Co-incidental C9orf72 expansion mutation-related frontotemporal lobar degeneration pathology and sporadic Creutzfeldt−Jakob disease
Abstract
Background
The C9orf72 hexanucleotide expansion mutation is the most common cause of genetic frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS) and combined FTD-ALS. Its underlying neuropathology combines TDP-43 pathology and dipeptide repeat protein (DPR) deposits and may also associate with other neurodegeneration-associated protein aggregates. Herein we present a unique combination of C9orf72 mutation with sporadic Creutzfeldt−Jakob disease (CJD) in a 74-year-old patient with rapidly progressive dementia.
Methods
Detailed neuropathological examination including immunohistochemistry for several proteinopathies. Genetic analysis was conducted by repeat primed polymerase chain reaction (PCR). Furthermore, we analyzed additional C9orf72 mutation carriers for prion−protein (PrP) deposits in brain tissue and screened the cerebellar cortex of other CJD cases for p62/DPR neuronal inclusions to assess the frequency of combined pathologies.
Results
Postmortem brain examination of a patient with a rapidly progressive neurological deterioration of 8 months’ duration confirmed the diagnosis of CJD. She harbored valine homozygosity at PRNP codon 129. In addition, a frontotemporal lobar degeneration (FTLD)-pattern with TDP-43 protein aggregates and p62+/C9RANT+ positive inclusions along with a high degree of Alzheimer-related pathology (A3B3C3) were identified. The suspected C9orf72 expansion mutation was confirmed by repeat-primed PCR. Screening of 13 C9orf72 cases showed no pathological PrP aggregates and screening of 100 CJD cases revealed no other C9orf72 expansion mutation carriers.
Conclusion
A combination of a C9orf72 expansion mutation-related FTLD with sporadic CJD in the same patient is rare. While the rarity of both diseases makes this concurrence most likely to be coincidental, questions regarding a potential link between these two neurodegenerative pathologies deserve further studies.
INTRODUCTION
The GGGGCC hexanucleotide repeat expansion mutation in the non-coding region of chromosome 9 open reading frame 72 (C9orf72) gene is the most common genetic cause of familial and sporadic frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS) and FTD-ALS [1-3]. Clinical phenotypes are relatively broad, although those associated with FTD are commonly behavioral while ALS exhibit frequently bulbar and psychiatric symptoms [4-6]. Other phenotypes include parkinsonism, [4, 7, 8] Huntington's disease-mimics [9, 10] or Creutzfeldt−Jakob disease (CJD)-like phenotypes with rapidly progressive course [3][11].
Neuropathologically, a combination of phospho-TDP-43 and p62+/phospho-TDP-43− protein aggregates containing the aberrant C9orf72 protein product dipeptide repeat proteins (DPRs) is observed. DPRs are formed by repeat-associated translation, can be immunohistochemically visualized by specific antibodies, [12, 13] and can be easily identified in the cerebellum and hippocampus, which represent useful screening regions for identifying C9orf72 expansion mutation carriers [14].
We describe herein a unique constellation of sporadic CJD and C9orf72 expansion mutation. We additionally screened other CJD brains for potential C9orf72 expansion mutation and screened C9orf72 expansion mutation carriers for CJD pathology.
METHODS
Neuropathological examination
Hematoxylin−eosin, Luxol fast blue stains and immunohistochemistry were performed on formalin-fixed and paraffin-embedded tissue blocks applying several primary antibodies (Table 1). The DAKO EnVision© detection kit (Dako, Glostrup, Denmark) was used for visualization of antibody reactions.
