Increased Afterload in Patients With Acute Reduction in Left Ventricular Ejection Fraction Following Mitral Valve Transcatheter Edge-to-Edge Repair
Funding: The authors received no specific funding for this work.
ABSTRACT
Aim
The mechanisms and clinical importance of acute reduction (ARD) in left ventricular (LV) function following transcatheter edge-to-edge repair (TEER) for mitral regurgitation (MR) remains unclear. This study aimed to non-invasively evaluate the cardio-mechanical parameters, including end-systolic elastance (Ees) and arterial elastance (Ea), to explore their association with ARD following mitral TEER.
Methods and Results
We performed a retrospective analysis of serial transthoracic echocardiography (TTE) studies before and after mitral TEER. Cardio-mechanical parameters were evaluated non-invasively using a modified single-beat method. After the exclusion of nine patients requiring intravenous catecholamine infusion, the study cohort comprised 49 consecutive patients (25 men; mean age: 81 ± 9 years) with successful mitral TEER. ARD in LV function was defined as a decrease in LV ejection fraction (LVEF) of ≥5 points following the procedure by TTE, which was reported in 18 patients. The hospitalization period was longer in patients with ARD in LVEF than in those without ARD (5.5 days vs. 4 days, p = 0.031). Following improvement in MR, Ea increased (1.54 ± 0.49 mmHg/mL vs. 1.84 ± 0.55 mmHg/mL, p = 0.004). Linear regression analysis revealed a correlation between Δtotal stroke volume (SV) and ΔEa (r = 0.614, p < 0.0001). Notably, ΔEa was higher in patients with ARD in LVEF than in those without ARD in LVEF (0.60 ± 0.73 mmHg/mL vs. 0.14 ± 0.39 mmHg/mL, p = 0.006).
Conclusion
ARD in LVEF after mitral TEER was reported in a substantial proportion of patients and may have prognostic implications. Evaluating cardio-mechanical parameters may aid in understanding complex hemodynamics and guiding treatment strategies for patients with MR undergoing TEER.
1 Introduction
Mitral valve (MV) transcatheter edge-to-edge repair (TEER) is a safe and viable alternative to MV surgery in high-risk patients with severe primary and secondary mitral regurgitation (MR) [1-4]. However, acute reduction (ARD) in left ventricular (LV) function may develop in a substantial number of patients undergoing TEER [5-10]. Moreover, recent studies on ARD in LV function after MV TEER emphasized the importance in predicting long-term prognosis [7, 9, 10]. This phenomenon, referred to as “afterload mismatch”, could be attributed to the immediate loss of low-resistance leak into the left atrium, leading to an acute change in LV loading conditions. However, no clear clinical evidence demonstrating the role of “afterload mismatch” in the mechanisms of an ARD in LV function following mitral TEER has not yet been seen.
Invasive ventricular pressure–volume (PV) analysis is the reference method for assessing cardiac mechanics, which can elucidate the pathophysiological mechanisms of various types of heart failure, including myocardial and valvular heart diseases, and is beneficial for monitoring the efficacy of therapeutic interventions [11]. Sunagawa demonstrated that cardiac stroke volume (SV) is determined by the interaction between ventricular end-systolic elastance and arterial elastance [12]. Ventricular end-systolic elastance (Ees), a measure of the contractility of the left ventricle, is defined by the slope of the end-systolic PV relationship and remains unaffected by preload changes. Arterial elastance (Ea) is a reliable index of vascular load and is determined by the compliance, impedance, and resistance of the arterial system [13]. End-systolic pressure corresponds to the maximal ejection pressure because end-systole occurs as LV relaxation begins. As the invasive measurement of Ees and Ea in varying preloads and ventricular end-systolic pressures is challenging in clinical practice [14], real-world data on these parameters are scarce.
To address the challenges of invasive measurements, simplified single-beat methods were developed by Chen et al. to estimate Ees, Ea, and ventricular-arterial coupling (VAC) values [15]. Using these methods, this study aimed to evaluate hemodynamic and cardio-mechanical parameters noninvasively and investigate the mechanisms of an ARD in LV function following MV TEER.
