Myocardial Dysfunction and Risk of Long COVID in Patients Recovered From Mild and Moderate COVID-19
Funding: The authors received no specific funding for this work.
ABSTRACT
Purpose
Numerous recovered COVID-19 patients exhibit persistent cardiovascular symptoms. However, the degree of myocardial dysfunction and its associated risk factors remain unclear. This study aims to evaluate myocardial dysfunction in recovered patients and pinpoint predictors of persistent cardiovascular symptoms.
Methods
We reviewed the echocardiograms of patients who recovered from mild or moderate COVID-19 and presented with cardiovascular symptoms during the Omicron surge. Myocardial strain was analyzed in 546 patients before and after infection, and in 351 prepandemic healthy controls. Clinical follow-up at 12 months post-infection was used to evaluated symptom persistence, and multivariable logistic regression was used to identify independent predictors.
Results
Baseline characteristics showed no significant differences between patients and controls (all p > 0.05). Although the left ventricle global longitudinal strain (LVGLS) remained stable post-infection, significant reductions emerged in regional left ventricle longitudinal strains (LVLS) and all left atrial strains (LAS) (all p < 0.05). Persistent cardiovascular symptoms affected 16.5% (90/546) of patients at 1-year follow-up. Multivariate analysis showed that only LA conduit strain (OR = 0.919, 95% CI: 0.857, 0.985, p = 0.017) and basal inferoseptal LVLS (OR = 0.883, 95% CI: 0.792, 0.986, p = 0.026) correlated with persisting cardiovascular symptoms.
Conclusion
Our findings demonstrate that subclinical but persistent COVID-19-associated myocardial dysfunction is characterized by regional LVLS impairment and LAS reduction. The identified strain parameters (LAScd and basal inferoseptal LVLS) serve as novel imaging markers for stratifying patients at risk of persistent cardiovascular symptoms. These results advocate for targeted echocardiographic surveillance and early intervention strategies in post-COVID care pathways.
Trial Registration
ClinicalTrials.gov identifier: NCT06170307
1 Introduction
The Omicron variant of SARS-CoV-2 is more transmissible yet less virulent than previous prevalent variants. Clinical manifestations have evolved from pneumonia to upper respiratory tract infection [1]. Although most COVID-19 patients have experienced mild symptoms during the Omicron surge, studies have estimated that 4.5%–36.6% of all COVID-19 patients continue to experience symptoms more than 3 months after infection, a condition referred to as “post-COVID” or “long-COVID” [2]. Concerns exist regarding undetected myocardial injury, especially in patients with persistent symptoms after COVID-19. A novel predictive marker for long—COVID is highly desirable. Such a novel biomarker could be crucial for devising future prevention and treatment strategies.
Two-dimensional speckle tracking echocardiography (2D-STE) has demonstrated excellent consistency and feasibility in the quantitative evaluation of myocardial function, and is comparable to cardiovascular magnetic resonance feature tracking (CMR-FT) [3]. Several studies have demonstrated that COVID-19-induced systemic hyperinflammation significantly impairs cardiovascular function, increasing thrombosis risk and triggering myocarditis and myocardial damage through mechanisms distinct from those of myocardial infarction pathways [4, 5]. Nevertheless, the existing research results on subclinical myocardial injury detected by 2D-STE in COVID-19 patients remain inconclusive [6, 7]. There is a dearth of studies that compare the echocardiographic changes from baseline in recovered COVID-19 patients and explore the factors associated with persistent symptoms [8].
In this observational study, we retrospectively analyzed the serial 2D-STE data of recovered patients presenting with persistent cardiovascular symptoms. The primary objectives of this study are to evaluate the alterations in myocardial function and to identify the risk factors associated with persistent cardiovascular symptoms after recovery.
2 Materials and Methods
2.1 Study Population
Patients who recovered from mild or moderate COVID-19 and presented to our hospital for echocardiography due to cardiovascular symptoms (including palpitations, chest pain, shortness of breath, fatigue, or orthostatic tachycardia) between December 20, 2022 and January 20, 2023 (during the Omicron surge) were retrospectively reviewed. The included patients had undergone baseline echocardiography within 1 year before infection, as identified by the echocardiographic laboratory database. Age-, sex-, and risk factor-matched adults before the COVID-19 epidemic were selected as healthy controls. For risk factors, we considered smoking and drinking history, as well as prevalent comorbidities such as hypertension, diabetes, and dyslipidemia. We ensured similar distributions of these factors between the case and control groups to reduce confounding factors and strengthen the validity of the study.
