressing medication-refractory tremor and akinetic–rigid syndrome.
INTRODUCTION
Parkinson disease (PD) manifests as a movement disorder with cardinal features of bradykinesia, resting tremor, and/or rigidity. Additionally, PD tremor can include postural and kinetic tremor, alongside resting tremor, all linked to disordered rhythmic activity in the basal ganglia and cerebellothalamocortical circuitry, along with irregular interactions between circuits [1]. Magnetic resonance-guided focused ultrasound (MRgFUS) offers a novel approach to PD treatment. This therapy targets specific areas such as the ventral intermediate nucleus (VIM), subthalamic nucleus, and pallidothalamic tract (PTT) to alleviate tremor symptoms. However, given the diverse clinical manifestations of PD, including variations in tremor subtypes and other cardinal features, a single-target approach with MRgFUS may not address all patient needs effectively [2]. For instance, whereas VIM thalamotomy may effectively address posture/kinetic tremor, it may not adequately target rigidity and bradykinesia. Similarly, procedures targeting PTT or the subthalamic nucleus might have limited effectiveness against posture/kinetic tremor [3].
In a previous paper, we analyzed three individuals diagnosed with PD primarily exhibiting tremors, averaging 60.7 ± 6.0 years of age, who received stepwise dual-target MRgFUS therapy. This approach involved precisely targeting both the VIM and PTT using personalized brain magnetic resonance imaging (MRI) mapping. Evaluation of primary and secondary outcome measures occurred at baseline, 1 day, and 1 month posttreatment, revealing a notable decrease in both resting and posture/kinetic tremors following the MRgFUS procedure after 1 month [4]. Additionally, improvements were noted in rigidity, bradykinesia, and non-motor symptoms scale. Importantly, no noticeable adverse effects were observed during the 1-month follow-up [4].
Here, we aimed to investigate further consistency of the efficacy and safety of MRgFUS in treating tremor-dominant PD with akinetic–rigid features by gathering data from PD patients with a 1-year follow-up period.
MATERIALS AND METHODS
This case series study examines 10 patients diagnosed with tremor-dominant medication-resistant PD who underwent MRgFUS treatment alongside standardized care. Inclusion criteria required patients to have a follow-up exceeding 1 year posttreatment. The study cohort comprised 10 idiopathic PD patients exhibiting both resting and postural/kinetic tremors, with a mean age of 66.7 ± 3.2 years, consisting of eight men (Table 1). PD diagnosis was confirmed based on the UK Brain Bank Clinical Criteria through clinical history and examination conducted by neurologists specializing in movement disorders. Inadequate medication trial was determined by either a lack of response to therapeutic doses, poor drug efficacy, or the emergence of side effects during dose titration. Notably, three subjects from a previous report were also included in this follow-up. Among the participants, six exhibited dominant symptoms on the right side. In this analysis, all patients provided informed consent after receiving comprehensive information regarding the treatment, its outcomes, and associated risks. They explicitly granted written inform consent of their anonymized data. The study adhered to the standards outlined in the Declaration of Helsinki and received approval from the China Medical University Hospital, Taiwan (IRB CMUH111-REC3 014).