2 Methods
We retrospectively analyzed consecutive patients who had undergone TEER for MR at the Cardiology Division, Department of Internal Medicine, Tokai University School of Medicine Hospital (Isehara, Kanagawa Prefecture, Japan) between April 2018 and July 2024. Patients who had undergone serial transthoracic echocardiography (TTE) examinations before and immediately after the TEER procedure within 3 days were included. Patients with unsuccessful TEER due to technique or device-related issues, intravenous catecholamine infusion or cardiopulmonary supportive devices during TTE, lack of follow-up data 6 months after the procedure, or poor TTE echogenicity were excluded. The Institutional Review Board for Clinical Research of Tokai University School of Medicine approved this observational study protocol. The study was conducted in accordance with the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all the patients upon admission.
Clinical data, including predisposing factors, medications, and mid-term all-cause mortality following TEER, were obtained from medical records. TEER for MR was performed using the MitraClip system, guided by two-dimensional (2D) and three-dimensional (3D) transesophageal echocardiography (TEE), along with fluoroscopy [1]. The predefined procedural endpoint required the successful implantation of at least one clip, a reduction in severity of MR to ≤ 2+, as assessed by visual evaluation on intraprocedural TEE, and no residual V wave in the pulmonary artery wedge pressure waveform, as evaluated by right heart catheterization at the end of the procedure. Technical and device success was based on the Mitral Valve Academic Research Consortium (MVARC) criteria [16]. Technical success was defined by the following criteria: (1) the absence of procedural death; (2) successful access, delivery, and retrieval of the device delivery system; (3) successful deployment and correct positioning of the first intended device; and (4) freedom from emergency surgery or reintervention related to the device or access procedure. Device success was defined as: (1) the absence of procedural death or stroke; (2) proper placement and positioning of the device; (3) freedom from unplanned surgical or interventional procedures related to the device or access procedure; and (4) continued safety and performance of the device as intended.
All TTE images acquired at our institution were transferred via an institutional wireless network to a moving picture server in the Medical Ultrasound Laboratory (PrimeVitaPlus, Nihon Kohden Corp., Shinjyuku-ku, Japan). Therefore, study reviews and conventional measurements were performed in the medical ultrasound laboratory following the current TTE guidelines [17-20] and interpreted by cardiologists. LV and left atrial volumes were assessed using biplane disk summation [19]. MR severity was graded using a multiparametric approach according to current guidelines, including qualitative, semiquantitative, and quantitative parameters [20]. MV morphology served as a qualitative parameter. The presence of systolic pulmonary vein flow reversal usually indicates severe MR. The vena contracta cross-sectional area and effective regurgitant orifice area according to the proximal isovelocity surface area method are quantitative parameters with thresholds for severe MR (4+) of effective regurgitant orifice area ≥40 mm2. The total SV was calculated as the difference between the LV end-systolic volume and LV end-diastolic volume using biplane disk summation. Forward SV was measured using quantitative pulsed Doppler imaging of the LV outflow tract in the absence of severe aortic regurgitation. In patients with severe aortic regurgitation, the right ventricular outflow tract was used. The SV and cardiac output were indexed by dividing the body surface area of the patients. Changes in TTE and cardiac mechanics parameters were calculated as the difference between the baseline value and post-procedure value (Δvalue). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) measured using the brachial cuff method within 1 h prior to the TTE were obtained from the medical records. Based on the presence or absence of ARD in LV function, patients were divided into two subgroups. ARD in LV function was defined as a decrease in LV ejection fraction (LVEF) of ≥5 points, which corresponds to the value of the standard deviation in LVEF measurements [17].
A simplified PV loop for each subgroup before and after TEER was illustrated non-invasively using the averaged values of the obtained parameters, as previously described [21].
