Effectiveness and safety of monthly versus quarterly fremanezumab for migraine prevention: An Italian, multicenter, real-life study
Abstract
Background and Purpose
Fremanezumab, a monoclonal antibody targeting the calcitonin gene-related peptide for migraine prevention, is available in monthly (225 mg) and quarterly (675 mg) doses. Previous studies showed efficacy and safety for both regimens, but a real-life comparison is lacking. This study aimed to compare the effectiveness and safety of monthly and quarterly fremanezumab in a real-life setting.
Methods
This Italian, prospective, multicenter study enrolled 95 migraine patients. During a 3-month treatment period, patients received either monthly or quarterly fremanezumab (49 monthly, 46 quarterly). A 6-month treatment period involved 79 patients (43 monthly, 36 quarterly). Monthly headache (MHD) and migraine days (MMD), number of days (AMD) and pills (AMP) of acute medication intake, and Headache Impact Test (HIT-6), Migraine Disability Assessment (MIDAS) test, and Numeric Rating Scale (NRS) scores were recorded at baseline and after 3 and 6 months of treatment. Adverse events (AEs), responder rates, and medication overuse were also investigated.
Results
Both monthly and quarterly treatments led to significant reductions in MMD, MHD, AMP, AMD, HIT-6, MIDAS, and NRS scores after 3 and 6 months. The monthly regimen exhibited a slightly greater reduction in MMD and MHD after the first quarter, with no significant difference observed after 6 months. The most common AE was transient injection-site reaction, without between-group differences. Responder rates and resolution of medication overuse did not significantly differ between the groups.
Conclusions
Both monthly and quarterly regimens were effective and safe, with a tendency for an advantage of the monthly regimen only in the first quarter of treatment.
INTRODUCTION
Migraine is a highly disabling neurological disease with a global prevalence of 14%–15%, affecting particularly young women [1]. Increased recognition of the huge personal and social impact of migraine has raised interest in the development of new treatments. Since 2020, the field of migraine preventive therapy has been completely renewed by the introduction of monoclonal antibodies (mAbs) specifically developed for migraine. Their target is calcitonin gene-related peptide (CGRP), which is a neuropeptide involved in migraine pathophysiology [2].
Fremanezumab is a humanized monoclonal antibody that binds CGRP and prevents its biological activity. Two dose regimens are approved: subcutaneous administration of fremanezumab 225 mg monthly and 675 mg quarterly [3]. The efficacy and safety of monthly and quarterly fremanezumab were first demonstrated in two pivotal 12-week phase 3 randomized, double-blind, placebo-controlled efficacy studies, in patients with episodic migraine (HALO EM) and chronic migraine (HALO CM) [4, 5]. The FOCUS trial showed that fremanezumab was effective over a 12-week treatment period in patients with documented failure to up to four migraine preventive medication classes [6]. The exploratory post hoc analysis including data from the HALO EM, HALO CM, and FOCUS studies showed that quarterly and monthly treatment with fremanezumab significantly reduced not only headache frequency but also headache severity and duration of the remaining attacks in patients with episodic and chronic migraine [7]. Monthly and quarterly fremanezumab was found to be effective also in reducing an overuse of acute medications compared to placebo [8].
Although randomized controlled trials (RCTs) showed that the two dose regimens achieved comparable efficacy and safety results, there are no studies that have conducted a direct statistical comparison between the two dosages. An exposure-response model, using data from RCTs, simulated an efficacy comparison between the two dosage regimens, predicting the achievement of clinical benefit with both dosages in episodic and chronic migraine patients [9]. A recent systemic review and meta-analysis of RCTs showed that there were no significant differences in reduction of migraine frequency between the two dose regimens [10].
Real-life studies confirmed the effectiveness and safety of monthly and quarterly fremanezumab in both chronic and episodic migraine patients, and also in patients with multiple previous failures and diverse comorbidities [11-15]. The real-life FRIEND study suggested a potential clinical advantage for the monthly regimen, although only few patients received quarterly fremanezumab and no statistical analyses were conducted [13, 14]. A recent Japanese study compared the effectiveness of monthly and quarterly fremanezumab, yielding comparable results over a 6-month period, but without evaluating changes in disability or safety outcomes [15].
