Efficient CRISPR/Cas9-mediated knockin of reporter genes in rats at ROSA26 by pronuclear microinjection
Takaya Abe and Ken-ichi Inoue contributed equally to this work.
Abstract
The genetic modification of rats is a key technology for advancing biomedical research on human diseases. CRISPR/Cas9-mediated genome editing enables the generation of knockout rats in a single step, without the need for embryonic stem cells, by directly injecting genome editing components into zygotes. This simplifies the process, reduces costs, and accelerates gene function analysis in rats. However, the insertion of a gene cassette into a target site has remained inefficient, limiting the generation of knockin (KI) rats. To overcome this issue, we developed an optimized method that covers the entire process from zygote harvesting with superovulation to timed microinjection, ensuring the consistent generation of KI rats. We successfully generated four different fluorescent reporter lines at the ROSA26 locus in rats. Our study provides detailed, step-by-step protocols for donor vector design, zygote collection, microinjection, founder screening, and cryopreservation in rats.
1 INTRODUCTION
Genetic engineering of the rat genome is a critical technology in biomedical research for investigating the functions of genes in human diseases because rats have many advantages in physiological, toxicological, and pharmacological examinations compared with mice. However, the genetic approaches available for rats are largely behind those available for mice. In 2008, a method to establish rat embryonic stem cells (ESCs) was reported (Buehr et al., 2008; Li et al., 2008), and more than 20 years after it was developed for mice, the gene targeting technology via ESCs was established in rats (Kobayashi et al., 2012; Tong et al., 2010). Gene targeting in rat ESCs is known to be inefficient and has not become a practical method as it has in mice. At about the same time, genome editing technologies using ZFN (zinc finger nuclease) and TALEN (transcriptional activator-like effector protein and FokI nuclease) have been developed in rats (Geurts et al., 2009; Tesson et al., 2011). However, those technologies are still intractable because nucleases need to be generated for each target genomic sequence, which is laborious. A few years later, CRISPR/Cas9-mediated genome editing was established in various organisms including rats (Ma et al., 2014). Initially, a significant challenge in genome editing was that large DNA donor knockin (KI) was generally inefficient. To overcome that issue, we previously reported an efficient CRISPR-mediated KI method in mice. In that method, KI efficiency was improved by the microinjection of a CRISPR cocktail consisting of a gRNA-Cas9 protein complex and a donor vector during the pronuclear stage 3–4 zygotes (Abe et al., 2020). On the other hand, microinjection at a later stage dramatically decreases KI efficiency. We found that the time duration from microinjection to the nuclear envelope breakdown (NEB), which causes the diffusion of the CRIPSR cocktail, is the key to successful KI.
In this report, we describe modifications of the CRISPR-mediated KI method in rats that achieves a high-efficiency generation of four different fluorescent reporter lines at the ROSA26 locus, along with methods for donor vector design, zygote collection, microinjection, founder rat screening, and cryopreservation.
2 MATERIALS AND METHODS
2.1 The ROSA26 KI vector and gRNA
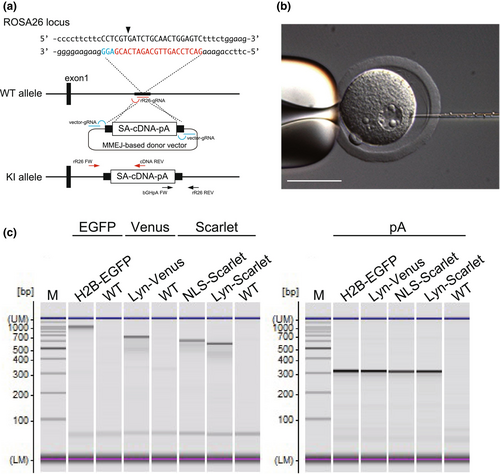
The ROSA26 locus in mice is well-known as a safe harbor locus for the ubiquitous expression of inserted genes. Mice with reporter genes at the ROSA26 locus are extensively used as powerful tools in mouse genetics, such as in vivo live imaging (Abe & Fujimori, 2013). The rat orthologue of the mouse ROSA26 locus has been identified, where a reporter gene can be ubiquitously expressed under the machinery of an endogenous non-coding RNA promoter (Kobayashi et al., 2012). We have previously demonstrated the generation of KI mice via two types of KI methods, the microhomology-mediated end joining (MMEJ)-based method and the homologous recombination (HR)-based method (Abe et al., 2020). In the MMEJ-based method, the donor vector and the gRNA site were designed to express a fluorescent reporter at the locus as a practical example. In the MMEJ-based KI method, three double-strand breaks (DSBs) are induced by CRISPR/Cas9: one at the genomic target site and two on the donor vector. The donor vector carries microhomology arms (40 bp) flanking the KI cassette, with two gRNA target sites at the ends of the microhomology arms. After the DSBs are induced, microhomology ends are created, followed by insertion of the gene cassette into the target site through the DNA repair machinery (Sakuma et al., 2016). We also attempted to use the HR-based method, which is known to be one of the standard methods for inserting a gene cassette into the genome.