Numerical data are presented as either means ± standard deviations or medians and ranges. Categorical variables are presented as numbers (%). Continuous variables between two unpaired groups were compared using the unpaired Student's t-test or Wilcoxon rank-sum test, depending on the normality of the distribution. Continuous variables between the two paired groups were compared using a paired Student's t-test. The differences in categorical variables between the two groups were analyzed using the chi-square test. Linear regression analysis was performed to assess significant correlations between the variables. All statistical analyses were performed using JMP 14.0.0 (SAS Institute, Cary, NC, USA), and p values of <0.05 indicated statistical significance.
3 Results
A total of 65 consecutive patients with MR who had undergone successful MV TEER during the study period were enrolled. Following the exclusion of 16 patients because of intravenous catecholamine infusion during TTE (n = 9), lack of available follow-up data (n = 4), and poor echogenicity (n = 3), 49 (25 men; mean age, 81 ± 9 years) were included (Table 1). Among them, ARD in LVEF was found in 18 patients (37%). No differences in baseline characteristics were observed between patients with and without ARD in LVEF. There were 30 patients with degenerative MR (primarily MR) and 19 patients with functional MR (secondary MR). The frequency of degenerative MR was not significantly different between patients with and without ARD in LVEF (78% [14/18] vs. 52% [16/30], p = 0.070). The median duration between TEER and post-TEER TTE was 1 day (interquartile range [IQR]: 1, 2). The median hospitalization period was longer in patients with ARD than in those without ARD (5.5 vs. 4 days, p = 0.031). Midterm mortality after TEER was not significantly different between the two groups (6% vs. 0%, p = 0.185). There was one patient with heart failure rehospitalization in those patients without ARD in LVEF (3%, 1/31), while there was no such patient in those with ARD in LVEF (0%, 0/18), which was not significantly different (p = 0.441).
| Reduction of LVEF ≥ 5% | ||||
|---|---|---|---|---|
| All (n = 49) | absent (n = 31) | present (n = 18) | p value | |
| Sex, Male, n (%) | 25 (51) | 12 (50) | 11 (69) | 0.240 |
| Age, year | 81 ± 9 | 79 ± 9 | 84 ± 6 | 0.064 |
| Height, cm | 157 ± 10 | 158 ± 12 | 156 ± 8 | 0.542 |
| Weight, Kg | 53 ± 8 | 53 ± 9 | 51 ± 7 | 0.436 |
| Body surface area, m2 | 1.50 ± 0.16 | 1.52 ± 0.19 | 1.48 ± 0.11 | 0.430 |
| Hypertension, n (%) | 18 (37) | 12 (39) | 6 (33) | 0.707 |
| Diabetes Miletus, n (%) | 6 (12) | 4 (13) | 2 (11) | 0.854 |
| Dyslipidemia, n (%) | 17 (35) | 13 (42) | 4 (22) | 0.162 |
| Atrial fibrillation, n (%) | 19 (39) | 12 (39) | 7 (39) | 0.990 |
| Pacemaker implantation, n (%) | 4 (8) | 2 (7) | 2 (11) | 0.567 |
| Coronary artery disease, n (%) | 11 (23) | 8 (26) | 3 (17) | 0.460 |
| Aortic valve prosthesis, n (%) | 3 (6) | 3 (10) | 0 (0) | 0.173 |
| Chronic pulmonary disease, n (%) | 2 (4) | 2 (6) | 0 (0) | 0.271 |
| Chronic kidney disease, n (%) | 15 (31) | 11 (35) | 4 (22) | 0.332 |
| Chronic heart failure, n (%) | 49 (100) | 31 (100) | 18 (100) | 1.00 |
| Peripheral artery disease, n (%) | 3 (6) | 2 (7) | 1 (6) | 0.890 |
| Cerebral vascular disease, n (%) | 3 (6) | 2 (7) | 1 (6) | 0.890 |
| ACEI or ARB, n (%) | 24 (49) | 14 (45) | 10 (56) | 0.483 |
| CCB, n (%) | 2 (4) | 1 (3) | 1 (6) | 0.691 |
| β-blocker, n (%) | 26 (53) | 18 (58) | 8 (44) | 0.357 |
| ARNI, n (%) | 16 (33) | 11 (36) | 5 (28) | 0.579 |
| SGLT2 inhibitor, n (%) | 26 (53) | 18 (58) | 8 (44) | 0.357 |
| MRA, n (%) | 25 (51) | 13 (42) | 12 (66) | 0.074 |
| Loop diuretics, n (%) | 34 (69) | 20 (65) | 14 (78) | 0.332 |
| Degenerative mitral valve pathology, n (%) | 30 (61) | 16 (52) | 14 (78) | 0.070 |
| Median hospitalization period, (IQR) | 4 (3.5–8) | 4 (3–7) | 5.5 (4–12.25) | 0.031 |
| Mortality in 6 months after the procedure, n (%) | 1 (2) | 0 (0) | 1 (6) | 0.185 |
- Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; CCB, calcium channel blocker; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; SGLT2, sodium-glucose cotransporter-2.