The inclusion criteria for the post-COVID group were (i) age between 18 and 70 years; (ii) COVID-19 diagnosed by RT-PCR and comprehensive analysis of their epidemiological history and clinical presentation; (iii) mild and moderate COVID-19 according to the Diagnosis and Treatment Protocol of Novel Coronavirus (Version 10) by the National Health Commission of the People's Republic of China [9]: Mild COVID-19 infection was defined as various symptoms but not dyspnea or abnormal chest CT findings; and moderate COVID-19 infection was defined as evidence of lower respiratory system disease either on clinical assessment or on chest CT but no oxygen saturation lower than 94% on room air; (iv) echocardiogram was performed 2–12 weeks after the diagnosis of infection; and (v) baseline echocardiogram within 1 year before infection, which could be indexed through an echocardiographic laboratory database.
We excluded the following patients: (i) those with multiple COVID-19 infections; (ii) patients with cardiotoxic drug history or severe systemic diseases like hyperthyroidism, pulmonary hypertension, severe COPD, malignancy, or renal failure; (iii) autoimmune diseases, morbid obesity, severe hypertension, diabetes with comorbidities; (iv) arrhythmias that could directly cause palpitations or hemodynamic compromise (e.g., atrial fibrillation/flutter, frequent ventricular premature beat, ventricular tachycardia, supraventricular tachycardia, and significant bradycardia), moderate or severe valvular disease, coronary heart disease (≥50% stenosis), cardiomyopathy, or congenital heart disease; and (v) those with poor echocardiogram quality (over 50% sub-optimal views).
2.2 Ethical Approval
The study has been approved by the Ethics Committee of Shandong First Medical University's First Affiliated Hospital (Ethics No. YXLL-KY-2023(136)). Due to the retrospective nature of the study, the need for informed consent from all subjects and/or their legal guardian(s) was waived by the Ethics Committee. Authors had access to information that could identify individual participants during or after data collection. The study was conducted in compliance with the Declaration of Helsinki as well as by local guidelines and regulations.
2.3 Data Collection
All patients underwent comprehensive echocardiographic examination before and after infection using the Philips Epiq7C ultrasound machine equipped with a 1–5 MHz transducer (S5-1), following the American Society of Echocardiography guideline. Left atrial volume (LAV), LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), and LV ejection fraction (LVEF) were measured using the modified biplane Simpson method. Doppler was used to measure the peak early-diastolic (E) and late-diastolic (A) velocity. Tissue Doppler e′ was measured both at the septum and lateral mitral annuli, and the averaged value was used to calculate the E/e′ ratio. M-mode was used to measure tricuspid annular plane systolic excursion (TAPSE). The 2D area change fraction of the right ventricle (RVFAC) was also measured. Body surface area (BSA) = 0.006H + 0.0128W − 0.1529 (H is height in m; W is weight in kg); LAVI = LAV/BSA; LVEDVI = LVEDV/BSA; LVESVI = LVESV/BSA; SI = (LVEDV − LVESV)/BSA.
Offline analysis for strain using TomTec Software (version 2.31). LA strain (LAS) measurements were taken during the reservoir, conduit, and contractile phases of LA, denoted as reservoir function (LASr), conduit function (LAScd), and contractile function (LASct), respectively. LV global longitudinal strain (LVGLS), LV global circumferential strain (LVGCS), and RV global longitudinal strain (RVGLS) were measured. Regional left ventricle longitudinal strain (LVLS) was assessed based on the American Heart Association 17-segment model, and RV-free wall strain (RVFWS) was calculated as the average of the strain of the RV-free wall. LA stiffness index (LASI) was calculated as the E/eʹ ratio to LASr × 100. These were completed by experienced research sonographers who were blinded to the aims of the study.
Simultaneously, clinical data, electrocardiogram (ECG), and high-sensitivity troponin I or troponin I (hs-TnI or TnI) of post-COVID on the day of visit were obtained through the hospital electronic medical record system.
Follow-up was carried out 12 months post-infection. We used a combination of telephone-administered questionnaires and electronic health record reviews. We recorded the following data: (1) persistent cardiovascular symptoms; (2) interval from presentation to the emergency department or hospitalization with cardiovascular symptoms; and (3) any major adverse cardiac events (MACEs) such as myocardial infarction, stroke, revascularization, or death.
2.4 Definition
Abnormal ECG refers to myocardial ischemia manifestations, including transient ST segment deviation, transient T wave change, Wellen's syndrome, and so on. Troponin abnormalities were classified as elevated over the upper reference limit of the 99th percentile.