Our study seeks to determine statistically significant differences between the two fremanezumab dosages in a real-world context, specifically assessing their effectiveness in reducing migraine and headache days and examining potential distinctions in reducing attack severity, analgesic consumption, disability, and safety.
METHODS
Study design and participants
This was a multicenter, prospective, real-life study involving three Italian Headache Centers located in Milan, Bologna, and Rome.
A total of 95 migraine patients were enrolled from February 2020 to April 2023; 50 attended the Headache Clinic at San Raffaele Hospital in Milan, 23 attended the IRCCS Institute of Neurological Sciences in Bologna, and 22 attended the Headache and Neurosonology Unit of Campus Bio-Medico in Rome. Inclusion criteria were as follows: age ≥ 18 years, diagnosis of migraine according to the International Classification of Headache Disorders, 3rd Edition (ICHD3) [16], and eligibility to treatment with anti-CGRP mAbs. According to the current national reimbursement policies, migraine prophylaxis with fremanezumab is fully reimbursed by the Italian National Health System for migraine patients who have at least 8 monthly migraine days (MMD), as well as moderate or severe migraine-related disability according to the Migraine Disability Assessment (MIDAS) questionnaire, and who have previously failed at least three classes of preventive medications, including tricyclics, anticonvulsants, beta-blockers and onabotulinum toxin A. Exclusion criteria were cardio- or cerebrovascular comorbidities and poor controlled blood hypertension.
All patients underwent a baseline visit during which monthly or quarterly fremanezumab was started according to the patient's preference. After the baseline evaluation (T0), office visits were attended after 3 (M3) and 6 (M6) months of treatment.
At T0, patients underwent a neurological examination and were instructed to fill in a paper headache diary recording headache and migraine days, acute medication intake, and pain intensity. Medication overuse headache (MOH) was diagnosed according to the ICHD3 criteria [16]. Patients with coexisting MOH were educated to withdraw from excessive intake of analgesics. Any concomitant prophylaxis was continued without changes.
Clinical variables, namely MMD, monthly headache days (MHD), and monthly number of days (AMD) and pills (AMP) of acute medication intake were recorded at T0, M3, and M6 using the headache diary and a semistructured clinical interview. For each clinical variable, we recorded the average across the past 3 months prior to the evaluation. At each time point, migraine pain intensity was quantified using the Numeric Rating Scale (NRS) [17], migraine-related disability was evaluated using the MIDAS [18] questionnaire, and migraine impact was assessed with the 6-item Headache Impact Test (HIT-6) [19]. Using the scores from NRS, MIDAS, and HIT-6, patients were categorized into various groups reflecting escalating levels of pain intensity, migraine-related disability, and impact [17-19]. The presence of adverse events (AEs) was investigated at follow-up visits.
Outcomes
The primary efficacy endpoint was to assess between-group difference in reduction of MMD and MHD from T0 to M3 and from T0 to M6. The primary safety endpoint was to assess between-group difference in AEs.
Secondary endpoints encompassed (i) to assess between-group differences in ≥30%, ≥50%, and ≥75% response rates at M3 and M6 (response rates were defined in reduction of MMD from T0 to M3 and from T0 to M6); (ii) to assess between-group differences in reduction of acute medication intake and in the resolution of MOH from T0 to M3 and from T0 to M6; (iii) to explore between-group differences in improvement of NRS, HIT-6, and MIDAS scores from T0 to M3 and from T0 to M6; and (iv) to assess between-group differences in ≥50% response rate in terms of reduction of MIDAS score from T0 to M3 and from T0 to M6. This latest secondary outcome has been defined based on the rules of the Italian Medicines Agency, which states that a ≥50% reduction in the MIDAS score after 3 and then after 6 months of treatment is mandatory to continue the therapy.