The changes in TTE parameters and cardiac mechanics before and after TEER are summarized in Table 2. Following improvement in MR after TEER, forward SV index and forward cardiac index improved (30 ± 10 mL/min/m2 vs. 35 ± 12 mL/min/m2, p = 0.035; 2.12 ± 0.65 L/min/m2 vs. 2.66 ± 1.03 L/min/m2 p = 0.003; respectively), whereas total SV was decreased (72 ± 21 mL vs. 58 ± 116 mL, p < 0.001). Additionally, Ea increased (1.54 ± 0.49 mmHg/mL vs. 1.84 ± 0.55 mmHg/mL, p = 0.004).
| Baseline | Post procedure | p value | |
|---|---|---|---|
| Systolic blood pressure, mmHg | 114 ± 20 | 111 ± 17 | 0.469 |
| Heart rate, bpm | 74 ± 16 | 78 ± 18 | 0.238 |
| Mitral regurgitation index, point | 3.1 ± 0.8 | 1.2 ± 0.7 | <0.0001 |
| Mitral regurgitant volume, mL/beat | 49 ± 19 | NA | NA |
| Effective regurgitant orifice area, mm2 | 34 ± 15 | NA | NA |
| Effective regurgitant orifice area ≥40 mm2, n (%) | 14 (29) | NA | NA |
| Mean mitral valve pressure gradient, mmHg | 1.5 ± 1.0 | 2.9 ± 1.6 | <0.0001 |
| LA volume index, mL/m2 | 93 ± 65 | 89 ± 62 | 0.761 |
| LV end-diastolic volume, mL | 142 ± 74 | 124 ± 71 | 0.226 |
| LV end-diastolic volume index, mL/m2 | 93 ± 44 | 82 ± 42 | 0.174 |
| LVEF, % | 57 ± 18 | 54 ± 18 | 0.436 |
| Total stroke volume, mL | 72 ± 21 | 58 ± 16 | <0.001 |
| Forward stroke volume index, mL/m2 | 30 ± 10 | 35 ± 12 | 0.035 |
| Forward cardiac index, L/min/m2 | 2.12 ± 0.65 | 2.66 ± 1.03 | 0.003 |
| LV mass index, g/m2 | 133 ± 41 | 124 ± 40 | 0.287 |
| Relative wall thickness | 0.35 ± 0.09 | 0.38 ± 0.10 | 0.211 |
| Tricuspid regurgitation pressure gradient, mmHg | 31 ± 14 | 30 ± 10 | 0.524 |
| s’, cm/s | 5.4 ± 2.0 | 5.4 ± 1.7 | 0.974 |
| Right atrial pressure, mmHg | 3.6 ± 1.7 | 3.1 ± 0.7 | 0.051 |
| Pes, mmHg | 103 ± 18 | 100 ± 15 | 0.464 |
| Ees, mmHg/mL | 2.04 ± 1.10 | 2.19 ± 1.28 | 0.540 |
| Ea, mmHg/mL | 1.54 ± 0.49 | 1.84 ± 0.55 | 0.004 |
| VAC (Ea/Ees) | 0.94 ± 0.57 | 1.07 ± 0.56 | 0.282 |
| Vo, mL | 6 ± 50 | 5 ± 51 | 0.973 |
- Note: Bold texts are indicating statistically significant.