2.5 Statistical Analysis
Data analysis was performed using SPSS 26.0. A two-tailed p value < 0.05 was considered statistically significant. Continuous data were tested for normal distribution using the Shapiro–Wilk test. Normally distributed variables (mean ± SD) were compared via one-way ANOVA (reported as H value) with Dunn's post-hoc test after Levene's test for variance homogeneity (applied to LV strain, LA, and RV strain). Non-normally distributed variables (median [IQR]) were analyzed by Kruskal–Wallis tests (reported as H value), followed by Dunn's post-hoc tests with Bonferroni adjustment for variables like gender and risk factors. Categorical variables (counts [%]) were compared using Chi-square or Fisher's exact tests for gender and risk factors, with results reported as χ2 values. To ascertain the independent predictors of persistent cardiovascular symptoms, we conducted a multivariable logistic regression analysis, which was performed by using the forward stepwise approach. The multivariable logistic regression included variables with p < 0.05 in the univariable logistic regression analysis. We excluded multicollinearity using a correlation coefficient r > 0.70. The receiver operating characteristic (ROC) area under the curve (AUC) was calculated to assess the predictive discriminatory power for persistent cardiovascular symptoms of strain parameters. The Youden method determined the optimal cutoff value.
2.6 Sample Size Determination
Based on the rule of thumb (Events Per Variable, EPV), considering eight independent variables and an event incidence of 16.5%, the minimum sample size is estimated to be between approximately 485 (assuming 10 events per independent variable) and 970 (assuming 20 events per independent variable).
3 Results
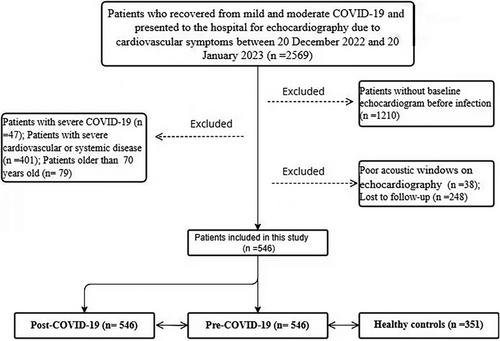
Initially, a retrospective review was conducted on the data of 2569 recovered COVID-19 patients who underwent echocardiography due to cardiovascular symptoms between December 20, 2022 and January 20, 2023. A total of 1210 patients without baseline echocardiograms, 47 with severe COVID-19, 236 with severe cardiovascular disease, 31 with severe arrhythmia, 134 with severe systemic disease, 248 unable to complete follow-up, 79 over 70 years old, and 38 with poor echocardiographic acoustic windows were excluded. After these exclusions, a total of 546 patients (aged 46.29 ± 12.29 years, 39.56% male) were finally included in this study. From the echocardiographic laboratory database, 351 age-matched (48.51 ± 10.37 years), sex-matched (41.88% male), and risk factor-matched pre-COVID adults served as healthy controls. The detailed selection process is presented in the flow chart shown in Figure 1.
3.1 Comparison Between the Post-COVID, Pre-COVID, and Control Groups
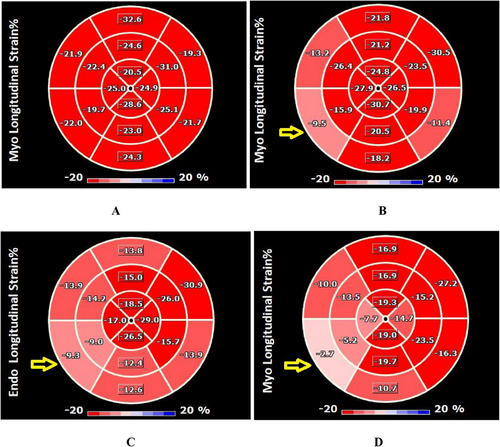
The pre-COVID and control groups showed no significant differences in any of the study variables (p > 0.05). Moreover, no discernible differences were observed in the global systolic function or structural parameters of the LV or RV among the three groups (p > 0.05). However, in the LV segmental analysis, compared with the control group and at baseline, LVLS in the basal and mid inferoseptal segments were lower in the recovered patients. Additionally, the mid anterolateral LVLS in these patients was lower than that in the control group (p < 0.05) (Figure 2). After infection, the LA diastolic function parameters such as E/e', LAVI, and LASI increased. Conversely, the LAS in all three phases (reservoir, conduit, contractile) was lower than those at baseline and in the control group (Table 1).