Statistical analyses
The distribution of demographic and clinical variables was tested using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Quantitative data were expressed as median and interquartile range (25th-75th percentile), and categorical data were expressed as frequency. Because the data were not normally distributed, between-group differences in demographic and clinical variables at baseline were assessed with Fisher exact or Mann–Whitney tests, as appropriate. Within-group differences in treatment effectiveness were assessed at M3 and M6 using the Wilcoxon signed-rank test. Between-group differences in treatment effectiveness after 3 and 6 months were assessed using the following models: linear mixed-effects models on log-transformed data for changes in MMD, MHD, AMD, and AMP; cumulative link mixed models for changes in MIDAS, HIT-6, and NRS scores; and generalized linear mixed-effects models for changes in MOH rates. The difference between groups regarding the presence of side effects and the responder rates at both M3 and M6 was assessed using the Fisher exact test. Statistical analyses were performed using IBM SPSS Statistic software, version 26, and R software, version 4.1.0. Results were tested at a threshold of p < 0.05, adjusted for the Bonferroni correction.
RESULTS
Clinical characteristics
Forty-nine patients underwent the monthly regimen and 46 the quarterly one. All patients completed a 3-month treatment period; 79 patients completed a 6-month treatment period (43 on monthly regimen and 36 on quarterly regimen). Three patients on the monthly regimen and seven patients on the quarterly regimen were lost to follow-up, and three patients on the monthly regimen and three on the quarterly regimen stopped treatment after 3 months due to ineffectiveness. Demographic and clinical features were similar between the two groups at baseline (Table 1). There were 36 (73%) women in the monthly group and 37 (80%) in the quarterly group (p = 1.0); the median age was 55 years and 51 years, respectively (p = 0.5). Twenty-six patients (53%) in the monthly group and 28 (61%) in the quarterly group had chronic migraine (p = 1.0). Patients with MOH were 26 (53%) in the monthly group and 26 (57%) in the quarterly group (p = 1.0). Four (8%) and six (13%) patients had migraine with aura in the monthly and in the quarterly group, respectively (p = 1.0). Patients using concomitant prophylaxis, including antidepressants, beta-blockers, and antiepileptic drugs, were 22 (45%) in the monthly group and 13 (28%) in the quarterly group (p = 0.8). Patients with comorbidities were 14 (29%) in the monthly group and 17 (37%) in the quarterly group (p = 1.0). Comorbidities included hypertension, dysthyroidism, and anxiety or depressive disorder. Median disease duration was 36 and 30 years, respectively (p = 1.0). Patients had failed a median of four and three preventive treatments prior to starting fremanezumab in the monthly and in the quarterly group, respectively (p = 1.0).
| Characteristic | Mtly group | Qtly group | Mtly vs. Qtly pa |
|---|---|---|---|
| Women/men | 36 (73%)/13 (27%) | 37 (80%)/9 (20%) | 1.0 |
| Median age, years [IQR] | 55 [47–64] | 51 [41–58] | 0.5 |
| Migraine with aura/migraine without aura | 4 (8%)/45 (92%) | 6 (13%)/40 (87%) | 1.0 |
| Chronic/episodic migraine | 26 (53%)/23 (47%) | 28 (61%)/18 (39%) | 1.0 |
| Median disease duration, years [IQR] | 36 [21–45] | 30 [23–40] | 1.0 |
| Comorbidities | 14 (29%)/35 (71%) | 17 (37%)/29 (63%) | 1.0 |
| Concomitant prophylaxis | 22 (45%) | 13 (28%) | 0.8 |
| Past preventive treatments failed, n [IQR] | 4 [3–4] | 3 [3–5] | 1.0 |
| Median monthly headache days [IQR] | 15 [11–20] | 15 [10–21] | 1.0 |
| Median monthly migraine days [IQR] | 15 [11–20] | 15 [10–20] | 1.0 |
| Median monthly acute medication pills [IQR] | 15 [12–22] | 18 [11–26] | 1.0 |
| Median monthly acute medication days [IQR] | 13 [11–18] | 15 [10–20] | 1.0 |
| Medication overuse headache | 26 (53%) | 26 (57%) | 1.0 |
| Median MIDAS score [IQR] | 84 [48–114] | 74 [50–110] | 0.5 |
| MIDAS categories | |||
| Little or no disability | 0 | 0 | |
| Mild disability | 0 | 0 | |
| Moderate disability | 6 | 2 | |
| Severe disability | 43 | 44 | |
| Median HIT-6 score [IQR] | 66 [63–71] | 67 [64–69] | 1.0 |
| HIT-6 categories | |||
| Little or no impact | 0 | 1 | |
| Some impact | 2 | 1 | |
| Substantial impact | 2 | 2 | |
| Severe impact | 45 | 42 | |
| Median NRS score [IQR] | 8 [7–9] | 8 [7–8] | 1.0 |
| NRS categories | |||
| Mild pain | 2 | 1 | |
| Moderate pain | 16 | 19 | |
| Severe pain | 31 | 26 | |
- Note: Measures are reported as median [IQR; 25th–75th percentile] or n (%).