| Characteristics | ||||||
|---|---|---|---|---|---|---|
| N | Male:female | Disease duration, years | Age, years | Skull density ratio | ||
| 10 | 8:2 | 11.2 ± 3.4 | 66.7 ± 3.2 | 0.48 ± 0.02 | ||
| Items | Visit | |||||
|---|---|---|---|---|---|---|
| Pretreatment | 1-day follow-up | 1-month follow-up | 3-month follow-up | 6-month follow-up | 1-year follow-up | |
| LEDD, mg | 889 ± 187 | 810 ± 125 | 860 ± 94 | 860 ± 94 | 860 ± 94 | 860 ± 94 |
| CRST (A + B), treated side (med. off) | 16.0 ± 1.9 | 4.0 ± 0.8*** | 3.1 ± 0.5*** | 4.7 ± 1.8*** | 4.0 ± 1.4*** | 3.4 ± 1.5*** |
| UPDRS score (med. off)a | ||||||
| Part I | 2.7 ± 0.6 | 1.3 ± 0.4 | 1.2 ± 0.4* | 1.4 ± 0.4 | 2.2 ± 0.4 | 2.3 ± 0.6 |
| Part II | 14.0 ± 2.1 | 4.5 ± 1.2** | 6.5 ± 1.2** | 8.5 ± 1.6 | 9.1 ± 1.6 | 9.1 ± 1.9 |
| Part III, treated side | ||||||
| Resting tremor | 2.4 ± 0.4 | 0.1 ± 0.1*** | 0.8 ± 0.4* | 0.7 ± 0.4** | 0.4 ± 0.2*** | 0.4 ± 0.2*** |
| Action tremor | 2.5 ± 0.3 | 0.6 ± 0.2*** | 0.8 ± 0.2*** | 0.8 ± 0.2*** | 0.7 ± 0.2*** | 0.7 ± 0.2*** |
| Rigidity | 3.5 ± 0.7 | 0.4 ± 0.2*** | 0.7 ± 0.3** | 0.9 ± 0.3** | 1.0 ± 0.3** | 1.1 ± 0.2** |
| Motor subsection | 5.2 ± 0.6 | 1.1 ± 0.3*** | 1.2 ± 0.2*** | 1.8 ± 0.5** | 1.5 ± 0.3** | 1.1 ± 0.2** |
| Part III, treated side, total | 13.6 ± 1.5 | 2.2 ± 0.5*** | 3.5 ± 0.7*** | 4.2 ± 0.9*** | 3.6 ± 0.7*** | 3.6 ± 0.6*** |
| Part III, total | 33.8 ± 4.0 | 15.0 ± 2.1** | 17.8 ± 3.7** | 19.3 ± 4.1* | 16.1 ± 2.2** | 14.8 ± 2.5** |
| Part IV, n = 1 | 8.0 | 4.0 | 4.0 | 4.0 | 4.0 | 2.0 |
| HY scoreb | 2.7 ± 0.4 | 1.7 ± 0.2*** | 1.7 ± 0.2*** | 2.1 ± 0.3*** | 2.2 ± 0.3*** | 2.0 ± 0.2*** |
| PDQ-39 scorec | 37.5 ± 5.5 | N/A | 19.1 ± 3.8* | 18.2 ± 3.8** | 20.0 ± 4.1* | 18.3 ± 3.5** |
| Non-motor symptoms scaled | 39.7 ± 6.2 | N/A | 18.7 ± 4.6* | 17.0 ± 4.0** | 18.0 ± 3.7** | 19.3 ± 4.7* |
| NPIe | 2.7 ± 0.9 | N/A | 1.3 ± 0.4 | 1.3 ± 0.4 | N/A | 1.3 ± 0.4 |
| CGI scoref | 6.4 ± 0.2 | 1.0 ± 0.0*** | 1.1 ± 0.1*** | 1.2 ± 0.1*** | 1.0 ± 0.0*** | 1.0 ± 0.0*** |
- Abbreviations: CGI, Clinical Global Impression; CRST, Clinical Rating Scale for Tremor (part A contains tremor at rest, posture, and action [hands and feet]; part B contains action tremor of treated hand during writing, drawing, and water pouring [range from 0 to 20]); HY, Hoehn and Yahr scale; LEDD, levodopa equivalent daily dosage; med., medication; N/A, not available; NPI, Neuropsychiatric Inventory; PDQ-39, 39-item Parkinson's Disease Questionnaire; UPDRS, Unified Parkinson's Disease Rating Scale.
- a UPDRS part I ranges from 0 to 16. Part II ranges from 0 to 52. Motor subsections encompass evaluations of finger taps, hand grips, and hand pronate/supinate, with scores ranging from 0 to 12. Part III, specific to the treated side, includes assessments for tremor at rest (hand and feet), action tremor (hand), and rigidity (upper and lower extremity) and a motor subsection. Part IV scores range from 0 to 16.
- b HY score ranges from 0 to 5.
- c PDQ-39 ranges from 0 to 156.
- d Non-motor symptoms scale includes other issues such as mental health, memory problems, and pain, ranging from 0 to 360.
- e NPI in the first patient; ranges from 0 to 144.
- f CGI ranges from 1 to 7; lower scores are better.
- * p < 0.05,
- ** p < 0.01,
- *** p < 0.001 by paired t-test compared with pretreatment condition.