- Abbreviations: Ea, effective arterial elastance; Ees, end-systolic elastance; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; NA, not applicable; Pes, end-systolic pressure; VAC, ventricular arterial coupling.
As shown in Figure 1, linear regression analyses revealed significant correlations between ΔLVEF and Δtotal SV, and ΔLVEF and ΔEa (r = 0.601, p<0.0001; r = 0.550, p<0.0001; respectively); however, ΔLVEF and ΔEes were not correlated (p = 0.789). In contrast, Δtotal SV and ΔEa were strongly correlated (r = 0.614, p < 0.0001).
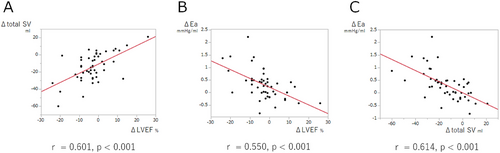
Comparisons of baseline values and their changes (Δvalue) between patients with and without ARD in LVEF are summarized in Table 3. Although baseline parameters were comparable between the two groups, Δtotal SV was larger in patients with ARD in LVEF than in those without ARD in LVEF (−21 ± 17 mL vs. −10 ± 14 mL, p = 0.011). Moreover, ΔEa was higher in patients with ARD in LVEF than in patients without ARD in LVEF (0.60 ± 0.73 mmHg/mL vs. 0.14 ± 0.39 mmHg/mL, p = 0.006). However, Δtotal SV and ΔEa were not significantly different between patients with ARD in LVEF and patients without ARD in LVEF (−21 ± 20 mL vs. −13 ± 15 mL, p = 0.238; 0.45 ± 0.66 mmHg/mL vs. 0.15 ± 0.46 mmHg/mL, p = 0.161; respectively) probably due to small sample size, when limited to patients in a subgroup of degenerative MR (n = 30), as shown in Supplemental Table 1.
| Reduction of LVEF ≥ 5% | |||||
|---|---|---|---|---|---|
| Absent (n = 31) | Present (n = 18) | p value | Odds ratio | 95% confidence interval | |
| Systolic blood pressure, mmHg | 115 ± 21 | 112 ± 18 | 0.516 | ||
| Heart rate, bpm | 74 ± 16 | 76 ± 16 | 0.808 | ||
| Mitral regurgitation index, point | 2.9 ± 0.7 | 3.3 ± 0.8 | 0.123 | ||
| Effective regurgitant orifice area, mm2 | 32 ± 12 | 38 ± 19 | 0.209 | ||
| LV end-diastolic volume, mL | 152 ± 84 | 124 ± 49 | 0.194 | ||
| LV end-diastolic volume index, mL/m2 | 99 ± 49 | 84 ± 33 | 0.244 | ||
| LVEF, % | 53 ± 18 | 63 ± 15 | 0.072 | 1.035 | 0.998–1.079 |
| Total stroke volume, mL | 71 ± 22 | 73 ± 20 | 0.723 | ||
| Forward stroke volume index, mL/m2 | 33 ± 13 | 29 ± 12 | 0.324 | ||
| Forward cardiac index, L/min/m2 | 2.30 ± 0.82 | 2.04 ± 0.61 | 0.251 | ||
| s’, cm/s | 5.3 ± 2.7 | 5.6 ± 1.5 | 0.597 | ||
| Pes, mmHg | 104 ± 19 | 101 ± 16 | 0.557 | ||
| Ees, mmHg/mL | 1.98 ± 1.15 | 2.15 ± 1.04 | 0.619 | ||
| Ea, mmHg/mL | 1.58 ± 0.48 | 1.47 ± 0.51 | 0.475 | ||
| VAC (Ea/Ees) | 1.01 ± 0.68 | 0.77 ± 0.20 | 0.065 | 0.218 | 0.038–1.244 |
| V0, mL | 11 ± 56 | 3 ± 35 | 0.363 | ||
| Δ Systolic blood pressure, mmHg | −4 ± 22 | 1 ± 21 | 0.453 | ||