- Abbreviations: HIT-6, 6-item Headache Impact Test; IQR, interquartile range; MIDAS, Migraine Disability Assessment test; Mtly, monthly; NRS, Numeric Rating Scale; Qtly, quarterly.
- a Mann–Whitney test was used for continuous and ordinal variables; Fisher exact test was used for categorical variables. Statistical significance was set at p < 0.05, adjusted for the Bonferroni correction.
Reduction of migraine and headache frequency
After 3 as well as after 6 months of treatment with fremanezumab, both monthly and quarterly treated patients had a significant reduction of MHD and MMD. At M3, the median reduction of MHD was −9 for the monthly regimen and −7 for the quarterly regimen, whereas at M6 it was −10 and −8, respectively. We observed a trend in favor of the monthly regimen over the quarterly at M3 (p = 0.04) but not at M6 (p = 0.07). At M3, the median reduction of MMD was −8 for the monthly regimen and −7 for the quarterly regimen, whereas at M6 it was −10 and −8, respectively. We observed a slight advantage of the monthly regimen over the quarterly at M3 (p = 0.03) but not at M6 (p = 0.2; Table 2).
| Mtly group | Qtly group | Mtly vs. Qtly | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M3 | M6 | M3 vs. T0 pa | M6 vs. T0 pa | M3 | M6 | M3 vs. T0 pa | M6 vs. T0 pa | M3 vs. T0 pb | M6 vs. T0 pb | |
| MHD | 5 [3 to 9] | 5 [3 to 6] | <0.001c | <0.001c | 8 [5 to 13] | 7 [4 to 13] | <0.001c | <0.001c | 0.035c | 0.065 |
| Change from T0 | −9 [−6 to −13] | −10 [−7 to −12] | −7 [−2 to −10] | −8 [−3 to −11] | ||||||
| MMD | 5 [3 to 8] | 5 [3 to 6] | <0.001c | <0.001c | 8 [5 to 13] | 7 [4 to 13] | <0.001c | <0.001c | 0.034c | 0.217 |
| Change from T0 | −8 [−5 to −12] | −10 [−6 to −12] | −7 [−2 to −10] | −8 [−4 to −10] | ||||||
| AMP | 4 [2 to 9] | 4 [2 to 7] | <0.001c | <0.001c | 8 [3 to 13] | 6 [2 to 13] | <0.001c | <0.001c | 0.277 | 1.0 |
| Change from T0 | −11 [−8 to −15] | −11 [−9 to −17] | −9 [−4 to −15] | −9 [−5 to −16] | ||||||
| AMD | 4 [2 to 7] | 4 [2 to 6] | <0.001c | <0.001c | 6 [3 to 11] | 6 [2 to 12] | <0.001c | <0.001c | 0.087 | 0.289 |
| Change from T0 | −10 [−6 to −12] | −10 [−8 to −12] | −7 [−3 to −10] | −8 [−4 to −11] | ||||||
| MIDAS score | 8 [2 to 29] | 8 [3 to 17] | <0.001c | <0.001c | 20 [8 to 41] | 16 [4 to 45] | <0.001c | <0.001c | 1.0 | 1.0 |
| Change from T0 | −52 [−29 to −102] | −69 [−40 to −104] | −48 [−19 to −89] | −51 [−24 to −94] | ||||||
| MIDAS categories | ||||||||||
| Little or no disability |
18 |
15 |
6 | 10 | ||||||
| Mild disability | 10 | 11 |
7 |
6 |
||||||
| Moderate disability | 4 | 8 | 11 | 5 | ||||||
| Severe disability | 17 | 9 | 22 | 15 | ||||||
| HIT-6 score | 60 [54 to 64] | 56 [50 to 63] | <0.001c | <0.001c | 60 [54 to 64] | 58 [54 to 64] | 0.002c | <0.001c | 1.0 | 1.0 |
| Change from T0 | −9 [−4 to −12] | −9 [−5 to −16] | −7 [−2 to −13] | −10 [−3 to −19] | ||||||
| HIT-6 categories | ||||||||||
| Little or no impact | 5 | 9 | 7 | 7 | ||||||
| Some impact |
15 |
12 |
5 | 7 | ||||||
| Substantial impact | 4 | 4 | 9 | 9 | ||||||
| Severe impact | 25 | 18 | 25 | 13 | ||||||