2.1.1 Donor vectors
The MMEJ-based donor vector was designed to insert a gene cassette into intron 1 of the non-coding RNA at ROSA26. A DNA fragment consisting of the microhomology arms and the gRNA sites of the donor vector was synthesized (ThermoFisher Scientific, USA) and cloned into the KpnI and SacI sites of pBluescript SKII. The sequences of the microhomology arms and the vector gRNA site are as follows: 5′-arm (5′-GCA TCG TAC GCG TAC GTG TTT GGC CCT GGG CCT GGA AGA TTC CCT TCC CCC TTC TTC CCT CGT-3′), 3′-arm (5′-GAT CTG CAA CTG GAG TCT TTC TGG AAG ATA GGC GGG AGT CCC AAA CAC GTA CGC GTA CGA TGC-3′) (Figure S1). The gRNA site is indicated by underlines. To construct the donor vectors using Gateway technology, an adenovirus splicing acceptor (SA), the reading frame cassette A from the Gateway conversion system (ThermoFisher Scientific), and a bovine growth hormone polyadenylation sequence (bpA) were inserted between the microhomology arms (prR26-SA-bpA-DEST).
An HR-based donor vector was also designed. The sequences of the homology arms (263 bp and 287 bp) were cloned in pBluescript SKII (Figure S1), and the DNA fragment consisting of SA, loxP-Neo-tpA-loxP, the reading frame cassette A, and bpA obtained from pROSA26-STOP-DEST (Abe et al., 2011) was inserted between the homology arms (prR26-STOP-DEST).
The cDNA fragments of H2B-EGFP and Lyn-Venus were obtained from pcDNA3-H2B-EGFP and pcDNA3-Lyn-Venus, respectively (Abe et al., 2011). The Venus plasmid was a gift from Dr. Atsushi Miyawaki (Nagai et al., 2002). The pmScarlet-i C1 was a gift from Dr. Dorus Gadella (Addgene plasmid#85044) (Bindels et al., 2017) and was used for the generation of the NLS (SV40 nuclear localization signal)-Scarlet-i and the Lyn-Scarlet-i. The cDNAs of the fluorescent reporters were cloned into the pENTR2B vector (ThermoFisher Scientific) and then recloned into the prR26-SA-bpA-DEST (MMEJ) or prR26-STOP-DEST (HR) by the LR reaction of the Gateway system to generate the donor vectors.
The donor vectors were purified with an Endotoxin-free plasmid DNA isolation kit (TaKaRa, Japan, NucleoBond Xtra Mid EF, U0420) for microinjection. The purified vector (2 μg/μL) was stored at −20°C until used.
2.1.2 Guide RNA
The gRNA site (5′-GAC TCC AGT TGC AGA TCA CG-3′) at ROSA26 has been used for genome editing (Yoshimi et al., 2016). The genomic sequence of the gRNA site was confirmed by polymerase chain reaction (PCR) and Sanger sequencing using Wistar rat genomic DNA. The primers used are as follows: rR26 FW (5′-TGA GTT GTG GCA CTG AGG AAC GTG C-3′) and rR26 REV (5′-GCT GCA TAA AAC CCC AGG TGA GTG C-3′) (509 bp). rR26-crRNA (5′-GAC UCC AGU UGC AGA UCA CGg uuu uag agc uau gcu guu uug-3′), PITCh-crRNA3 (5′-GCA UCG UAC GCG UAC GUG UUg uuu uag agc uau gcu guu uug-3′) (Sakuma et al., 2016), and tracrRNA (5′-AAA CAG CAU AGC AAG UUA AAA UAA GGC UAG UCC GUU AUC AAC UUG AAA AAG UGG CAC CGA GUC GGU GCU-3′) were chemically synthesized (FASMAC Inc., Japan). crRNA (500 ng/μL) and tracrRNA (1 μg/μL) were diluted in RNase-free water and stored at −80°C until used.
2.2 Genome editing in zygotes
Genome editing was performed by microinjection of a CRISPR cocktail consisting of the gRNA-Cas9 protein complex and the donor vector prepared as described in section 2.1 into the pronuclei of Wistar rat zygotes.
2.2.1 Animals
Wistar rats, purchased from the Jackson Laboratory Japan, Inc., were maintained on a 12-hour light/dark cycle (light on 7:00 a.m./light off 7:00 p.m.) and provided with food and water ad libitum in a specific pathogen-free animal facility. All animal experiments were approved by the Institutional Animal Care and Use Committee of RIKEN Kobe Branch.