Procedures
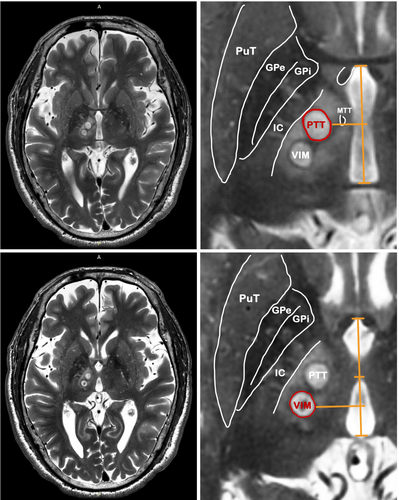
As described in our previous study, prior to MRgFUS therapy, participants underwent a 3-T MRI scan for target delineation, conducted 1 day in advance. High-resolution axial and sagittal T2-weighted images were acquired to identify the anterior commissure (AC) and posterior commissure (PC) and measure their separation. Subsequently, a three-dimensional fast-spin-echo-based double inversion recovery white matter null image was superimposed onto the T2-weighted image to detect and avoid critical regions such as the mammillothalamic tract and internal capsule. MRgFUS procedures were performed using a 1.5-T MRI system (GE Optima 450w GEM, GE Healthcare, Milwaukee, WI, USA) equipped with the ExAblate Neuro device (InSightec, Haifa, Israel). Target coordinates for thalamus-related structures, including the PTT and VIM contralateral to the more affected limb, were determined based on individual AC–PC lines. Standard targets for PTT and VIM were established following a previously documented protocol. We selected the PTT located at the midpoint of the AC–PC line, which is a region 4–10 mm lateral to the third ventricle wall and directly at the level of the AC–PC line. For the VIM, the target was set at a point one quarter of the AC–PC line anterior to the PC point, 11 mm lateral to the third ventricle wall, and 2 mm above the AC–PC line, with adjustments made as necessary [4].
Data collection
Primary outcome measurement was mentioned in our previous study [4] and included the off-medication status Clinical Rating Scale for Tremor (CRST) and Unified Parkinson's Disease Rating Scale (UPDRS) III, whereas secondary outcome measures consisted of UPDRS I, II, and IV, Hoehn and Yahr score, Neuropsychiatric Inventory [5], 39-item Parkinson's Disease Questionnaire (PDQ-39) [6], PD Non-Motor Symptoms Scale (NMSS) [7, 8], and Clinical Global Impression (CGI) [9].
Thorough assessments of each participant were conducted before, during, and after MRgFUS treatment, and any discomfort experienced was recorded. Primary and secondary evaluations for PD were conducted at various time points, including baseline, 1 day, 4 weeks, 12 weeks, 6 months, 12 months, and annually post-MRgFUS. Neuroimaging, comprising MRI and diffusion tensor imaging scans, was performed at baseline and after MRgFUS treatment. This report presents the 1-year data. The primary endpoints aimed to demonstrate improvements in tremor and cardinal features of PD, whereas the secondary endpoints focused on mitigating non-motor symptoms scale and enhancing overall quality of life [4].
Statistical analysis
Quantitative scores were subjected to statistical analysis using the paired t-test to compare them with baseline measurements. Mean values are presented with their corresponding SD. Significance was determined by a p-value < 0.05.
RESULTS
We present findings from a cohort of 10 patients diagnosed with idiopathic PD, exhibiting tremor and rigidity/bradykinesia symptoms (Table 1). Patients stated they encountered no problems in completing the tasks.
The maximum sonication power was 794.60 ± 72.62 W (range = 679–874) for PTT treatment and 809.20 ± 40.49 W (range = 765–865) for VIM treatment. The received energy was 17,002.10 ± 9525.62 J (range = 7564–35,000) for PTT treatment and 19,834.20 ± 8760.29 J (range = 13,003–36,033) for VIM treatment. Skull density ratio was 0.48 ± 0.02 (range = 0.40–0.59).