| NRS score | 6 [5 to 8] | 5 [5 to 7] | <0.001c | <0.001c | 7 [5 to 8] | 5 [4 to 8] | 0.006c | 0.001c | 0.8 | 1.0 |
| Change from T0 | −1 [0 to −3] | −2 [0 to −3] | −1 [0 to −3] | −2 [0 to −3] | ||||||
| NRS categories | ||||||||||
| Mild pain | 12 | 8 |
10 |
13 |
||||||
| Moderate pain | 24 | 29 | 19 | 14 | ||||||
| Severe pain | 13 | 6 | 17 | 9 | ||||||
- Note: Measures are reported as median and interquartile range (25th–75th percentile) or n.
- Abbreviations: AMD, acute medication days; AMP, acute medication pills; HIT-6, 6-item Headache Impact Test; MHD, monthly headache days; MIDAS, Migraine Disability Assessment test; MMD, monthly migraine days; Mtly, monthly; NRS, Numeric Rating Scale; Qtly, quarterly.
- a Wilcoxon test, statistical significance was set at p < 0.05, adjusted for the Bonferroni correction.
- b Linear mixed-effects models on log-transformed data for continuous data and cumulative link mixed models for ordinal data; statistical significance was set at p < 0.05, adjusted for the Bonferroni correction.
- c Statistically significant.
Safety
AEs were reported by one patient in the monthly group and by two patients in the quarterly group at M3, and by one patient in the monthly group and one patient in the quarterly group at M6. The most frequent AE was transient injection-site reaction, reported by two patients in the quarterly group at M3 and by one patient in the monthly group at M6; constipation was reported by one patient in the monthly group at M3 and by one patient in the quarterly group at M6. All adverse reactions were mild, and none required additional treatments or procedures. There was not a statistically significant difference in AE frequency between the two regimens at M3 (p = 0.6) or at M6 (p = 1.0).
Response rate
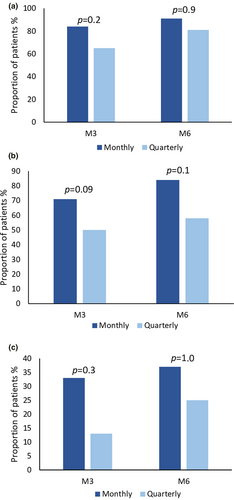
Figure 1 represents the distribution of 30%, 50%, and 75% responder rates in the two treatment groups at various time points. At M3, a ≥30% reduction of MMD was observed in 41 patients (84%) in the monthly group and in 30 patients (65%) in the quarterly group, without a statistically significant difference between the two groups (p = 0.2). A ≥50% reduction was observed in 35 patients (71%) in the monthly group and in 23 patients (50%) in the quarterly group, without a statistically significant between-group difference (p = 0.09); a ≥75% reduction in MMD was observed in 16 patients (33%) in the monthly group and in six patients (13%) in the quarterly group, without a statistically significant between-group difference (p = 0.3). At M6, a ≥30% reduction of MMD was observed in 39 patients (91%) in the monthly group and in 29 patients (81%) in the quarterly group, without a statistically significant difference between the two groups (p = 0.9). A ≥50% reduction was observed in 36 patients (84%) in the monthly group and in 21 patients (58%) in the quarterly group, without a statistically significant between-group difference (p = 0.1); a ≥75% reduction in MMD was observed in 16 patients (37%) in the monthly group and in nine patients (25%) in the quarterly group, without a statistically significant between-group difference (p = 1.0).