| Antibody name | Clone | Company | Dilution |
|---|---|---|---|
| Anti-α-synuclein | Clone 5G4 | Roboscreen, Leipzig, Germany | 1:4000 |
| Anti-βA4-amyloid | Clone 6F/3D | DAKO, Glostrup, Denmark | 1:100 |
| Anti-C9RANT | Polyclonal | Novus Biologicals, Centennial, CO, USA | 1:1000 |
| Anti-p62 | Clone 3/p62 lck ligand | BD Transduction Laboratories, Franklin Lakes, NJ, USA | 1:500 |
| Anti-phosphorylated tau | Clone AT8, pS202/pT205 | Thermo Scientific, Rockford, IL, USA | 1:200 |
| Anti-phosphorylated TDP-43 | Clone 11-9, pS409/410 | Cosmo Bio, Tokyo, Japan | 1:20,000 |
| Anti-PrP | Clone 12F10 | Cayman Chemical, Ann Arbor, MI, USA | 1:1000 |
Genetic analysis
C9orf72 repeat expansion mutation was analyzed by repeat-primed polymerase chain reaction (PCR) as described previously [15] and PRNP sequencing was performed as described.
Screening of C9orf72 mutation and CJD cases
To assess the potential co-existence of a prion disease in C9orf72 mutations carriers we screened 13 additional, unrelated, postmortem cases for pathological prion−protein deposits by immunohistochemistry (anti-PrP/12F10 antibody) in selected areas (frontal cortex, hippocampus, thalamus, cerebellum).
Furthermore, to identify further potential C9orf72 mutation carriers among prion diseases, we immunohistochemically screened 100 definite CJD cases with different histotypes (MM, MV, VV) for typical p62+/TDP-43− inclusions in the cerebellar cortex.
Ethical standards
The study was performed within OERPE in accordance with the Declaration of Helsinki, and was approved by the Ethics Committee of the Medical University of Vienna (EK 396-2011).
RESULTS
Clinical summary
A 74-year-old woman was admitted to the Neurology department due to confusion, ataxia and dizziness of a few weeks’ duration. An interview with her closest relatives did not indicate previous neurological symptoms. The patient's family history was unremarkable. Cranial magnetic resonance imaging (MRI), which included T2, fluid-attenuated inversion recovery (FLAIR) and T1 sequences with contrast, showed a small (3 cm in diameter) frontobasal tumor with a surrounding edema, suspicious for meningioma. Additionally, a mild temporal atrophy was noted. No obvious cortical or basal ganglia hyperintensities were reported, but images could not be reviewed. Although her symptoms could not be convincingly linked to the tumor itself, she was promptly operated. Histology revealed a classical meningothelial meningioma (WHO grade I). Postoperatively the patient did not improve but developed a rapidly progressive dementia, spasticity, bilateral Babinski sign, myoclonus and progressive stuporous state. A lumbar puncture 3 months after symptom onset showed positivity for 14-3-3 protein. On electroencephalography periodic sharp wave complexes were identified. MRI was not repeated. Due to increasing clinical suspicion of CJD, the surgical instruments were retrospectively identified and immediately discarded. The patient deteriorated further, presented several infections and fever episodes, evolved to akinetic mutism and finally died in a nursing home 8 months after disease onset with the final clinical diagnosis of “probable” CJD.
Neuropathological findings
Fixed brain weight was 948 g. Gross examination revealed a severe generalized atrophy with a frontobasal tissue defect caused by the surgical removal of the meningioma. On coronal sections, diffuse cortical and basal ganglia atrophy, enlargement of lateral ventricles and white matter rarefaction was found. The cerebellum revealed atrophy of the cerebellar peduncles, dentate nucleus and cerebellar cortex.
Histopathology and immunohistochemistry
Histopathological examination revealed severe neurodegenerative changes. The cerebral cortex and subcortical nuclei showed diffuse neuronal loss, prominent gliosis and spongiform change (Figure 1A,B). In the temporal cortex, a distinctive superficial laminar spongiosis was identified in addition to the severe cortical affection (Figure 1D). The cerebellar cortex showed severe loss of granule and Purkinje cells and Bergmann gliosis (Figure 1F). White matter showed marked rarefaction and gliosis (Figure 1G), accentuated in frontal and temporal lobes, and a degeneration of most white matter tracts including the pyramidal tract. A moderate cerebrovascular pathology with expanded Virchow−Robin spaces and perivascular pigment-laden macrophages was also observed.