2.2.2 Zygote collection
Three female rats were generally used to collect pronuclear stage zygotes for each microinjection round. Sensitivity to hormones is known to vary widely among rat strains, and effective reproductive treatment must be optimized for each strain. We optimized the dosages and timing of hormone administration for the Wistar strain rats. The practical schedule for zygote collection and the protocol from hormone administration to zygote collection is described in this section.
- 1000 IUpregnant mare's serum gonadotropin (PMSG) (ASKA Pharmaceutical, Japan) stock solution.
- 1000 IUhuman chorionic gonadotropin (hCG) (ASKA Pharmaceutical) stock solution.
- Female Wistar rats: 10 weeks old (The Jackson Laboratory Japan, Inc.)
- Male Wistar rats: from 12 to 48 weeks old (The Jackson Laboratory Japan, Inc.)
- Paraffin oil (Nacalai Tesque, Japan, 26137–85).
- Potassium simplex optimized medium (KSOM) (ARK Resource, Japan, Rat-KSOM).
- KSOM/0.1% hyaluronidase: Rat-KSOM with 0.1% hyaluronidase (Merck, USA, H3506).
- 35-mm dishes.
- Embryo manipulation pipets.
- Surgical instruments.
- Stereomicroscope.
- Wire net: a custom-made wire net creating approximately 1.5 cm of space from the bottom of the cage (Morita et al., 2023).
- 30G × 22-mm ophthalmic needle (NIPRO, Japan, 00–224).
- 1-mL syringe (Terumo, Japan, SS-01T).
- Incubator: 37°C, 5% CO2.
- Three days before the microinjection (Day 1), administer 50 IU PMSG to each female rat by intraperitoneal injection at 9:00 a.m. (Note: time points depend on the light–dark cycle).
- After 48 hours (Day 3), administer 50 IU hCG to each female rat by intraperitoneal injection.
- Seven hours after the hCG injection, mate each female rat with a male rat overnight using a cage equipped with a wire net.
- The day before zygote collection (Day 3), place four drops of 50 μL KSOM on a 35-mm dish covered with paraffin oil. Four dishes are needed for three pregnant females: three dishes for zygote collection (collection dish) and one dish for culture after the microinjection (culture dish).
- On the following day (Day 4), check for vaginal plugs either inside the vagina or that had fallen under the wire net to identify female rats that had copulated.
- Sacrifice the female rats that had copulated at 11:00 a.m.
- Dissect out an oviduct from each female rat and transfer it into 100 μL KSOM/0.1% hyaluronidase in the lid of a 3.5-cm dish.
- Fill a 1-mL syringe with KSOM/0.1% hyaluronidase and attach an ophthalmic needle.
- Insert the tip of the needle into the infundibulum and flush out zygotes.
- Allow the mixture to stand for 1–2 minutes until cumulus cells are removed.
- Transfer the pronuclear-stage zygotes to a drop of KSOM (collection dish) and repeat twice to remove the hyaluronidase.
- Incubate the zygotes in the remaining drop of KSOM (collection dish) at 37°C, 5% CO2 until microinjection (around an hour).
2.2.3 Time-lapse recording of pronuclear stage zygotes
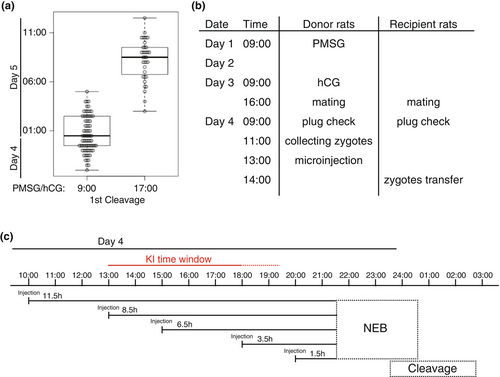
Pronuclear stage zygotes were collected from superovulated pregnant female rats and were cultured with KSOM. Time-lapse recordings of zygotes were performed using an A1-Ti confocal microscope (Nikon, Japan) equipped with an incubation culture system (Tokken, Japan) or an incubation imaging system LCV1000 (Olympus, Japan). Bright-field images were taken at intervals of 30 minutes. NIS-Elements (Nikon), MetaMorph (Universal Imaging Corporation, USA), or Fiji (Schindelin et al., 2012) was used to analyze images, and the time points of the first cleavage were plotted as beeswarm boxplot diagrams with RStudio (https://posit.co/download/rstudio-desktop/) (Figure 1a).
