Risk of aspiration pneumonia and hospital mortality in Parkinson disease: A systematic review and meta-analysis
Abstract
Background and Purpose
This study was undertaken to conduct a meta-analysis on the prevalence of aspiration pneumonia (AP) and hospital mortality in Parkinson disease (PD) as well as the risk of AP in PD patients compared to controls.
Methods
We searched MEDLINE and Embase from inception to 19 March 2024 to identify cross-sectional, cohort, and case–control studies comparing the frequency of AP and hospital mortality in PD patients. We computed risk ratios (RRs) with accompanying 95% confidence intervals (CIs) for each study and pooled the results using a random-effects meta-analysis.
Results
A total of 781 studies were initially screened, and 13 studies involving 541,785,587 patients were included. Patients with PD had >3 times higher risk of AP compared to controls (RR = 3.30, 95% CI = 1.82–6.00, p < 0.0001). This increased risk was similar in both cohort studies (RR = 3.01, 95% CI = 1.10–8.24, p = 0.03) and case–control studies (RR = 3.86, 95% CI = 3.84–3.87, p < 0.00001). The prevalence of AP in 12 studies was 2.74% (95% CI = 1.69–4.41), and hospital mortality was 10% in six studies (10.0%, 95% CI = 5.32–18.0). Prevalence of AP was higher in studies with smaller sample size (5.26%, 95% CI = 3.08–8.83 vs. 2.06%, 95% CI = 1.19–3.55, p = 0.02).
Conclusions
Our meta-analysis showed that patients with PD had >3 times higher risk of AP, with an average 2.74% prevalence and 10.0% hospital mortality. Early recognition and treatment of AP in PD patients will help reduce morbidity and mortality. A multidisciplinary holistic approach is needed to address the multifactorial causes of AP.
INTRODUCTION
Parkinson disease (PD) is a neurological disorder that causes bradykinesia, rigidity, and tremor [1], affecting >10 million individuals worldwide, and is one of the fastest growing chronic neurological conditions [2]. During the course of PD, >80% of patients will develop dysphagia [3]. In severe cases of dysphagia, the inhalation of oropharyngeal and gastric secretions leads to pulmonary distress from airway obstruction, pneumonia, and chemical pneumonitis, causing aspiration pneumonia (AP) and in severe cases mortality [4, 5]. In a study in Japan, AP accounted for >40% of emergency department admissions in patients with PD [6], and postmortem studies of PD patients found that AP was the primary cause of death in 30% of patients [7].
AP is a leading cause of morbidity and mortality in PD patients, with mortality doubling from 23.9% after 1 month to 65.2% after 1 year of incident AP [8]. Furthermore, the incidence of AP in PD patients has rapidly increased in recent years, with increasing prevalence in older age groups [9]. Although effective treatments for PD have improved the life expectancy of patients with PD [10], the risk of developing other PD-associated comorbidities and complications increases with age [9].
Every year, >17,000 people in America die from AP [11]; >20% of these deaths occurred in patients with neurological disorders such as PD [5]. Although AP is a highly aggressive form of pneumonia, it is preventable and can be treated when detected early [12]. In patients with PD, hospitalization further increases the risk of AP due to prolonged bedrest and immobility [8]. The resultant impact of AP leads to increased length of stay and hospitalization costs and worsens the patient's quality of life [13]. Knowledge of the prevalence of AP and high-risk subtypes of patients with PD will help clinicians in managing PD patients with high aspiration risk.
To date, there has been no meta-analysis that examines the prevalence of AP and hospital mortality in PD. To address this gap in knowledge, we conducted a systematic review and meta-analysis to explore the following outcomes: (i) the prevalence of AP and hospital mortality in PD and (ii) the risk of AP in PD patients compared to controls.
METHODS
This systematic review and meta-analysis was registered with PROSPERO (International Prospective Register of Systematic Reviews; CRD42024541076) and adheres to the reporting guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [14].
Data sources and searches
A systematic search was conducted in MEDLINE and Embase using Medical Subject Headings (MeSH) and keywords. Keywords and MeSH terms synonymous with “Parkinson's disease” and “aspiration pneumonia” formed the basis of the search strategy. The search period includes articles from inception to 19 March 2024. The search strategy was not limited by publication date. MEDLINE and Embase include articles from 1946 and 1947, respectively, to the present. Only full text articles published in the English language were included. The full search strategy and search terms are included in Table S1. References were imported into EndNoteX9 for the initial removal of duplicates. The last queries on MEDLINE and Embase were run on 19 March 2024.