| Δ Heart rate, bpm | 5 ± 13 | 2 ± 13 | 0.518 | ||
| Δ LV end-diastolic volume, mL | −19 ± 21 | −16 ± 24 | 0.660 | ||
| Δ LV end-diastolic volume index, mL/m2 | −13 ± 14 | −11 ± 16 | 0.620 | ||
| Δ Total stroke volume, mL | −10 ± 14 | −21 ± 17 | 0.011 | 0.949 | 0.904–0.988 |
| Δ Forward stroke volume index, mL/m2 | 5 ± 10 | 6 ± 13 | 0.645 | ||
| Δ Forward cardiac index, L/min/m2 | 0.59 ± 0.95 | 0.44 ± 0.92 | 0.990 | ||
| Δs’ | −0.03 ± 2.4 | −0.17 ± 1.9 | 0.764 | ||
| Δ Pes, mmHg | −4 ± 20 | 1 ± 19 | 0.451 | ||
| Δ Ees, mmHg/mL | 0.19 ± 1.17 | 0.25 ± 1.52 | 0.882 | ||
| Δ Ea, mmHg/mL | 0.14 ± 0.39 | 0.60 ± 0.73 | 0.006 | 5.086 | 1.573–22.359 |
| Δ VAC (Ea/Ees) | 0.05 ± 0.46 | 0.24 ± 0.40 | 0.166 | ||
| Δ V0, mL | −6 ± 38 | 9 ± 20 | 0.137 | ||
- Abbreviations: Ea, effective arterial elastance; Ees, end-systolic elastance; LV, left ventricular; LVEF, left ventricular ejection fraction; Pes, end-systolic pressure; VAC, ventricular arterial coupling.
Simplified PV loops before and after TEER are illustrated in Figure 2A. The Ea values before and after TEER are shown in Figure 2B. By TEER, Ea was unchanged from 1.58 ± 0.48 mmHg/mL to 1.72 ± 0.48 mmHg/mL in patients without ARD in LVEF (p = 0.254), while Ea was increased from 1.47 ± 0.51 mmHg/mL to 2.07 ± 0.60 mmHg/mL in patients with ARD in LVEF (p = 0.003).
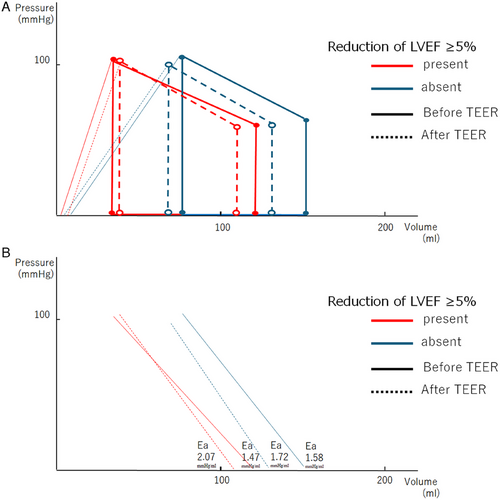
4 Discussion
In this study, hemodynamic and cardio-mechanical parameters associated with ARD in LVEF following successful MV TEER were assessed using non-invasive methods. The main findings are as follows: (1) ARD in LVEF occurred in a substantial proportion of patients (37%, 18/49) after successful TEER for MR; (2) PV loop analysis was applicable in all patients both with and without ARD in LVEF, which revealed that MV TEER led to increased Ea and unchanged Ees. Moreover, both Δtotal SV and ΔEa demonstrated a significant correlation with ΔLVEF; (3) ARD in LVEF was associated with a decrease in total SV and an increase in Ea after TEER; and (4) ARD in LVEF was associated with short-term outcomes such as longer hospital stays after the procedure.
ARD in LVEF after TEER for MR has been widely reported, with incident rates ranging between 10% and 67%, depending on the criteria for ARD in LVEF (1%–28%) [5-10]. In this study, we defined ARD in LVEF as a reduction of ≥5 points, and the observed incident rate was 37%, which aligned with the findings of previous studies. Moreover, we adopted this definition from the value of standard deviation in LVEF assessments. Therefore, we believe that our criteria for ARD in patients with low LVEF are appropriate.