2.2.4 Microinjection
Piezo-assisted microinjection, which can improve the birth rates of injected zygotes, was used rather than conventional microinjection (Abe et al., 2020). The details of the piezo-assisted microinjection setup, including fabrication of the injection and holding needles, have been described previously (Abe et al., 2023). CRISPR cocktails were prepared just before injection as follows:
- Paraffin oil (Nacalai Tesque, 26137–85).
- PB1 medium (500 mL) NaCl, 4 g; KCl, 0.1 g; CaCl2, 0.06 g; KH2PO4, 0.1 g; MgCl2・6H2O, 0.575 g; Na2HPO4, 0.575 g; sodium pyruvate, 0.018 g; D-glucose, 0.5 g; Penicillin G, 0.0375 g; BSA, 1.5 g.
- Polyvinylpyrrolidone (PVP) medium: 12% PVP (Merck, P5288) in PB1.
- KSOM (Rat-KSOM, ARK Resource).
- crRNA stock solution: resuspend at 500 ng/μL in nuclease-free water and store at −80°C.
- tracrRNA stock solution: resuspend at 1 μg/μL in nuclease-free water and store at −80°C.
- Nuclease-free water (Promega, USA, P1193).
- Cas9 protein (ThermoFisher Scientific, A36497).
- Donor vector: dilute at 10 ng (or 100 ng)/μL in nuclease-free water before use.
- For the MMEJ-based method, prepare 9 μL of the premixture by mixing 1 μL rR26-crRNA (500 ng/μL), 1 μL PITCh-gRNA3 (500 ng/μL), 2 μL tracrRNA (1 μg/μL), 1 μL donor vector (10 or 100 ng/μL), and 4 μL nuclease-free water on ice. For the HR-based method, prepare 9 μL of the premixture by mixing 1 μL rR26-crRNA (500 ng/μL), 1 μL tracrRNA (1 μg/μL), 1 μL donor vector (10 ng/μL), and 6 μL nuclease-free water on ice.
- Add 1 μL Cas9 protein (1 μg/μL) to 9 μL of the premixture and gently mix. The final concentrations are as follows:
- The MMEJ-based method involves crRNA (50 ng/μL each), tracrRNA (200 ng/μL), donor vector (1 or 10 ng/μL), and Cas9 protein (100 ng/μL).
- The HR-based method involves crRNA (50 ng/μL), tracrRNA (100 ng/μL), donor vector (1 ng/μL), and Cas9 protein (100 ng/μL).
- Centrifuge at 13,000 g for 15 min at 4°C.
- Keep on ice until use.
- Place the following four 5-μL drops in an injection chamber: the first drop is the CRISPR cocktail, the second drop is KSOM, the third drop is PVP medium, and the fourth drop is KSOM covered with paraffin oil.
- Set the injection chamber on the stage of a micromanipulator.
- Fill with the CRISPR cocktail of the first drop into the injection needle.
- Transfer zygotes to the second drop. The number of zygotes depends on how many zygotes you can inject within 30 minutes.
- Hold each zygote with a holding needle and inject the cocktail into the pronucleus of the zygote.
- Repeat the injection for the other zygotes.
- Transfer all injected zygotes into a KSOM drop covered with paraffin oil (culture dish) and incubate until zygote transfer.
2.2.5 Zygote transfer
The injected zygotes are transferred to pseudopregnant recipient rats soon after injection. We describe the preparation of recipients and the zygote transfer into the oviduct through the infundibulum. Approximately 30 zygotes (15 in each oviduct) are transferred to each recipient rat.
- Rat Vaginal Impedance Checker (Muromachi Kikai Co., Ltd., Japan, MK-12-B).
- Vasectomized male rats (12–18 weeks old, The Jackson Laboratory Japan, Inc.): vasectomized in-house.
- Female rats (10–16 weeks old, The Jackson Laboratory Japan, Inc.)
- Isoflurane inhalation solution (Pfizer, USA).
- Anesthetic machine (Shinano, Japan, SN-487-0T).
- Surgical instruments.
- Stereomicroscope.
- Wound clips (Becton Dickinson, USA, Autoclip wound closing system, 427639, 427631).
- A sonic vibrator for rats (NATUME SEISAKUSHO, Japan, KN-595-50).
Protocol
- Vasectomized Male Method: on the day before microinjection, identify proestrus female rats using the vaginal impedance checker, then mate them with vasectomized male rats in a cage equipped with a wire net.
- The next morning, check for vaginal plugs either inside the vagina or that had fallen under the wire net to confirm copulation. If using the sonic vibrator method, induce pseudopregnancy on this day as previously described (Kaneko et al., 2020).
- Anesthetize the recipient rats with isoflurane inhalation: 4–5% for induction, 2–3% for maintenance.
- Make an incision in the ovarian bursa near the fimbria ovarica and transfer 15 injected zygotes into the oviduct through the fimbria ovarica using a capillary.