Study selection
Covidence was used to manage study selection. Two authors (W.Y.C. and C.K.M.C.) reviewed each reference in a blinded manner, and any disagreements were resolved through discussion or referred to a third independent author for the final decision (J.D.J.W.). The review was carried out in two stages; first, the titles and abstracts were reviewed and second, the full texts of selected references were retrieved and reviewed. Original studies, published in English, discussing AP in adults with PD were included. Accepted study designs included case–control, cross-sectional, and cohort studies. Randomized controlled trials, non-peer-reviewed articles, review articles (including other systematic reviews and meta-analyses), editorials, letters to the editor, and conference abstracts were excluded. Studies involving animal or nonhuman studies were also excluded.
Data extraction and quality assessment
Two investigators (C.K.M.C. and J.D.J.W.) independently extracted information from the included studies using Covidence. The data collected included authors, year of publication, total number of participants, age and sex of study participants, sample size, type of treatment, and AP outcome. Regarding discrepancies, a third author (W.Y.C.) was consulted to make the final decision regarding the data extraction process. The Newcastle–Ottawa Quality Assessment Scale was used to assess the risk of bias of the included studies. [15] Two investigators (C.K.M.C. and J.D.J.W.) independently reviewed all included studies and rated them based on the following domains: selection of study group, comparability of selected groups, and measurement of outcome of interest (Table S2). Subsequently, gradings for each domain were compared between the two authors; in the case of disagreements, a third independent author (W.Y.C.) was consulted, and a consensus was reached through discussion. The Newcastle–Ottawa scale was subsequently converted to AHRQ (Agency for Healthcare Research and Quality) standards (good, fair, or poor quality studies). A study with ≥7 points was considered “good,” 2–6 points “fair,” and ≤1 point “poor” quality [15, 16].
Data synthesis and analysis
All analyses were undertaken using Review Manager version 5.4.1 and RStudio version 4.3.3. Prevalence estimates of AP and hospital mortality were calculated by pooling the study-specific estimates using random-effects models. Pooled risk ratios (RRs) were meta-analyzed using the Mantel–Haenszel method. The level of significance is defined as p < 0.05. The choice between the fixed-effect and random-effects models was made depending on the I2 index and Cochran Q-test p-value. An I2 index of <25% is indicative of low heterogeneity, 25%–75% moderate heterogeneity, and >75% high heterogeneity. In cases with minimal heterogeneity, a fixed-effect model was used. Otherwise, a random-effects model was used. All results were presented as their effect sizes with the accompanying 95% confidence intervals (CIs), along with the p-values where applicable. In addition, to further examine for differences among studies with different sample sizes, we conducted subgroup analysis according to the sample size of the studies. Studies with >2000 PD patients were considered large, whereas studies with <2000 PD patients were considered small.
RESULTS
Overview
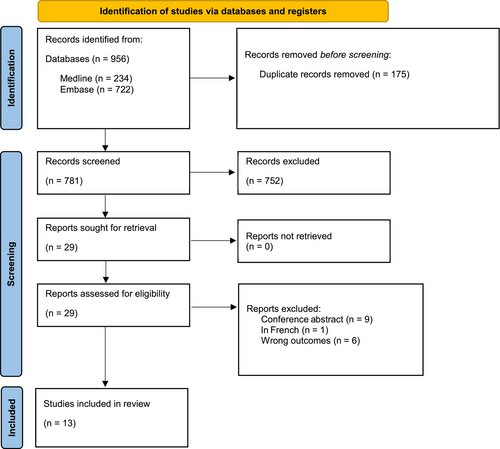
A total of 956 studies were found after searching MEDLINE and Embase. Among these, 175 were duplicates and 781 studies remained following duplicate removal. The study team screened the titles and abstracts of these studies and included 29 studies for further review. The study team retrieved the full texts of all 29 studies, and a further 16 studies were excluded upon full text review. The study selection process and reasons for excluding the 16 studies are illustrated in a PRISMA-P 2020 flow diagram (Figure 1). Finally, 13 studies involving 541,785,587 patients (PD and non-PD) were included in the final analysis [6, 8, 9, 17-26].