Reduction in acute medication intake and cessation of medication overuse
At M3 and at M6, both groups had a significant reduction of AMP and AMD, with no significant between-group differences (AMP: M3 p = 0.3, M6 p = 1.0; AMD: M3 p = 0.09, M6 p = 0.3; Table 2). At M3, AMP decreased by −11 and −9 in the monthly and in the quarterly group, respectively, and AMD decreased by −10 and −7, respectively. At M6, AMP decreased by −11 and −9 and AMD decreased by −10 and −8 in the monthly and in the quarterly group, respectively (Table 2).
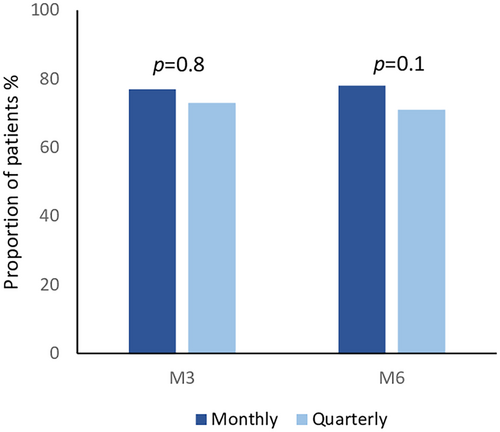
Among patients with MOH, we observed a cessation of abuse in 20 patients in the monthly group (77%) and in 19 patients (73%) in the quarterly group at M3; 18 of 23 (78%) patients with MOH in the monthly group who completed a 6-month period of treatment reported a cessation of treatment overuse at M6; the same result was observed in 15 (71%) of 21 patients in the quarterly group. No statistically significant difference between the two regimens emerged at M3 (p = 0.8) or at M6 (p = 0.1; Figure 2).
Reduction in NRS, HIT-6, and MIDAS scores
After 3 months of treatment as well as after 6 months of treatment with fremanezumab, both groups of patients had a significant reduction of NRS, HIT-6, and MIDAS scores (Table 2). At M3, MIDAS score decreased by −52 and −48, respectively: HIT-6 score decreased by −9 and −7, respectively; NRS score decreased by −1 in both groups. At M6, MIDAS score decreased by −69 and −51, respectively; HIT-6 score decreased by −9 and −10, respectively; NRS score decreased by −2 in both groups. We did not observe a statistically significant distinction between the two treatment regimens at M3 (NRS score: p = 0.8, HIT-6 score: p = 1.0, MIDAS score: p = 1.0) or at M6 (NRS score: p = 1.0, HIT-6 score: p = 1.0, MIDAS score: p = 1.0; Table 2).
A ≥50% reduction in MIDAS score was observed in 44 patients (90%) in the monthly group and in 41 patients (89%) in the quarterly group at M3, without a statistically significant between-group difference (p = 1.0), and at M6 the same result was reported by 39 patients (91%) in the monthly group and by 32 (89%) in the quarterly group, without a statistically significant between-group difference (p = 1.0).
DISCUSSION
Our multicenter, prospective, real-life study showed that both monthly (225 mg) and quarterly (675 mg) regimens of fremanezumab are effective in reducing migraine frequency, acute medication intake, migraine severity, and related disability in a real-life setting. Furthermore, we observed that monthly and quarterly fremanezumab provided a clinical benefit also in patients with difficult-to-treat migraine who had previously failed up to five migraine preventive treatments, as previously observed in RCTs [4-6].