- Relocate the ovary and fat pad into the abdomen and close the skin using wound clips.
- Repeat the procedure to transfer the injected zygotes into the other rats.
- After surgery, the rats should be kept warm with a heat lamp and monitored until they recover from the anesthesia.
2.3 Genotyping of founder rats
The resulting F0 rats were genotyped by PCR and sequencing, and the germline transmission was confirmed in the next generation by crossing them with wild-type rats. In this section, two kinds of DNA isolation methods are described. One method is used for the F0 rats to yield purified genomic DNA, allowing long-term storage for further analyses. The other method is a quick DNA extraction method used for the routine PCR genotyping of descendants.
2.3.1 Purification of genomic DNA
- Proteinase K (Roche, Switzerland, 3115887,001) stock solution: resuspend at 10 mg/mL in water and store at −20°C.
- Lysis buffer: 10 mM Tris–HCl (pH 7.5), 10 mM EDTA, 10 mM NaCl, 0.1% SDS, 1 mg/mL proteinase K.
- RNase A (Nacalai Tesque, 30141,014) stock solution: 1 mg/mL RNase A, 10 mM Tris–HCl (pH 7.5), 15 mM NaCl.
- Transfer a tail tip (1 mm) or E12.5 embryos to a 1.5-mL tube with 300 μL lysis buffer and incubate at 55°C overnight.
- Add RNase A (1 mg/μL) for an hour at 37°C.
- Perform phenol–chloroform treatment, followed by ethanol precipitation.
- Resuspend genomic DNA in TE (Tris-EDTA) buffer.
- Use 20–50 ng of the genomic DNA per 25 μL PCR.
2.3.2 Extraction of genomic DNA
- Alkaline lysis buffer: 50 mM NaOH.
- Neutralization buffer: 100 mM Tris–HCl (pH 8.0).
- Transfer a tail tip (1 mm) to a 0.5 mL tube and add 180 μL alkaline lysis buffer.
- Boil for 10 min at 95°C.
- Add 20 μL of the neutralization buffer and vortex.
- Centrifuge at 13,000 g for 5 min
- Use 2 μL of the DNA extract per 25 μL PCR.
2.3.3 Genotyping PCR
- Go-Taq Master Mix (Promega, USA, M7122).
- Microchip Electrophoresis system (Shimadzu, Japan, MultiNa).
- MultiNa DNA-1000 kit (Shimadzu).
- Primers: rR26 FW (5′-TGA GTT GTG GCA CTG AGG AAC GTG C-3′), bGHpA FW (5′-GGG GGA GGA TTG GGA AGA CAA TAG C-3′), rR26 REV (5’-GCT GCA TAA AAC CCC AGG TGA GTG C-3′), EGFP REV (5’-CAC GCT GAA CTT GTG GCC GTT TAC G-3′), Scarlet REV (5’-CAT GAA CTC CTT GAT CAC TGC CTC G-3′), Venus REV (5’-TTC AGG GTC AGC TTG CCG TAG GTG G-3′), rR26 REV4 (5′-AGG GAA TGC CAG TGC TCT GTC TAG G-3′), SA REV (5’-CGG CCT CGA CTC TAC GAT ACC GTC G-3′).
- Prepare PCR mixtures using the following primers:
- PCR#1 (5′-side of H2B-EGFP allele): rR26 FW and EGFP REV (1028 bp).
- PCR#2 (5′-side of Lyn-Venus allele): rR26 FW and Venus REV (704 bp).
- PCR#3 (5′-side of NLS-Scarlet allele): rR26 FW and Scarlet REV (635 bp).
- PCR#4 (5′-side of Lyn-Scarlet allele): rR26 FW and Scarlet REV (587 bp).
- PCR#5 (3′-side of R26-KI allele): bGHpA FW and rR26 REV (324 bp).
- PCR#6 (5′-side of R26R-H2B-EGFP allele): rR26 FW and SA REV (445 bp).
- PCR#7 (3′-side of R26R-H2B-EGFP allele): bGHpA FW and rR26 REV4 (608 bp)
- Add 20–50 ng genomic DNA or 2 μL DNA extract to each PCR tube.
- Analyze PCR products by agarose gel electrophoresis or a Microchip electrophoresis system according to the instrument's instructions.
2.4 Imaging of embryos and adult tissues
The reporter rat lines were further evaluated for the characteristics of the fluorescent reporter in vivo. Each male KI rat (12–18 weeks old) was crossed with a female wild-type rat (10–16 weeks old) or a female KI rat to collect embryos. Preimplantation stage: one-cell stage zygotes were collected at the E1.5 stage from oviducts and were cultured to the blastocyst stage in a 200 μL drop of KSOM (ARK Resource) covered with paraffin oil on a 35-mm glass-bottom dish in an atmosphere of 5% CO2 at 37°C. Images of two-cell, eight-cell, and blastocyst stage embryos were taken using an A1-Ti confocal microscope (Nikon) (filter sets used: a band-pass filter 525/50 for GFP and a band-pass filter 595/50 for RFP) equipped with an incubation culture system (Tokken).