| Control (n = 351) | Pre-COVID (n = 546) | Post-COVID (n = 546) | F/H/χ2 | p | |
|---|---|---|---|---|---|
| Age (years) | 48.51 ± 10.37 | 45.72 ± 11.94 | 46.29 ± 12.29 | 4.138 | 0.121 |
| Male, n (%) | 147 (41.9%) | — | 216 (39.6%) | — | 0.568 |
| BSA (m2) | 1.72 ± 0.18 | 1.76 ± 0.40 | 1.79 ± 0.23 | 1.784 | 0.170 |
| HR (betas/min) | 75.86 ± 10.72 | 76.56 ± 10.02 | 79.17 ± 13.67 | 2.527 | 0.082 |
| Hypertension, n (%) | 68 (19.37%) | 54 (15.38%) | 89 (16.30%) | 0.379 | 0.825 |
| Diabetes, n (%) | 34 (9.68%) | 19 (3.47%) | 19 (3.47%) | 3.344 | 0.219 |
| Dyslipidemia, n (%) | 15 (4.27%) | 9 (1.65%) | 12 (2.19%) | 1.927 | 0.420 |
| Smoking history, n (%) | 52 (14.81%) | 67 (12.27%) | 66 (12.09%) | 0.530 | 0.821 |
| Alcohol intake, n (%) | 36 (10.25%) | 85 (15.57%) | 80 (14.65%) | 1.642 | 0.450 |
| Echocardiography | |||||
| LVEDVI (mL/m2) | 40.45 ± 16.61 | 46.99 ± 11.03 | 50.22 ± 16.25 | 0.492 | 0.612 |
| LVESVI (mL/m2) | 18.79 ± 6.41 | 17.74 ± 5.96 | 18.81 ± 7.27 | 0.239 | 0.788 |
| LVSI (mL/m2) | 31.85 ± 7.17 | 29.41 ± 6.53 | 32.03 ± 6.7 | 1.753 | 0.176 |
| LVM (g) | 108.59 ± 35.30 | 115 ± 33.80 | 110.71 ± 33.74 | 0.379 | 0.685 |
| LVEF (%) | 62.82 ± 5.80 | 62.44 ± 6.85 | 62.90 ± 6.32 | 0.056 | 0.946 |
| E/A | 0.96 (0.79, 1.23) | 1.10 (0.77, 1.33) | 1.06 (0.77, 1.34) | 0.063 | 0.367 |
| E/e′ | 7.8 (6.68, 9.35) b | 7.68 (6.38, 9.55) b | 8.33 (6, 10.52) a | 7.666 | 0.022 |
| LAVi (mL/m2) | 21.98 (17.7, 27.65) b | 23.33 (19.88, 29.11) b | 25.89 (19.6, 31.76) a | 7.285 | 0.026 |
| TAPSE (mm) | 22.55 ± 3.73 | 22.90 ± 3.64 | 22.61 ± 3.93 | 0.3 | 0.741 |
| RVFAC (%) | 47.82 ± 7.68 | 46.50 ± 8.25 | 48.29 ± 7.58 | 1.183 | 0.308 |
| LV strain | |||||
| Basal anterior (%) | −20.96 ± 5.37 | −19.71 ± 5.50 | −19.30 ± 6.17 | 2.161 | 0.117 |
| Basal anteroseptal (%) | −15.33 ± 5.29 | −14.93 ± 6.43 | −14.62 ± 5.40 | 0.275 | 0.76 |
| Basal inferoseptal (%) | −15.6 ± 5.21b | −15.09 ± 4.21b | −14.00 ± 4.12a | 4.258 | 0.015 |
| Basal inferior (%) | −16.95 ± 5.05 | −16.30 ± 4.96 | −15.30 ± 6.09 | 1.634 | 0.197 |
| Basal inferolateral (%) | −17.54 ± 5.67 | −18.33 ± 5.61 | −16.41 ± 5.47 | 2.000 | 0.137 |
| Basal anterolateral (%) | −22.26 ± 5.53 | −22.56 ± 5.92 | −22.76 ± 6.10 | 0.144 | 0.866 |
| Mid anterior (%) | −20.42 ± 5.79 | −19.42 ± 5.42 | −19.91 ± 5.74 | 1.022 | 0.361 |
| Mid anteroseptal (%) | −20.66 ± 5.09 | −20.15 ± 5.21 | −19.96 ± 5.03 | 0.428 | 0.652 |
| Mid inferoseptal (%) | −19.74 ± 5.06b | −18.97 ± 5.03b | −18.13 ± 4.14a | 3.817 | 0.023 |
| Mid inferior (%) | −20.19 ± 4.74 | −19.39 ± 5.30 | −19.18 ± 5.17 | 1.028 | 0.359 |
| Mid inferolateral (%) | −18.88 ± 5.73 | −19.46 ± 5.42 | −17.51 ± 5.50 | 1.979 | 0.14 |
| Mid anterolateral (%) | −21.14 ± 6.68b | −20.01 ± 6.40 | −18.15 ± 6.03 | 3.364 | 0.036 |
| Apical anterior (%) | −17.28 ± 7.38 | −16.39 ± 7.75 | −17.76 ± 8.37 | 0.708 | 0.493 |
| Apical septal (%) | −19.26 ± 5.61 | −18.38 ± 5.55 | −20.04 ± 4.91 | 1.743 | 0.177 |
| Apical inferior (%) | −22.31 ± 5.82 | −21.71 ± 6.37 | −22.20 ± 7.02 | 0.315 | 0.73 |
| Apical lateral (%) | −20.15 ± 6.25 | −18.68 ± 5.66 | −19.53 ± 5.40 | 2.009 | 0.136 |
| LVGLS (%) | −18.66 ± 3.52 | −18.03 ± 3.24 | −17.91 ± 3.33 | 1.390 | 0.251 |