Characteristics of included studies
Nine studies used either state or national databases, whereas the remaining four studies were based on single-centre databases. Of the 13 studies, seven were conducted in the USA, and Ireland, India, Taiwan, South Korea, Canada, and Japan produced one each. In terms of study design, there were 12 retrospective studies (seven cohort, four case–control, and one cross-sectional) and one prospective cohort study (Table 1). In total, 12 studies reported the prevalence of AP among PD patients. Of these 12 studies, five compared the prevalence of AP between PD and non-PD patients. Among these five studies, three were case–control studies [8, 17, 26], whereas the remaining two were retrospective cohort studies [9, 23]. All five studies included patients with PD matched to controls without PD. Won et al. and Guttman et al. were matched to controls in a 1:4 and 1:2 ratio, respectively, by gender and age [8, 26]. George et al. matched patients to controls in a 1:1 ratio by gender, age, ethnicity, and Elixhauser Comorbidity Index using coarsened exact matching [17]. Using the Newcastle–Ottawa Scale, nine of the studies included were of good quality and four studies were of fair quality (Table S2).
| Study (year) | Study location | Database | Study design | Parkinson disease participants/patients | Control participants/patients | ||||
|---|---|---|---|---|---|---|---|---|---|
| Participants, n | Gender, male, % | Age, years, mean (SD) | Participants, n | Gender, male, % | Age, years, mean (SD) | ||||
| Akbar (2015) | USA | National | Retrospective cohort study | 5,665,710 | 51.69 | 77.13 (0.08) | 532,531,602 | 45.05 | 70.07 (0.01) |
| Di Luca (2021) | USA | National | Retrospective cohort study | 334,395 | 53 | 77.73 (9.82) | NA | NA | NA |
| Fernandez (2002) | USA | National | Retrospective cohort study | 15,186 | 40.4 | NA | NA | NA | NA |
| Fujioka (2016) | Japan | Single-centre | Retrospective cohort study | 136 | NA | 75 | NA | NA | NA |
| George (2023) | USA | State | Retrospective cohort study | 35,457 | 58.23 | 81 (75–86)a | 35,457 | 58.23 | 81 (75–86)a |
| Guttman (2004) | Canada | State | Retrospective cohort study | 15,306 | NA | NA | 30,612 | NA | NA |
| Kelly (2016) | Ireland | National | Retrospective cohort study | 13,660 | NA | NA | NA | NA | NA |
| Mahajan (2016) | USA | National | Retrospective cohort study | 3,015,645 | 53.2 | 78 (72–84)a | NA | NA | NA |
| Martinez-Ramirez (2015) | USA | Single-centre | Retrospective cross-sectional study | 212 | 60.8 | 74.1 (10.1) | NA | NA | NA |
| Paul (2019) | India | Single-centre | Prospective cohort study | 50 | 82 | 71.8 | NA | NA | NA |
| Pepper (1999) | USA | National | Retrospective cohort study | 234 | 98.7 | 71.1 (7.6) | 40,979 | 97.7 | 64.1 (10.8) |
| Won (2021) | South Korea | National | Retrospective cohort study | 10,159 | 38.3 | NA | 39,574 | 38.1 | NA |
| Wu (2016) | Taiwan | National | Retrospective case–control | 1213 | 51.8 | 62.9 (17.0) | NA | NA | NA |
- Abbreviation: NA: not applicable.
- a Data are given as age, years, median (interquartile range).
Primary outcome: AP
Prevalence of AP
Twelve studies [6, 8, 9, 17, 18, 20-26] involving 9,107,313 PD participants and 354,064 events of AP were pooled, and the prevalence was found to be 2.74% (95% CI = 1.69–4.41). The I2 index was 100%, and the Cochran Q-test was significant at p < 0.0001 (Figure 2).
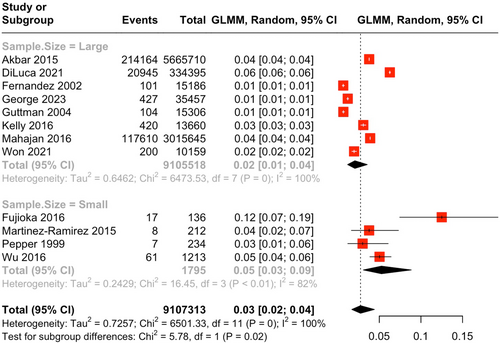
A subgroup analysis based on study sample size was conducted. Studies with larger sample size (n = 8) demonstrated a prevalence of 2.06% (95% CI = 1.19–3.55), whereas studies with smaller sample size (n = 4) demonstrated a prevalence of 5.26% (95% CI = 3.08–8.83; Figure 2). The subgroup differences demonstrated a significantly higher prevalence of AP among PD patients in studies with smaller sample size (p = 0.02).