Post-implantation stage and adult tissues: E14.5 embryos and adult tissues of R26-NLS-Scarlet KI rats were collected in phosphate-buffered saline Images were acquired using a Leica M165FC stereomicroscope equipped with epifluorescence optics and a Leica DFC310FX camera (filter set used: excitation 545/30 nm and emission 620/60 nm for Scarlet). Images were assembled using Photoshop software (Adobe Systems, USA).
2.5 Freezing and thawing of zygotes
For lineage preservation of the established rat lines, one-cell stage zygotes were cryopreserved using the simple vitrification method, and both the freezing and subsequent thawing of the zygotes were performed as previously described (Taketsuru & Kaneko, 2013). The detailed protocol is provided in the Data S1.
3 RESULTS
3.1 Timing of microinjection
We have previously reported that the developmental stage of zygotes used for microinjection is crucial for the efficiency of generating KI mice. To achieve this, we used a microinjection method with frozen in vitro fertilization (IVF) zygotes, which was highly efficient not only for standardizing the developmental stages of zygotes but also for precisely controlling them (Figure S2) (Abe et al., 2020). However, rat reproductive technologies have not been well-established compared with those in mice, and it is technically inconsistent to use frozen IVF zygotes for genome editing. Hence, zygotes were collected through the natural mating of female rats following superovulation treatment. As the correlation between hormone administration timing and fertilization timing has not been addressed in detail, to evaluate whether hormone administration timing influences fertilization timing, superovulation was performed using PMSG to induce follicular development at two different time points, 9:00 a.m. or 5:00 p.m., and hCG was administered to induce ovulation 48 h after PMSG administration. We observed the timing of the first cleavages to compare developmental progress between the two conditions using time-lapse recording. The results show that the first cleavages in the 5:00 p.m. administration group were shifted approximately 8 hours later compared with the 9:00 a.m. administration group (Figure 1a). That result suggested that the timing of fertilization could be controlled to a certain extent by adjusting the timing of the hormone administration. Although it was not precisely controlled in the same way as in mouse IVF (Figure S2), about half of the zygotes were contained within a range of approximately 3 h (Figure 1a).
We also investigated the timing of NEB, which is an important indicator for determining the appropriate microinjection timing for efficient KI using time-lapse recording. After fertilization, the paternal and maternal pronuclei gradually moved closer together, and the first cleavage was observed approximately 2 h after NEB (Movie S1, Figure S3). Based on these results, we decided to administer hormones at 9:00 a.m. and start microinjections at 1:00 p.m., which is approximately 8.5 h before NEB (Figure 1b,c). Our results in mice suggested that this timing would provide sufficient time for KI events to occur in the pronucleus (Abe et al., 2020).
3.2 Generation of KI lines
Four MMEJ-based KI donor vectors (H2B-EGFP, NLS-Scarlet, Lyn-Venus, and Lyn-Scarlet) were generated to express fluorescent probes at the ROSA26 locus (Figure 2a). Each donor vector was co-injected with the CRISPR cocktail into the pronucleus of a zygote (Figure 2b and Movie S2). When the concentration of the donor vector was 1 ng/μL, offspring were obtained from 24.4% to 30.0% of the injected zygotes for each vector (Table 1). KI alleles were identified by PCR (Figure 2c) and were further confirmed by sequencing, resulting in the successful generation of all four reporter lines, with KI efficiencies ranging from 6.3% to 28.6% of pups (from 1.7% to 8.6% of injected zygotes). We found that a 10 ng/μL donor vector concentration in the CRISPR cocktail decreased the birth rate of F0 rats to 1.5%–12% (Table 1). This is because DNA concentration in the CRISPR cocktail affects the survival rate of the embryos (Table S1). Therefore, we decided to use the CRISPR cocktail at a concentration of 1 ng/μL of the donor vector to generate KI rats for further investigations. The germline transmission was confirmed in their offspring, and all established KI lines were maintained as homozygous without any abnormal defects. As a result, we successfully demonstrated the generation of four KI lines using the MMEJ-based KI method. In addition, we also employed the HR-based KI method, which is one of the standard KI methods, to generate Cre reporter rats (R26R-H2B-EGFP) at the ROSA26 locus. From a single round of microinjection, a PCR-positive rat was obtained with 5.6% of its pups being PCR-positive (Figure S4). The R26R-H2B-EGFP line will be reported elsewhere with its phenotype.