| LVGCS (%) | −26.55 ± 5.62 | −26.20 ± 5.61 | −28.38 ± 5.72 | 2.396 | 0.093 |
| LA strain | |||||
| LASr (%) | 41.7 ± 9.90b | 39.00 ± 9.24b | 36.28 ± 6.07a | 28.342 | <0.001 |
| LAScd (%) | 27.39 ± 8.64b | 24.80 ± 8.50b | 19.46 ± 5.29a | 17.74 | <0.001 |
| LASct (%) | 18.31 ± 5.77b | 17.82 ± 4.52b | 15.20 ± 5.47a | 10.306 | <0.001 |
| LASI (%) | 0.18 ± 0.07b | 0.21 ± 0.08b | 0.25 ± 0.06a | 16.05 | <0.001 |
| RV strain | |||||
| RVGLS (%) | −21.99 ± 4.07 | −20.95 ± 3.96 | −22.42 ± 5.20 | 2.777 | 0.064 |
| Free (%) | −26.22 ± 5.49 | −25.08 ± 5.70 | −26.09 ± 6.60 | 1.304 | 0.273 |
- Abbreviations: BSA, body surface area; LAScd, left atrial conduit strain; LASI, left atrial stiffness index; LASr, left atrial reservoir strain; LAVI, left atrial volume index; LASct, left atrial contractile strain; LVEDVI, left ventricle end-diastolic volume index; LVEF, left ventricle ejection fraction; LVESVI, left ventricle end-systolic volume index; LVGCS, left ventricle global circumferential strain; LVGLS, left ventricle global longitudinal strain; RVFAC, 2D area change fraction of the right ventricle; RVGLS, right ventricle global longitudinal strain; TAPSE, tricuspid annular plane systolic excursion.
- a p < 0.05, compared with the pre-COVID group
- b p < 0.05, compared with the post-COVID group.
3.2 Subgroup Comparison of Troponin
On the day of echocardiographic evaluation, 67% of the participants (366/546) underwent testing for hs-TnI or TnI. Notably, only 7% of these individuals had abnormal hs-TnI or TnI results. Owing to the disparities in detection techniques, we were precluded from conducting a pooled linear regression analysis. As an alternative, subgroup comparisons were carried out. The findings indicated that, with respect to abnormal and normal hs-TnI or TnI, there was no statistically significant difference in global longitudinal strain or regional strain parameters between the two groups (Table 2).
| Aroponin− | Aroponin+ | p | |
|---|---|---|---|
| Basal anterior (%) | 19.57 ± 5.76 | 19.80 ± 5.23 | 0.824 |
| Basal anteroseptal (%) | 15.01 ± 6.55 | 14.28 ± 4.59 | 0.526 |
| Basal inferoseptal (%) | 13.88 ± 4.23 | 14.52 ± 3.67 | 0.405 |
| Basal inferior (%) | 15.97 ± 5.24 | 16.46 ± 5.29 | 0.611 |
| Basal inferolateral (%) | 18.90 ± 5.63 | 17.15 ± 5.63 | 0.367 |
| Basal anterolateral (%) | 22.58 ± 5.90 | 22.69 ± 6.21 | 0.917 |
| Mid anterior (%) | 19.52 ± 5.67 | 19.59 ± 5.22 | 0.938 |
| Mid anteroseptal (%) | 19.99 ± 5.17 | 20.56 ± 5.12 | 0.556 |
| Mid inferoseptal (%) | 18.21 ± 4.30 | 18.75 ± 4.57 | 0.504 |
| Mid inferior (%) | 19.22 ± 5.29 | 19.80 ± 5.15 | 0.554 |
| Mid inferolateral (%) | 18.98 ± 5.52 | 19.18 ± 5.43 | 0.844 |
| Mid anterolateral (%) | 19.39 ± 6.64 | 20.42 ± 5.03 | 0.376 |
| Apical anterior (%) | 16.92 ± 8.14 | 15.81 ± 6.87 | 0.446 |
| Apical septal (%) | 18.58 ± 5.59 | 19.45 ± 4.87 | 0.387 |
| Apical inferior (%) | 21.83 ± 6.66 | 21.80 ± 5.97 | 0.979 |
| Apical lateral (%) | 18.71 ± 5.59 | 19.53 ± 5.62 | 0.424 |
| LVGLS (%) | 17.92 ± 3.42 | 18.34 ± 2.50 | 0.478 |
| LVGCS (%) | 26.34 ± 5.77 | 28.09 ± 5.23 | 0.096 |
- Abbreviations: LVGCS, left ventricle global circumferential strain; LVGLS, left ventricle global longitudinal strain.