RR of AP
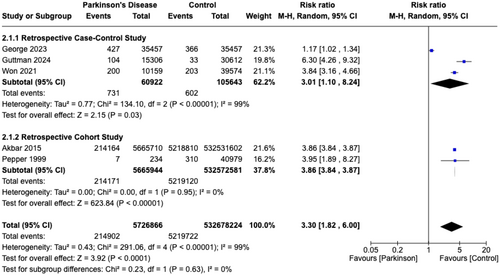
Pooled RRs of five studies [8, 9, 17, 23, 26] revealed significantly greater risk of AP in PD patients (RR = 3.30, 95% CI = 1.82–6.00, p < 0.0001) compared to controls (non-PD patients), with an I2 index of 99.0% (Figure 3). The five studies involved 538,405,090 patients, with 5,726,866 patients in the PD group and 532,678,224 in the control group. Although patients with PD have a greater risk of developing AP in retrospective cohort studies (RR = 3.01, 95% CI = 1.10–8.24, p = 0.03) compared to retrospective case–control studies (RR = 3.86, 95% CI = 3.84–3.87, p < 0.00001), the difference was not significant (p = 0.63).
Secondary outcome: hospital mortality
Prevalence of hospital mortality
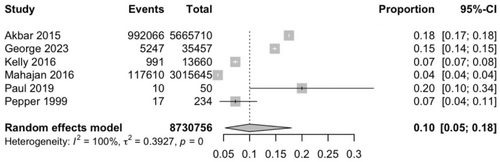
Six studies [9, 17, 19-21, 23] involving 8,730,756 PD participants and 1,115,941 events of hospital mortality were pooled, and the prevalence was found to be 10.0% (95% CI = 5.32–18.0). The I2 index was 100%, and the Cochran Q-test was significant at p < 0.0001 (Figure 4).
DISCUSSION
Although AP is a sequelae of PD-related motor complications, its actual prevalence and relative risk have not been systematically examined. To address this gap in knowledge, we conducted the first systematic review and meta-analysis on this topic, involving 13 studies and 541,785,587 patients. We found that patients with PD were at >3 times higher risk of AP (RR = 3.30, 95% CI = 1.82–6.00, p < 0.0001) compared to controls. In addition, the prevalence of AP and hospital mortality in patients with PD was 2.74% (95% CI = 1.69–4.41) and 10.0% (95% CI = 5.32–18.0), respectively. A subgroup analysis found that studies with a larger sample size (>2000 cases; 2.06%, 95% CI = 1.19–3.55) had a significantly lower prevalence of AP than studies with a smaller sample size (<2000 cases; 5.26%, 95% CI = 3.08–8.83).
AP is a common complication affecting patients with PD. Compared to controls, our study found that patients with PD are at an increased risk of AP (RR = 3.30, 95% CI = 1.82–6.00, p < 0.0001). This could be due to various factors, primarily stemming from impairments in swallowing and respiratory function.
First, dysphagia is a nonmotor symptom of PD that arises from dysfunction of the supramedullary swallowing system due to dysfunction of the dopaminergic neural network mainly in the basal ganglia and frontal lobe [27]. The weakness of muscles for swallowing and reduction of saliva swallowing can result in aspiration of food, liquid, or gastric content into the respiratory system, causing inflammation and infection of the lungs and airways [28]. Furthermore, swallowing difficulties in PD can result from the accumulation of Lewy bodies and alpha-synuclein, which are implicated in PD pathogenesis. These proteins can affect peripheral motor nerves innervating pharyngeal muscles, leading to dysphagia and further increasing the risk of AP due to poor swallowing efforts [27]. A combination of dysphagia and poor swallowing efforts can lead to the accumulation of saliva in the mouth, which further increases the risk of AP due to an increased source of liquid in the oropharyngeal cavity [29].
Second, PD increases the risk of AP through respiratory muscle weakness and an impaired cough reflex. PD patients often experience respiratory muscle weakness, which is attributed to the accumulation of alpha-synuclein in the medulla oblongata, leading to dysfunction of the respiratory centre [30]. This weakness can result in inadequate control of respiratory muscles and impaired airway protection, especially during swallowing. Additionally, PD patients may have an impaired cough reflex, which is thought to be due to expiratory muscle weakness secondary to a reduction in central neural drive [31]. This reduction in neural drive can affect the cough reflex before progressing to involve sensory pathways, resulting in an airway that is less well protected from aspirates entering the conducting system of the respiratory system [31].