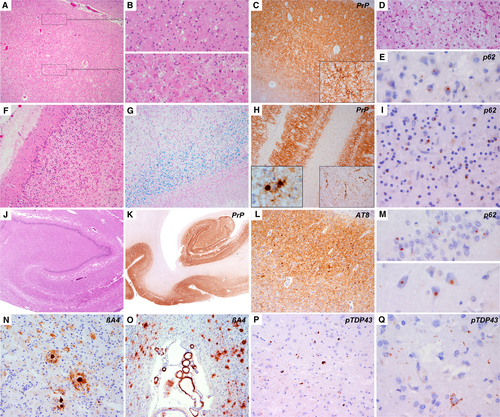
Immunohistochemistry showed prominent pathological prion−protein (PrP) deposits in a diffuse-synaptic pattern throughout cortical layers, subcortical and brainstem nuclei, and the cerebellar cortex (Figure 1C,H,K). Occasionally perineuronal immunoreactivity was seen in deep layers of the parietal cortex (Figure 1C, inset). The frontal white matter, basal ganglia, thalamic and cerebellar white matter showed some plaque-like deposits and coarse deposits along axons and around vessels (Figure 1H, inset). No Kuru-type or florid plaques were identified [16, 17].
There was a mild degree of concomitant phospho-TDP-43 pathology in cortical, subcortical and brainstem regions with diffuse-granular and compact neuronal cytoplasmic inclusions, occasional thin dystrophic neurites and fine dot-like neuropil immunoreactivity (Figure 1P,Q) [18-20]. Intranuclear inclusions were lacking. There were also widespread small, p62+ neuronal cytoplasmic inclusions in cortical neurons and granule cells of the cerebellum and hippocampal dentate gyrus (Figure 1E,I,M), suggestive of a C9orf72 repeat expansion mutation. These were also immunoreactive for C9RANT (repeat-associated non-ATG translation) peptides or DPR. Their distribution pattern did not match that of phospho-TDP-43. As this was an archival case, these findings were observed in a second histopathological assessment 13 years later.
Moreover, we observed high load of Alzheimer's disease neuropathological change (ADNC) (A2B3C3) [21] with tau-positive neurofibrillary pathology in limbic and cortical regions (Figure 1K), granular fuzzy astrocytes in line with aging-related tau astrogliopathy (ARTAG) pathology in amygdala, temporal white matter and parietal cortex, [22] frequent neuritic plaques and moderate amyloid angiopathy without capillary involvement. No α-synuclein aggregates were identified.
Genetic analysis
C9orf72 genotyping revealed a typical saw-tooth pattern expanding over the currently used cut-off of 30 repeats, indicating a pathogenic hexanucleotide repeat expansion. Amplicon-length analysis was performed using flanking PCR to increase sensitivity and specificity of results, as described elsewhere [23]. Sequencing of PRNP showed no mutations and valine homozygosity was detected at codon 129.
Screening of C9orf72 mutation and CJD cases
Immunohistochemical analysis of 13 additional C9orf72 mutation carriers showed no pathological PrP aggregates or CJD-suggestive lesions. The screening of 100 definite sporadic CJD (sCJD) for p62-positive cytoplasmic inclusions in the cerebellar cortex revealed no other cases suspicious of C9orf72 expansion mutation.
Retrospective analysis of stored cerebrospinal fluid (CSF) samples (−80°C) revealed total tau levels of >2171 pg/ml (normal levels <500 pg/ml); real-time quaking-induced conversion (RT-QuIC) was negative.
DISCUSSION
We present a patient with a unique constellation of definite sporadic CJD carrying a C9orf72 expansion mutation with FTLD neuropathological features, two neurodegenerative diseases which by themselves represent rare diseases. The patient presented typical symptoms of CJD and had no family history of a neurological disorder. While 14-3-3 protein and total tau levels were increased in the CSF, a retrospectively performed RT-QuIC assay for the pathological PrP was negative. Although the long storage time of 13 years might have influenced the results, some studies have shown very stable RT-QuIC results after short- and long-term (8–9 years) storage and after repetitive thawing/freezing cycles [24].