| Lines | Insert sizes (kbp) | Donor vector (ng/μL) | No. of injected zygotes | No. of pups | No. of KI | KI/zygotes | KI/pups |
|---|---|---|---|---|---|---|---|
| R26-H2B-EGFP | 1.7 | 1 | 59 | 16 (27.1%) | 1 | 1.7% | 6.3% |
| R26-NLS-Scarlet | 1.3 | 10 | 78 | 2 (2.6%) | 1 | 1.3% | 50.0% |
| 1 | 82 | 20 (24.4%) | 2 | 2.4% | 10.0% | ||
| R26-Lyn-Venus | 1.4 | 10 | 131 | 2 (1.5%) | 1 | 0.8% | 50.0% |
| 1 | 72 | 19 (26.4%) | 4 | 5.6% | 21.1% | ||
| R26-Lyn-Scarlet | 1.3 | 10 | 25 | 3 (12.0%) | 0 | 0 | 0 |
| 1 | 70 | 21 (30.0%) | 6 | 8.6% | 28.6% |
- Note: Donor vector (ng/μL), concentration of the donor vector in the CRISPR cocktail; No. of pups, the number of pups (the percentage of the offspring out of the injected zygotes); KI/Zygotes, the percentage of KI rats out of the injected zygotes; KI/Pups, the percentage of KI rats out of the F0 rats.
To identify the optimal time window for KI, we compared the time course of KI efficiency at the time points of 10:00 a.m., 1:00 p.m., 3:00 p.m., 6:00 p.m., and 8:00 p.m. with the R26-Lyn-Venus donor vector (Table 2). According to the time-lapse recording, 8:00 p.m. was estimated to be approximately 1.5 hours before NEB, which was close to NEB (Figure 1c). In the trials, embryos were collected at the E12.5 stage, and the KI efficiencies were analyzed by PCR. As a result, the KI efficiency at the 10:00 a.m. and 8:00 p.m. microinjections decreased compared with at 1:00 p.m., 3:00 p.m., and 6:00 p.m., suggesting that not only the later stage (8:00 p.m.) but also the earlier stage (10:00 a.m,) falls outside the appropriate timing (Tables 1 and 2). The number of collected embryos also declined at the 8:00 p.m. microinjection. These results suggested that the appropriate time window for KI is between 1:00 a.m. and 6:00 a.m.
| Injection timing | No. of injected zygotes | No. of embryos | No. of KI | KI/zygotes | KI/embryos |
|---|---|---|---|---|---|
| 10:00 a.m. | 56 | 13 (23.2%) | 0 | 0 | 0 |
| 1:00 p.m. | 30 | 6 (20.0%) | 2 | 6.7 | 33.3 |
| 3:00 p.m. | 30 | 7 (23.3%) | 2 | 6.7 | 28.6 |
| 6:00 p.m. | 30 | 15 (50.0%) | 2 | 6.7 | 13.3 |
| 8:00 p.m. | 60 | 8 (13.3%) | 2 | 3.3 | 25.0 |
- Note: No. of embryos, the number of E12.5 embryos (the percentage of the embryos out of the injected zygotes); KI/Zygotes, the percentage of KI embryos out of the injected zygotes; KI/Embryos, the percentage of KI embryos out of the collected embryos.
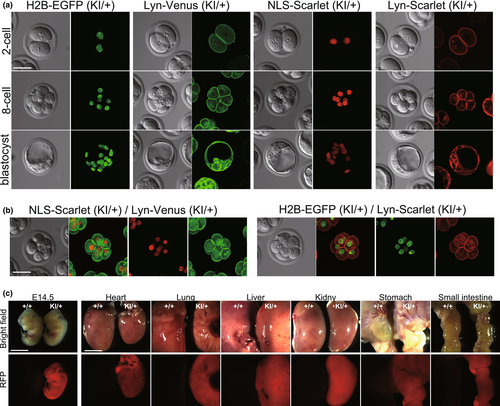
3.3 Localization of fluorescent fusion proteins in embryos
To evaluate the cellular localization of fluorescent probes and their signal intensities, we observed KI embryos from the two-cell stage to the blastocyst stage. As a result, all fluorescent signals were detectable from the two-cell stage and were precisely localized at the nucleus and cell membrane (Figure 3a). The dual labeling of nuclei and cell membranes was also observed by crossing with GFP and RFP lines (Figure 3b). We further analyzed the Scarlet signals in R26-NLS-Scarlet embryos at E14.5 and in several adult tissues (heart, lung, liver, kidney, stomach, small intestine). The Scarlet signals were detected in the entire embryos and in all tissues examined with a relatively bright intensity (Figure 3c). Taken together, we successfully established four fluorescent reporter lines labeling nuclei and cell membranes. The fluorescent probes were ubiquitously expressed in peri-implantation stages and in adult tissues.