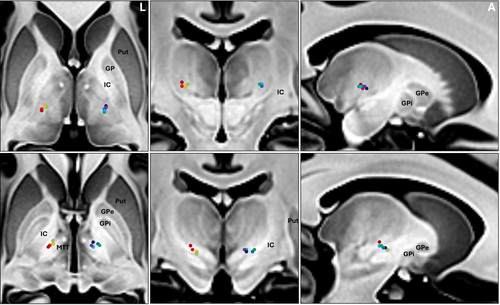
The lesion sizes were 138.21 ± 41.15 mm3 for PTT and 163.47 ± 38.01 mm3 for VIM within 24 h posttreatment via magnetic resonance (MR) T2-weighted images. Figure 1 shows sample post-24 h MR images of the final patient. Figure 2 displays MR images depicting lesions in nine patients after stepwise dual-target MRgFUS. One patient (FUS-PD001) was not included in the lesion analysis due to the unavailability of the post-24 h MR images.
Primary outcome measures
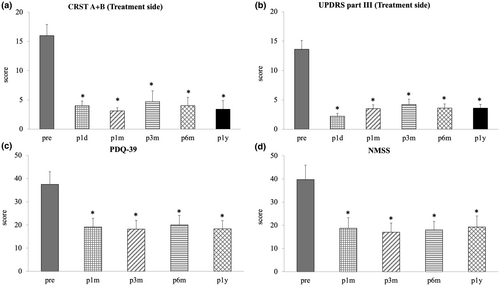
In this study, 10 idiopathic PD patients presenting both resting and postural/kinetic tremors (mean age = 66.7 ± 3.2 years, eight men) were included with levodopa equivalent daily dose of 889 ± 187 mg (Table 1). Notably, CRST (parts A + B) scores for the treated hand demonstrated substantial improvement at 1-day, 1-month, 3-month, 6-month, and 12-month follow-ups during patients' off-medication status, with improvements of 75% ± 4%, 81% ± 3%, 76% ± 6%, 79% ± 5%, and 83% ± 5%, respectively (all p < 0.001 by paired t-test).
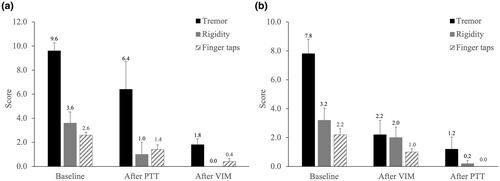
Moreover, Movement Disorder Society UPDRS III scores on the treated side, encompassing resting tremor, action tremor, motor subsection (includes finger taps, hand grips, and hand pronate/supinate), and rigidity, exhibited significant improvement at 1-day (83% ± 3%), 1-month (73% ± 5%), 3-month (70% ± 5%), 6-month (73% ± 4%), and 12-month (72% ± 5%) follow-ups (all p < 0.001 by paired t-test). Figure 3 depicts changes in motor UPDRS scores at 1-year follow-up, whereas Figure 4 shows average changes in clinical scores throughout the procedure, with PTT or VIM as the first target.
Secondary outcome measures
The PDQ-39 score exhibited a noteworthy decrease at 1 month posttreatment (baseline 37.5 ± 5.5 vs. 1-month follow-up 19.1 ± 3.8, p = 0.01), as well as at 3-month (18.2 ± 3.8, p = 0.01), 6-month (20.0 ± 4.1, p = 0.02), and 12-month (18.3 ± 3.5, p = 0.01) follow-ups.
Similarly, the NMSS demonstrated a significant reduction at 1 month posttreatment (baseline 39.7 ± 6.2 vs. 1-month follow-up 18.7 ± 4.6, p = 0.01), along with 3-month (17.0 ± 4.0, p = 0.01), 6-month (18.0 ± 3.7, p = 0.01), and 12-month (19.3 ± 4.7, p = 0.02) follow-ups.
Furthermore, the CGI scale indicated a notable improvement of 84.3% ± 0.4%, 82.9% ± 1.3%, 81.2% ± 2.1%, 84.3% ± 0.4%, and 84.3% ± 0.4% at 1-day, 1-month, 3-month, 6-month, and 12-month follow-ups, respectively (baseline = 6 vs. 1-day, 1-month, 3-month, 6-month, and 12-month follow-up = 1, all p < 0.001 by paired t-test).