3.3 Follow-Up
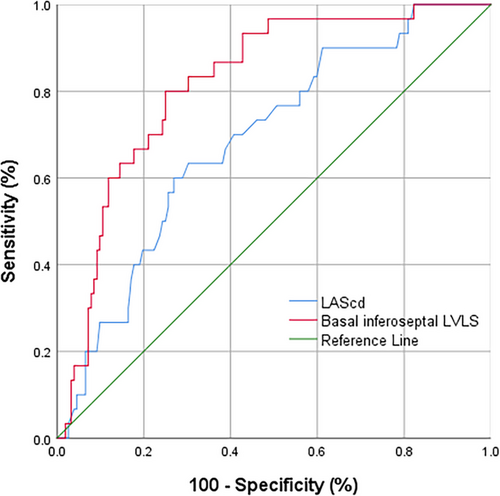
Persistent cardiovascular symptoms were observed in 16.48% of patients, mainly presenting as palpitations (11.72%) and chest pain (7.69%). Forty-eight patients (8.79%) sought medical attention due to these symptoms, with no major adverse cardiovascular events (MACEs) (Table 3). In univariable logistic regression analysis, age, BSA, hypertension, LAV, basal inferoseptal LVLS, LASr, LAScd, and LASI were identified as univariate predictors (p < 0.05). However, in the univariable analysis, a strong correlation was noted between LASr and LAScd (r = 0.77; p < 0.001), as well as between LASr and LASI (r = −0.67; p < 0.001). To prevent overfitting in the multivariable analysis, only LAScd was incorporated. After further adjustment for age, BSA, hypertension, and LAV through stepwise regression, basal inferoseptal LVLS (OR = 0.883, 95% CI: 0.792–0.986, p = 0.026) and LAScd (OR = 0.919, 95% CI: 0.857–0.985, p = 0.017) remained as significant predictors in the multivariate model (Table 4). ROC analysis determined that an LAScd ≤ 24.98% (AUC = 0.70, p = 0.017, sensitivity = 69%; specificity = 64%) and a basal inferoseptal LVLS ≤ −12.56% (AUC = 0.82, p = 0.026, sensitivity = 80%, specificity = 75%) were the optimal thresholds for predicting persistent symptoms (Figure 3).
| Total (n = 546) | |
|---|---|
| Post-COVID duration (week) | 50.24±13.01 |
| Persistent cardiovascular symptoms | 90 (16.48%) |
| Palpitations | 64 (11.72%) |
| Chest pain | 42 (7.69%) |
| Shortness of breath | 33 (6.04%) |
| Tachycardia | 30 (5.49%) |
| Hypotonia | 12 (2.20%) |
| Hospital visit | 48 (8.79%) |
| MACEs | 0 (0%) |
- Abbreviation: MACEs, major adverse cardiac events.