Previous RCTs and real-life studies demonstrated that fremanezumab is effective for migraine prevention [4-15]. A network meta-analysis of RCTs focusing on migraine preventive treatments targeting the CGRP found both fremanezumab 225 and 675 mg to be effective in reducing MMD, MHD, and AMD compared to placebo. This was accompanied by a significantly higher responder rate of ≥50% and ≥75% [20]. Additionally, a recent meta-analysis pooled data from RCTs, suggesting no substantial difference in mean change from baseline in MMD over a 12-week treatment period between the two fremanezumab dosing regimens, in both patients with episodic migraine and those with chronic migraine [10].
Our primary efficacy endpoint was to assess any difference between the two regimens in terms of reduction of migraine and headache frequency. We observed a slight advantage of the monthly regimen in reducing MHD and MMD at M3. However, this finding was not confirmed when comparing responder rates (≥30%, ≥50%, and ≥75%) in terms of MMD reduction between the two dosage groups.
The slightly greater reduction of MMD we observed at M3 for the monthly regimen over the quarterly is in line with the FRIEND and FOCUS studies, where the monthly regimen was associated with higher probability of responsiveness over a 12-week treatment period compared to the quarterly one [6, 13, 14]. However, this was only a descriptive result, as a direct, statistical-based comparison between the two regimens was not performed in the two studies. On the other hand, a recent observational retrospective real-life Japanese study including 75 patients treated with monthly fremanezumab and 52 treated with quarterly fremanezumab for 6 months found no significant differences between the two regimens in reducing MMD from baseline to each month of treatment, for both episodic and chronic migraine [15]. The different analytical approaches employed in the two studies, specifically assessing the monthly reduction in migraine days in the Japanese study versus evaluating the quarterly average reduction in migraine days in our study, could account for the observed difference in outcomes.
It is worth noting that the advantage of the monthly regimen over the quarterly we found was limited only to the first quarter of treatment, as no significant between-group differences emerged at M6. Moreover, whereas in patients receiving monthly fremanezumab the clinical response remained steady from M3 to M6, in patients receiving quarterly fremanezumab we observed an amelioration in clinical outcomes from M3 to M6. There is evidence indicating that concentrations of fremanezumab are higher and show greater accumulation in the initial months of treatment for the 225-mg monthly administration compared to the quarterly 675-mg administration [21]. Fremanezumab concentrations approach steady-state conditions in approximately 6 months for both the 225-mg subcutaneous monthly and 675-mg subcutaneous quarterly dosing regimens [21]. Both these aspects could explain the observed slight advantage of monthly administration over quarterly only in the first 3 months of therapy, an advantage that is no longer apparent at the 6th month, when both therapeutic regimens achieve steady state.
We could also speculate that the slight clinical advantage of the monthly regimen over the quarterly after the first 3 months of treatment is due to a wearing-off effect of quarterly fremanezumab. Wearing-off effect is defined as a reduced effect of a medication in the last days of the dose interval before the next scheduled dose [22]. The wearing-off effect of fremanezumab, whether administered on a monthly or quarterly basis, has previously been investigated with negative results. A subanalysis of data from a long-term, phase 3 RCT conducted by Blumenfeld et al. showed that there were no significant increases in the mean weekly migraine days observed between weeks 1–2 and weeks 11–12 in both the initial quarter of treatment (months 1–3) and the second one (months 4–6) with either monthly or quarterly fremanezumab [22]. A recent real-life Japanese study conducted on 101 migraine patients receiving monthly or quarterly fremanezumab found no significant increase in weekly migraine days in the week before the next dosing at any time point over 9 months [23]. In our study, we measured each clinical variable at M3 and M6 by averaging the values over the preceding 3 months. As a result, the absence of data for each specific month in the first and second quarters prevents us from drawing firm conclusions about the potential impact of the wearing-off effect of quarterly fremanezumab on the observed slight clinical advantage associated with monthly administration over the quarterly one.