Studies evaluating cardiomechanics with non-invasive PV loop analysis in MV TEER are scarce [21, 22], which reported no significant differences in Ees, Ea, and VAC before and after a successful TEER procedure. However, our findings indicate that Ea increased and Ees and VAC remained unchanged after MV TEER. Additionally, total SV was reduced following TEER, which corroborated the findings of a previous study [20]. Moreover, ΔEa and Δtotal SV were significantly correlated. Imasaka et al. reported a significant increase in Ea after MV surgery for severe MR [23]. Therefore, we postulated that the changes in Ea following treatment may be influenced by the magnitude of the reduction in total SV.
The mechanisms underlying ARD in LVEF after mitral TEER remain unclear, and existing evidence describing this phenomenon is limited [5-10]. ARD in LVEF has been more commonly observed in patients undergoing MV surgery for MR [24, 25]. Predictors of ARD in LVEF after the procedure include a large left ventricle [5], low LVEF [9, 10], large left atrial pressure V-wave [9], severe MR [10], and global longitudinal strain rate [25]. Given the smaller sample size, our study did not identify any baseline parameters predicting ARD in LVEF. However, our findings demonstrated significant associations between a decrease in total SV and ARD in LVEF and an increase in Ea and ARD in LVEF after TEER. This novel finding suggests that in patients with large left ventricles, impaired LV function, and severe MR, abruptly increased LV afterload following treatment may pose a substantial reduction in total SV, resulting in ARD in LV function. This further supported the role of “afterload mismatch” in the mechanisms of an ARD in LV function following MV TEER.
Recent studies on ARD in LVEF after MV TEER emphasized the importance of ARD in LVEF in predicting long-term prognosis [7, 9, 10]. Our findings revealed no differences in all-cause mortality in the midterm, which might be inconclusive due to the smaller sample size. However, our findings demonstrated the short-term prognostic value of hospital stay after TEER, another novel finding. Therefore, close monitoring and tailored medical care are recommended for patients exhibiting ARD in LVEF shortly after mitral TEER to optimize recovery and mitigate short-term complications.
This study had several limitations. First, the sample size in our cohort was relatively small, and the data obtained from a single-center clinical practice limited the volume of prognostic information and statistical power. Therefore, the results may not provide strong evidence to support our conclusions. Moreover, complete subgroup analyses, especially regarding the etiology of MR, could not be accomplished. Second, due to the retrograde fashioned study design, some crucial information, such as the precise value of systemic blood pressure at the time of TTE, was not able to be obtained from the medical records. This may somewhat involve inaccuracy in the results. Third, the possibility of a potential selection bias in patient enrollment, which limits the generalizability of our findings. Fourth, we cannot exclude possible influences of sedation during the procedure on peri-procedural hemodynamics and cardiac function. Despite these limitations, we believe that our data can be useful for generating hypotheses and guiding the development of larger multicenter studies.
In conclusion, the hemodynamic and cardio-mechanical parameters associated with ARD in LVEF following successful MV TEER were assessed. A substantial proportion of patients exhibited ARD in LVEF after TEER, which may have prognostic implications. In such circumstances, simplified single-beat PV loop analysis is a valuable tool, and evaluating Ees, Ea, and VAC may provide critical insights into the complex hemodynamics and clinical management of patients with MR undergoing TEER. However, further clinical investigations are warranted to validate these results.
Acknowledgments
The authors have nothing to report.
Ethics Statement
The study protocol was approved by the Institutional Review Board for Clinical Research of Tokai University School of Medicine. The study was conducted in accordance with the principles outlined in the Declaration of Helsinki.
Conflicts of Interest
Dr. Ohno has served as a proctor for Medtronic. Dr. Kamioka has served as a proctor for Edwards Life Sciences. The other authors have no potential conflicts of interest to declare.
Open Research
Data Availability Statement
The datasets generated and analyzed in this study are not publicly available because of patient privacy concerns.