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age (years) | 1.041 (1.006, 1.078) | 0.021 | ||
| Male | 1.022 (0.460, 2.274) | 0.957 | ||
| HR (beats/min) | 0.999 (0.972, 1.026) | 0.934 | ||
| BSA (m2) | 0.579 (0.364, 0.922) | 0.021 | ||
| Hypertension | 0.345 (0.160, 0.973) | 0.044 | ||
| Diabetes | 2.704 (0.637, 11.474) | 0.177 | ||
| Dyslipidemia | 1.713 (0.172, 17.046) | 0.646 | ||
| Smoking | 0.870 (0.289, 2.858) | 0.919 | ||
| Alcohol intake | 6.264 (0.817, 48) | 0.077 | ||
| Aroponin+ | 1.333 (0.473, 3.759) | 0.586 | ||
| Abnormal EGG | 0.952 (0.394, 2.305) | 0.914 | ||
| Pneumonia | 1.952 (0.830, 4.591) | 0.126 | ||
| LVEF (%) | 0.991 (0.945, 1.040) | 0.722 | ||
| E/e’ | 1.121 (0.953, 1.319) | 0.169 | ||
| LAV (mL) | 1.033 (1.006, 1.061) | 0.016 | ||
| LAVI (mL/m2) | 0.993 (0.965, 1.023) | 0.659 | ||
| TAPSE (mm) | 0.955 (0.883, 1.032) | 0.241 | ||
| RVFAC (%) | 1.002 (0.976, 1.028) | 0.902 | ||
| Basal inferoseptal (%) | 0.849 (0.764, 0.943) | 0.002 | 0.883 (0.792, 0.986) | 0.026 |
| Mid inferoseptal (%) | 0.923 (0.839,1.016) | 0.1 | ||
| Mid anterolateral (%) | 0.940 (0.88, 1.005) | 0.068 | ||
| LVGLS (%) | 0.945 (0.834, 1.072) | 0.379 | ||
| LVGCS (%) | 1.017 (0.950, 1.088) | 0.636 | ||
| LASr (%) | 0.906 (0.848, 0.967) | 0.003 | ||
| LAScd (%) | 0.903 (0.845, 0.964) | 0.002 | 0.919 (0.857, 0.985) | 0.017 |
| LASct (%) | 0.994 (0.923, 1.071 | 0.875 | ||
| LASI (%) | 3.735 (1.015, 4.227) | 0.014 | ||
| Free (%) | 1.003 (0.960, 1.048) | 0.9 | ||
- Abbreviations: BSA, body surface area; CI, confidence interval.; LAScd, left atrial conduit strain; LASct, left atrial contractile strain; LASI, left atrial stiffness index; LASr, left atrial reservoir strain; LAVI, left atrial volume index; LVEF, left ventricle ejection fraction; LVGCS, left ventricle global circumferential strain; LVGLS, left ventricle global longitudinal strain; OR, odds ratio; RVFAC, 2D area change fraction of the right ventricle; TAPSE, tricuspid annular plane systolic excursion.
4 Discussion
After the respiratory system, the cardiovascular system is most severely affected by COVID-19 [10]. Myocardial injury related to COVID-19 typically occurs within 4 weeks of infection. Nevertheless, some patients still experience persistent or new cardiovascular symptoms 3 months post-infection. However, whether myocardial dysfunction persists after recovery remains unclear. This study revealed that (i) post-COVID patients had no significant global systolic dysfunction but had a reduction in segmental LVLS in non-coronary artery distributions; (ii) recovered patients presented with reduced left atrial strain and evidence of left ventricular diastolic dysfunction; and (iii) the incidence of persistent cardiovascular symptoms was 16.48%, with no MACEs. Multivariable logistic regression analysis revealed LAScd and basal inferoseptal LVLS as independent predictors of persistent cardiovascular symptoms.
The acute stress caused by COVID-19 can trigger myocardial inflammation either through direct invasion of cardiomyocytes or via indirect damage caused by cytokine storms and inflammatory mediators, thereby affecting cardiac structure and function [11, 12]. Strain analysis via echocardiography strongly correlates with the levels of lymphocytic infiltrates in endomyocardial biopsy (EMB) samples and the amount of edema detected by CMR [13]. 2D-STE, regarded as a “digital biopsy”, is likely to become an important diagnostic tool for myocarditis and other myocardial injuries [14]. Multiple publications have reported on 2D-STE findings following COVID-19 infection: Cannata et al. [6] reported that LVGLS was abnormal in 1/3 of post-COVID patients and was associated with exercise endurance and MACEs. Akbulut et al. [7] noted that at 6 months after infection, there was no significant difference in LVGLS or RVGLS between recovered patients and COVID-19-free matched controls. However, none of those studies compared baseline echocardiographic data to rule out preinfectional abnormalities. In this study, the parameters of COVID-19 patients before infection were not different from those of COVID-19-free controls. Moreover, LVEF, LVGLS, and RVGLS after recovery were not significantly different from those of the control group or before infection, which is consistent with Young's findings [8]. Nevertheless, we found that LVLS of the basal and mid inferoseptal regions were lower in recovered patients than in controls and at baseline. Qiao et al. [15] reported that late gadolinium enhancement in patients with severe COVID patients with persistent cardiovascular symptoms after infection was most common in the interventricular septum, implying that the myocardium of the interventricular septum was earlier or more susceptible to inflammation and systemic stress.
The lack of correlation between troponin elevation and myocardial strain abnormalities may reflect distinct pathophysiological pathways in post-COVID cardiac sequelae: Troponin release primarily indicates cardiomyocyte injury or necrosis, which appears infrequent (7% in our cohort) and potentially transient in long-COVID. Regional strain alterations likely arise from diffuse myocardial inflammation or microvascular dysfunction, processes not directly mirrored by troponin levels. This hypothesis aligns with recent CMR studies demonstrating late gadolinium enhancement-negative myocardial edema in long-COVID patients with preserved troponin [16].