According to RCTs, fremanezumab is safe and well tolerated and the most common AEs are transient injection-site reactions [4-6]. A recent meta-analysis showed that the most frequent AEs associated with fremanezumab were injection site erythema, induration, and pruritus [24]. Transient injection-site reactions were found to be the most common AE also in real-life studies [11-14]. Similar to previous RCTs and real-life data, we found that the most common AEs were injection-site reactions, both for monthly and quarterly fremanezumab, without significant difference between the two regimens.
The present study did not reveal differences between the groups in the reduction of AMP and AMD, or in the cessation of MOH. A recent single-center real-life Italian study showed that monthly fremanezumab is effective in reducing MOH, but data about the quarterly regimen are missing, as patients treated with quarterly fremanezumab were not included [25]. To the best of our knowledge, our study is the first to evaluate cessation of MOH in migraine patients treated with fremanezumab, exploring both dose regimens.
The two dose regimens were also equally effective in reducing headache severity, evaluated with NRS score, and migraine-related disability and impact, evaluated with MIDAS and HIT-6 scores. No between-group differences emerged when considering the ≥50% response rate in terms of reduction of MIDAS score.
Our study is not without limitations. First is the small number of patients included. Second, the observational period of treatment is quite short. Finally, we started one of the two dose regimens of fremanezumab without a randomization but based on patients' preferences.
To the best of our knowledge, our study is the first to compare the effectiveness of the two dose regimens of fremanezumab in clinical practice not only in terms of reduction of migraine frequency but also in terms of reduction of acute medication intake, migraine severity, and related disability, using a systematic statistic approach. This real-life study provides evidence supporting the effectiveness of both monthly and quarterly fremanezumab over 3 and 6 months of treatment in a real-world context. A slight advantage of the monthly regimen was observed after the first 3 months of treatment in terms of reduction of migraine frequency, possibly due to a faster and greater rate of accumulation compared to the quarterly administration. This advantage, however, evens out after 6 months of treatment, when both dosages reach an equivalent steady state. Further real-life studies with a larger sample size are necessary to validate our results.
CONFLICT OF INTEREST STATEMENT
R.M. reports personal fees from Eli Lilly, Lundbeck, Teva, Pfizer, and AbbVie for participating on advisory boards and in speaker activities. I.C. reports personal fees for speaker activity from Eli Lilly. F.V. has received travel grants or honoraria for advisory boards, speaker panels, or clinical investigation studies from Allergan-AbbVie, Amgen, Angelini, Eli Lilly, Lundbeck, Novartis, Pfizer, and Teva. C.A. has received travel grants and honoraria from Novartis, Eli Lilly, Lusofarmaco, Laborest, Allergan, and Almirall. S.C. has received travel grants or honoraria for advisory boards, speaker panels, or clinical investigation studies from AbbVie, Angelini, Eli Lilly, Lundbeck, Novartis, Pfizer, and Teva. V.F. has received travel grants or honoraria for advisory boards and speaker panels from AbbVie, Eli Lilly, Lundbeck, Pfizer, and Teva. B.C. has received travel grants or honoraria for advisory boards, speaker panels, or investigation studies from Novartis, Teva, Eli Lilly, Pfizer, and Lusofarmaco. M.F. is editor-in-chief of the Journal of Neurology and associate editor of Human Brain Mapping, Neurological Sciences, and Radiology. He has received compensation for consulting services from Alexion, Almirall, Biogen, Merck, Novartis, Roche, and Sanofi; for speaking activities from Bayer, Biogen, Celgene, Chiesi Italia, Eli Lilly, Genzyme, Janssen, Merck-Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and Teva; for participation on advisory boards from Alexion, Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Genzyme, and Takeda; and for scientific direction of educational events from Biogen, Merck, Roche, Celgene, Bristol-Myers Squibb, Eli Lilly, Novartis, and Sanofi-Genzyme. He receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, the Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). The other authors declare no competing interests.
ETHICS STATEMENT
The study was conducted according to the Declaration of Helsinki and to principles of good clinical practice. The study was approved by local ethical committees, and all participants signed informed consent.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