Adverse events
Patients reported temporary procedure-related headache, pain, and dizziness during treatment, which resolved by follow-up assessments. No major adverse events were noted during the treatment or throughout the 1-year follow-up (Table 2).
| Event | n (%) |
|---|---|
| Lesion-relateda | |
| Finger paresthesia | 0 |
| Orofacial paresthesia | 0 |
| Ataxia | 0 |
| Hemiparesis | 0 |
| Dysmetria | 0 |
| Mild vocal change | 0 |
| Unsteady gait | 0 |
| MRI- or ultrasonography-related, all transientb | |
| Scalp numbness | 0 |
| Headache | 3 (30) |
| Dizziness or vertigo | 5 (50) |
| Head pain or heat sensation | 2 (20) |
| Stomach pain, nausea, or emesis | 0 |
| Tinnitus | 0 |
| Periorbital swelling | 0 |
| Neck, back, or shoulder pain | 1 (10) |
| Decline in mental status | 0 |
| Pin site pain | 2 (20) |
| Anxiety | 0 |
| Lightheadedness | 0 |
| Right-sided ecchymosis | 0 |
| Spot in visual field | 0 |
- Abbreviation: MRI, magnetic resonance imaging.
- a From the creation of a lesion.
- b From the procedure environment.
DISCUSSION
After a 1-year follow-up, we observed a significant reduction in PD tremor and akinesia/rigidity following stepwise dual-target MRgFUS treatment in 10 PD patients. Notably, no noticeable adverse effects were detected during the 1-year follow-up period. In our previous study, we highlighted the feasibility of MRgFUS in managing resting, posture/kinetic tremor, rigidity, and bradykinesia concurrently in PD patients, all within a single procedure [4]. Here, we further showed the consistency of the efficacy and safety by MRgFUS in PD treatment at 1-year follow-up (Table 1).
MRgFUS is an option for patients with PD. The treatment targets various anatomical structures using MRgFUS; however, a standardized target has yet to be established. In a prospective, sham-controlled randomized clinical trial study conducted by Bond et al. [10], 27 refractory tremor PD patients were enrolled. Of these patients, 20 were randomly assigned to the MRgFUS thalamotomy, whereas seven were assigned to the sham treatment. The study utilized CRST parts A and B, as well as the motor UPDRS, to assess hand tremors in a double-blinded manner. After 3 months, the patients were unblinded [2, 10]. Improvements in treated hands, mainly in postural or action tremor, followed by resting tremors, were observed in the treatment group. However, no mention was made in the report regarding improvements in other primary PD symptoms such as rigidity or bradykinesia following the VIM procedure.
A prospective open-label study investigated the enduring effects of unilateral MRgFUS subthalamotomy in patients with asymmetrical PD. Over a 36-month follow-up period, 32 patients were evaluated, revealing sustained improvements in the UPDRS motor part III score for the treated hemibody in the off-medication state, with a notable 52.3% reduction from baseline to 3 years. However, the study lacked detailed information regarding tremor subtypes, and baseline tremor scores were relatively moderate (3.7 ± 1.9), with a potential range from 0 to 16. Additionally, adverse events such as dyskinesia, weakness on the treated side, speech disturbances, facial weakness, and gait disturbances were reported in the active-treatment group, highlighting the importance of considering these factors when assessing the efficacy and safety of MRgFUS subthalamotomy for PD treatment [11].
There is also a retrospective observational analysis of a series of 51 patients with chronic and therapy-resistant PD who underwent PTT ablation at a single center. Of these patients, nine had tremor-dominant PD, five had akinetic–rigid PD, and 37 had mixed PD. The study showed improvements in parkinsonian features, including tremor, rigidity, and bradykinesia. Still, the analysis did not provide details about tremor subtypes, and the severity of tremor at baseline was relatively moderate (4.7 ± 3.4 in 12) [3]. Although the overall effect on tremor was significant, the impact of PTT on severe or specific subsets of tremor could not be concluded from the study.