Evaluating LA structure and function can help determine LV diastolic function. In line with our findings, Goerlich et al. [17] reported that compared with healthy individuals, post-COVID patients had elevated LAVI. Hamdy et al. [18] investigated 60 patients with persistent dyspnea 1 month after recovery and reported that the E/e' ratio, which reflects the LV filling pressure, was greater in patients with persistent dyspnea than in controls. Morris et al. [19] studied patients with LV diastolic dysfunction (LVDD) and elevated LV filling pressure and found that only 1/3 of patients had abnormal LAVI, whereas 60% had abnormal LAS, suggesting that LAS is a sensitive marker of early LVDD. LAS includes reservoir, conduit, and contractile strains. Zein Elabdeen et al. [20] reported that symptomatic COVID-19 patients exhibited lower LAS in all three phases (reservoir, conduit, and contractile) compared to asymptomatic patients. Similarly, all LASs of symptomatic patients in this study were lower than those of the controls and at baseline. LASI is a new index reflecting LA compliance derived from the LAS, which is obtained from the E/e ratio and LASr. LASI has been used clinically to distinguish HFrEF from HFpEF [21], and HFpEF from LVDD [22]. In this study, LASI in the post-COVID group was greater than that in the control group and pre-COVID group, indicating that LV diastolic function was impaired in patients recovering from mild or moderate COVID-19 and that LA remodeling was associated with increased stiffness and reduced compliance.
The primary component of LA function is thought to be its reservoir function [23]. LASr has predictive value for HFpEF, atrial fibrillation, and other cardiovascular diseases [24]. However, recent research has shown that LAScd has recently emerged as a powerful method for assessing LV diastolic dysfunction [25, 26]. Additionally, LAScd emerged as the strongest predictor of 5-year all-cause mortality in patients with chronic kidney disease [27], whereas the conduit phase outperformed other LAS parameters in predicting clinical outcomes in patients with aortic stenosis [28]. Thus, the conduit phase should not be viewed as a passive period but rather as a dynamic phase that is strongly modulated by ventricular relaxation as well as atrial and ventricular compliance, affected early and persistently during LA remodeling, and therefore proposed as an early marker of LA dysfunction [29].
The risk of long COVID in severe patients can be estimated using the PASC score (a clinical symptom-based score combined with an antibody signature) or another calculated score [30]. Since 90% of patients were not medically seen or were isolated during acute infection because of a mild or moderate disease course, an exact estimation of risk factors (especially laboratory parameters) for these patients is impossible. By thoroughly evaluating cardiovascular risk factors, troponin, ECG, and echocardiography in patients recovering from mild or moderate COVID-19, we showed that only basal inferoseptal LVLS and LAScd were associated with persistent cardiovascular symptoms in this study. LAScd was the only LAS parameter related to the 6-min walk distance and submaximal exercise capacity in patients with aortic stenosis [28], and it was closely correlated with post-COVID patients’ exercise duration [18]. These findings suggest that LA dysfunction should not be considered an innocent bystander of global cardiac pathology and conduit dysfunction is associated with functional limitations and may reflect an underlying inflammatory process parallel to hemodynamics. Our thresholds for LAScd (24.98%) and basal inferoseptal LVLS (−12.56%) are both higher than the lower limits of normal values (23% for LAScd, −10% for basal inferoseptal) [31, 32], which is consistent with the characteristics of the transitional stage of subclinical injury. Clinicians could use these thresholds to identify high-risk patients for targeted follow-up.
5 Limitations
This was a single-center study with moderate sample size. To verify the validity and generalizability of the results, larger multicenter studies are needed. Owing to the retrospective nature of this investigation, patients self-reported their cardiovascular symptoms 1 year after infection. This could introduce recall bias into the symptom descriptions, and self-selection bias could influence the likelihood that participants would experience symptoms. Although there is no widely available and effective treatment for long COVID, certain medications may be effective for some patients. Because this study did not account for the potential impact of therapy, the results cannot accurately reflect the likelihood of persistent cardiovascular symptoms in all patients. Cardiac biomarker assessment was incomplete: only 67% of participants underwent troponin testing, and the tests were not uniform. Although subgroup analyses revealed no strain-biomarker associations, selection bias cannot be fully excluded.
6 Conclusions
For patients who have recovered from COVID-19, changes in LA structure and function should receive particular attention during routine echocardiography, particularly for the evaluation of LAScd and basal inferoseptal LVLS, which has predictive value for persistent cardiovascular symptoms.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Open Research
Data Availability Statement
The data presented in this study are available from the corresponding author on request.