For the relatively short clinical course of 8 months, the brain was severely affected and involved also the frontotemporal regions. This exceeds what would be expected in a CJD VV subtype patient with such a short clinical history, suggesting that this severe neuropathological phenotype most likely represents superimposed CJD pathology on FTLD-TDP pathology. There was a degeneration of white matter tracts including the corticospinal tract, which while occurring in CJD, [25, 26] including its panencephalopathic forms, [27] is also frequently observed in FTLD-ALS.
The distribution of CJD pathology was very severe and a mixed VV2+1 histotype was plausible due to cortical and subcortical involvement, prominent cerebellar degeneration, focal perineuronal and plaque-like deposits, and prominent synaptic PrP pathology, [16, 17] and probably overlapped with FTLD pathology. Indeed, genetic analysis revealed valine homozygosity at PRNP-codon 129 with no mutations. Similarly, the distribution of TDP-43 pathology was consistent with that described in C9orf72-FTLD. This might be indistinguishable from sporadic FTLD-TDP cases, [1, 2, 6] and may follow a type A, type B or a type A/B pattern, as in our case [1, 2, 19]. Typically, DPR and phospho-TDP-43 pathologies do not follow the same distribution pattern [1, 2, 6]. In our case, the co-existence of CJD-PrP and C9orf72-FTLD-TDP-43 did not seem to influence the distribution of each other's pathology, but exacerbated the overall degenerative pathology, which was very severe.
We found no hints suggestive of prion disease in 13 additional C9orf72 mutation carriers and no hints for C9orf72 expansion in 100 additional sCJD. However, whether PrP, DPRs and TDP-43 influence each other and trigger their mutual misfolding and deposition, a mechanism that has been described for other neurodegenerative proteinopathies, remains unclear and merits further investigation [28, 29]. Particularly, it would be of interest to know whether DPR pathology alters the cellular secretion of PrP, inducing a multilaminar synaptic damage and leading to diffuse-synaptic PrP deposits instead of laminar and plaque-like deposits as expected for the VV2 subtype.
In any case, mixed neurodegenerative pathologies are increasingly recognized [30, 31]. Concomitant pathologies have been described in C9orf72 mutation carriers, and in CJD [5, 14, 32-36]. In CJD, their overall frequency or extent, especially of ADNC, do not seem to exceed that of normal aging, [34] but may show an atypical tau distribution in the hippocampus [35-37]. The relationship of prion diseases with tau pathology varies between etiological forms and merits further studies [36, 38]. Recently, the interaction between the cellular prion−protein and amyloid–beta oligomers has arisen interest due to the potential mediation of synaptic toxicity, making PrPc a potential therapeutic target of Aβ pathology [39, 40]. In contrast to other neurodegenerative conditions, [41-46] the relationship with TDP-43 protein is less clear, as postmortem studies have found only single CJD cases with limbic TDP-43 [33, 47, 48]. To our knowledge no cases of co-incidental CJD in C9orf72 expansion mutation carriers have been described to date.
In conclusion, this case broadens the clinicopathological spectrum of C9orf72 mutation and CJD, reinforces the value of thorough neuropathological examination in neurodegenerative diseases, and stresses the usefulness of systematic screening of concomitant proteinopathies including the C9orf72 expansion mutation independently of other neuropathological diagnoses [14]. Finally, it also poses the question of a potential link between these two rare neurodegenerative disorders.
ACKNOWLEDGMENTS
The Austrian Reference Center for Human Prion Diseases (OERPE) is funded by the Austrian Federal Ministry of Social Affairs, Health, Care and Consumer Protection. E.G. holds a grant from “Medizinisch-Wissenschaftlichen Fonds des Burgermeisters der Stadt Wien” (Project No. 18097). We thank Prof. Günther Kleinpeter and Prof. Agnes Wechsler-Fördös for excellent management.
DISCLOSURE OF CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