Other key factors that could lead to PD patients having greater risk of AP include postural instability, poor mobility, and side effects of PD medications. Postural instability in PD patients is caused by deficits in dopaminergic neurons, degeneration of the cholinergic system, and comorbid white matter disease [32]. This instability can make it difficult for patients to sit or stand upright, increasing the risk of aspiration when lying down, as lying down alters the position of the larynx and epiglottis, reducing the effectiveness of the epiglottis valve. PD medications, such as anticholinergics, can also contribute to the risk of aspiration with increased sedation and cognitive impairments, which has been linked to a higher risk of AP [32]. All of these are further compounded by the effects of aging. Another potentially preventable factor includes inappropriate assisted feeding and/or poor habits in executing swallowing, where food particles get pooled at the back of the tongue or the posterior pharyngeal space.
Based on pooled RRs from 12 studies, the prevalence of AP in patients with PD was 2.74% (95% CI = 1.69–4.41). In a subgroup analysis, we found that studies with a larger sample size (2.06%, 95% CI = 1.19–3.55) had a significantly lower prevalence of AP than studies with a smaller sample size (5.26%, 95% CI = 3.08–8.83). This could be for a number of reasons. First, of the four studies classified as small sample size, three were from a single-centre database. Studies comparing trials conducted in single-centre compared to multi-centre trials found that single-centre studies tend to report larger treatment effect, with potential reporting bias [33]. Second, higher incidence of AP in studies with smaller sample size may be expected, as studies with smaller sample size are inherently prone to type 1 and type 2 errors [34]. As a result, the larger CIs seen in these studies help to explain the variability [35].
Pooled RRs from six studies found that hospital mortality rate in patients with PD was 10.0% (95% CI = 5.32–18.0). The patient type and study setting may explain the differing hospital mortality rates between the studies. Paul et al. reported 20% hospital mortality rates in patients with PD that were in the intensive care unit (ICU) [19]. Patients in the ICU are critically ill and therefore are at higher risk of acquiring infections and experiencing desaturation. Moreover, Pepper et al. examined patients with PD that were admitted for elective bowel resection, cholecystectomy, or radical prostatectomy and reported hospital mortality rates of 7% [23]. Patients who are considered for elective operations are fit for surgery and not expected to succumb during the course of the operation and the hospital stay.
The mean age at onset in PD is usually approximately 60–65 years, and patients may have concurrent comorbidities [36, 37]. Studies by Won et al. and George et al. found that patients with PD have a greater comorbid burden, with higher rates of concomitant cardiovascular, oncological, and respiratory conditions than controls [8, 17]. Among the six studies that examined hospital mortality, the mean age of patients ranged from 71 [23] to 81 [17] years, and none of the studies excluded patients with pre-existing comorbidities. In the study by George et al., patients with PD were more likely (odds ratio = 1.11, 95% CI = 1.06–1.16) to die during the course of hospitalization than controls, with the admissions complicated by increased risk of AP and delirium. In comparison, Akbar et al. conversely found that patients with PD had lower hospital mortality rates than controls (22.47% vs 17.51%) [9]; it is postulated that greater awareness of swallowing dysfunction among PD patients can result in early detection, diagnosis of AP, and timely treatment. Furthermore, the hospital mortality rates in patients with AP has declined significantly in recent years despite increasing incidence of AP in recent years [9], possibly highlighting the effectiveness of greater awareness, early recognition, and timely treatment for AP.
The cause of hospital mortality in PD patients may be multifactorial; however, a postmortem study of PD patients found that AP was the primary cause of death in 30% of patients [8]. In comparison, AP-associated death was reported in 2.3% of all mortality in the USA during 1999–2017. Ensuring timely treatment and good discharge advice for patients with AP is pivotal, as Won et al. found that after the first occurrence of AP, the mortality rate of PD patients increases from 23.9% at 1 month to 65.2% at 1 year and 91.8% after 5 years [8]. With a 2.74% prevalence of AP and increased risk of developing AP in PD patients (RR = 3.30, 95% CI = 1.82–6.00, p < 0.0001) compared to controls, it is essential for physicians to mitigate the development of AP while instituting proper treatment for AP once it develops and to provide effective discharge advice for patients with PD.