In our previous study, we reported on three patients who underwent stepwise dual-target MRgFUS to alleviate tremor symptoms, rigidity, and bradykinesia in PD. This study marks the first publication addressing the lesioning of both the VIM and PTT in a single procedure. As demonstrated in our initial report, although rigidity, bradykinesia, and resting tremor significantly improved following PTT treatment, vigorous action and postural tremors persisted, leading to considerable disability, whereas VIM subsequently diminished it [4]. Here, we further presented 10 PD patients in this study. After that, we chose our first target based on the most disabling symptom either by the observation of the physicians or by the statement of the clinical history. VIM was the first target for troublesome tremor, whereas PTT lesioning would be chosen for akinetic–rigid syndrome. As can be observed (Figure 4), the tremor improved greatly after the VIM lesioning, whereas akinetic–rigid symptom improved majorly after PTT treatment. Throughout the 1-year follow-up period (Figure 3), symptom control remained stable, without any significant adverse effects. It is evident that stepwise dual-target MRgFUS played a crucial role in providing satisfactory relief from cardinal symptoms for our patients.
It is imperative to tailor the selection of treatment targets based on each patient's unique clinical presentation. Given that many PD patients are elderly and may struggle with prolonged treatment procedures, prioritizing the resolution of their most troublesome symptoms becomes paramount. Consequently, identifying and addressing the symptom causing the most distress should guide the decision-making process when selecting the initial treatment target.
In line with our previous study, which involved dual VIM and PTT lesioning, the results revealed marked improvements in non-motor symptoms scales, such as mental health and pain (Table 1). However, in single VIM study, there were no notable enhancements in cognition, mood, or behavior documented [12]. This suggests that targeting both VIM and PTT lesions at crucial network nodes may be more effective in disrupting pathological circuits associated with non-motor symptoms scale in PD patients. One possible explanation is the correlation between rigidity and pain [13, 14]. As muscle tone decreases, the relief from muscle overcontraction may be associated with a reduction in pain. Due to the intricate pathogenesis of pain [13] and non-motor symptoms scale in PD, further investigation is warranted to elucidate the underlying mechanisms of these findings and to reconcile any disparities in quality of life outcomes compared to previous research.
The study's limitations include a small sample size despite recruiting more patients, highlighting the need for larger studies to comprehensively assess MRgFUS in PD. Additionally, the relatively short follow-up period limits conclusions about long-term efficacy, necessitating further investigations with extended follow-up durations. Lastly, the study did not address potential carryover effects during intraoperative clinical score measurements, which should be considered in future research.
In conclusion, the findings of this report underscore the consistent improvement observed in PD cardinal features following stepwise dual-target MRgFUS treatment. This innovative procedure holds promise as a safe and effective option for addressing hemisymptoms in PD. Looking ahead, further research and larger scale studies are warranted to validate and expand upon these encouraging results, paving the way for advancements in PD treatment.
AUTHOR CONTRIBUTIONS
Jui-Cheng Chen: Conceptualization; methodology; software; data curation; supervision; formal analysis; validation; investigation; writing – original draft; writing – review and editing; resources. Chun-Ming Chen: Conceptualization; investigation; methodology; validation; software; formal analysis; data curation; supervision; writing – review and editing; writing – original draft. Yu Aoh: Conceptualization; investigation; methodology; validation; software; formal analysis; data curation; supervision. Ming-Kuei Lu: Conceptualization; investigation; methodology; validation; formal analysis; software; data curation; supervision; resources; writing – review and editing; writing – original draft. Chon-Haw Tsai: Conceptualization; investigation; methodology; validation; formal analysis; software; data curation; supervision; writing – review and editing; writing – original draft; resources.
ACKNOWLEDGMENTS
This study is supported in part by grants from the Ministry of Science and Technology (MOST111-2314-B-039-073-MY2, MOST-111-2314-B-039-080-, NSTC 112-2314-B-039-004-, NSTC 113-2314-B-039-020-) and the China Medical University Hospital, Taiwan (DMR-110-098, DMR-111-223, DMR-113-194). J.-C.C. is supported in part by China Medical University Hsinchu Hospital (CMUHCH-DMR-112-002, CMUHCH-DMR-113-004, CMUHCH-DMR-111-014, CMUHCH-DMR-112-021, and CMUHCH-DMR113-011). We also thank Dr. Kevin Tsai, Yi-Chun Chen, Pei-Jon Wu, Ching-Wen Cho, Dr. Yu-Kai Cheng, Hoon-Ming Heng, and Dr. Paul Chen for their assistance to the study.
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.