As our meta-analysis includes studies published from 1999 to 2023, several confounding factors remain. Over the past 2 decades, the introduction of deep brain stimulation (DBS) and long-acting dopamine agonists has helped to improve the motor symptoms and swallowing function of patients with PD [38]. This could help to reduce the risk of aspiration pneumonia and prolong the life expectancy of patients with PD [38]. However, the proportion of patients with PD in our studies who have undergone DBS and the duration of pharmacological therapy remain unknown. The 2002 study by Fernandez et al. found that the prevalence of AP in patients with PD was 0.67%, whereas the 2021 study by Di Luca et al. found that the prevalence of AP in patients with PD was 6.3% [18,22]. The higher prevalence of AP in the 2021 study may be contributed by increased recognition of AP in patients with PD. Another possible reason is that advances in DBS have helped to improve the life expectancy of patients with advanced PD refractory to oral medications, although the severity of PD and proportion of patients who underwent DBS in the study were not published [38, 39]. Although a longitudinal cohort study by Jung et al. found that the prevalence of AP after DBS was 0.2% [40], there is a lack of studies investigating incidence of AP between different treatment modalities and various subtypes of DBS. The percentage of PD patients undergoing DBS surgery in most countries is still low, and therefore the effect of DBS on AP is likely to be relatively small [41]. Future research should investigate the differences in prevalence and risk of AP after different treatment regimens [40]. Therefore, as our meta-analysis includes studies across 2 decades, the advances in therapeutic intervention and recognition of AP in patients with PD could be potential confounders on prevalence rates.
This study has some limitations. First, there was heterogeneity across studies due to differing sample sizes. To mitigate this, we performed subgroup analyses according to sample size. Second, due to a lack of distinction between the various etiologies of pneumonia, several studies that reported other secondary causes of AP, hospital-acquired and community-acquired pneumonia, were excluded. In addition, patients who had previous AP were not excluded from the studies. Third, the relationship between other subtypes of parkinsonism and AP remains unclear, as our study focused exclusively on PD. Fourth, there is heterogeneity of controls in each study. Of the five studies comparing AP between patients with PD and controls, the control group in the three case–control studies was matched for at least gender and age. The control group of the remaining two cohort studies were matched only for non-PD diagnosis. In this meta-analysis, we conducted a subgroup analysis based on the two different study types, and the risk of AP remains significant in patients with PD compared to controls in both subgroups (Figure 3). As individual-level information for the studies was not available, we are unable to determine the propensity score. Future studies should conduct a propensity score matching based on gender, age, and medical comorbidities. Lastly, due to limited studies reporting hospital mortality between PD and controls, we were unable to perform a meta-analysis comparing hospital mortality rates in PD and controls. Future studies should investigate the cause of hospital mortality among patients with PD; this could provide clearer distinctions among the various etiologies of pneumonia. Correlation studies between the duration of hospital stay and incidence of AP may identify modifiable or preventable risk factors. Studies on the impaired immune system and identification of immune therapeutic targets and biomarkers can potentially help to screen at-risk patients and to institute novel therapies in those at higher risk [42, 43].
CONCLUSIONS
In conclusion, our meta-analysis showed that patients with PD had >3 times higher risk of AP, with an average 2.74% prevalence and 10.0% hospital mortality. Early recognition and treatment of AP in PD patients will help reduce the morbidity and mortality. A multidisciplinary strategic approach is needed to address the multifactorial causes of AP. Research on the link between AP and hospital mortality should be further examined.
AUTHOR CONTRIBUTIONS
Wei Yu Chua: Conceptualization; methodology; software; data curation; supervision; resources; project administration; formal analysis; validation; visualization; investigation; writing – original draft; writing – review and editing. Jia Dong James Wang: Conceptualization; methodology; writing – review and editing; writing – original draft; data curation; project administration. Claire Kar Min Chan: Conceptualization; writing – original draft; methodology; writing – review and editing; project administration; data curation. Ling-Ling Chan: Conceptualization; methodology; funding acquisition; supervision; resources; project administration; writing – review and editing; writing – original draft. Eng-King Tan: Conceptualization; methodology; software; data curation; supervision; resources; formal analysis; project administration; validation; visualization; writing – review and editing; investigation; funding acquisition; writing – original draft.
ACKNOWLEDGEMENTS
We thank the National Medical Research Council.
FUNDING INFORMATION
E.-K.T. (grant OF-LCG000207) and L.-L.C. (Clinician Scientist Award) are supported by the National Medical Research Council.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