3.4 Line preservation
Cryopreservation techniques are crucial for the long-term preservation of genetically engineered animal strains, which are valuable bioresources, and for the safe, easy, and widespread sharing of those strains through frozen embryo transport. We performed a previously reported simple vitrification method for rat embryos and cryopreserved our established reporter rat lines (Taketsuru & Kaneko, 2013). Homozygous female rats treated for superovulation by hormone administration were crossed with homozygous male rats resulting in an average of 42.7 to 82.5 zygotes obtained from each line and all collected zygotes were cryopreserved, with approximately 40 per tube. To assess the viability of the cryopreserved zygotes, 40 zygotes (R26-Lyn-Venus#2) were thawed. Thirty-seven of those (93%) survived and were transferred into the oviducts of pseudopregnant female rats, resulting in 19 pups obtained (51.4%) (Supporting Table S2). Thus, we have successfully cryopreserved rat zygotes and demonstrated that they can be re-established from the cryopreserved state.
4 DISCUSSION
Rats are valuable laboratory animals that have been widely used for many years because of their physiological similarities to humans, their ease of handling, and many well-established behavioral models. Genetic modifications make rats even more useful in studies of human diseases, drug testing, and complex biological processes. CRISPR/Cas9-mediated genome editing in zygotes allows the generation of genetically engineered rats in a single step. However, a gene cassette KI is inefficient and achievements in establishing reporter lines have been limited (Sato et al., 2022). To overcome that issue, we describe an efficient method for CRISPR-mediated KI through pronuclear microinjection in rat zygotes, demonstrating the generation of four different fluorescent reporter lines at the ROSA26 locus. These reporter lines will be useful for live imaging of cell dynamics, division, and interactions. They will also be invaluable for studying cell migration, transplantation, and death, providing insights into developmental biology, regenerative medicine, and cancer research. Recently, we have also established mCherry KI rats at the dopamine transporter gene with an efficiency of 19.2% pups (7.1% of injected zygotes) (Matsumoto et al., 2024), suggesting that this method would serve to generate KI rats at other loci.
In our previous report on CRISPR-mediated KI through pronuclear microinjection in mice, the time to NEB after microinjection was identified as a critical factor for highly successful KI, along with the use of the gRNA-Cas9 protein complex. In this study, we found that microinjection 8.5 h before NEB (at 1:00 p.m.) could consistently yield KI, while those 11.5 h and 1.5 h (at 10:00 a.m. and 8:00 p.m.) had a decreased KI efficiency and zygotic survival rate. It is unclear why microinjection at the time close to NEB negatively affected the zygotic survival rate. However, this result also supported the reliability of our method, in which microinjection was performed at 1:00 p.m. Furthermore, we showed that microinjection 6.5 h and 3.5 h before NEB (at 3:00 p.m. and 6:00 p.m.) could obtain KI. That result suggested that the time window for KI could be estimated to be at least between 1:00 p.m. and 6:00 p.m. This could be because there was an 8-h difference between the earliest and latest divisions (Figure 1a). Note that it is appropriate to perform the microinjection when the zygote transfer can be completed within regular working hours.
Meanwhile, the CRISPR-mediated KI method has room for further improvement because of the variability in KI efficiencies even at the same KI locus. One possible cause of this variability is the heterogeneity in zygote development as mentioned above. One resolution for this issue is IVF. Indeed, we have employed IVF to standardize the zygote stage in mice, and have successfully generated KI mice with high efficiency and reproducibility (Abe et al., 2020). However, reproductive engineering techniques in rats, including IVF, have not been well established compared with those in mice, and this needs to be improved in the future.
The gRNA-Cas9 protein complex used in this study likely induces DSBs shortly after microinjection, which is also believed to facilitate gene cassette insertion before NEB. Using nuclease (ZFN, TALEN, and Cas9) mRNAs requires time for translation into proteins, which may prevent completion of the gene cassette insertion before NEB. This could be one reason why genome-editing-mediated KI by microinjection has been inefficient. Further improvements in KI efficiency could be expected if zygote stages are standardized by IVF and a more precise microinjection timing is identified.
We demonstrated the CRISPR-mediated KI using MMEJ-based and HR-based donor vectors. These methods integrate a DNA cassette through different DNA repair pathways (Sakuma et al., 2016). The MMEJ-based KI method uses the MMEJ repair pathway, which is active from the M phase to early S phase, whereas the HR-based KI method uses the HR repair pathway, which is active from late S to G2 phase. It is known that, in the one-cell stage, the duration from G1 to early S phase is longer than that from late S to G2 phase in mice (Gu et al., 2018), which suggests that MMEJ-based KI may have the advantage of a longer time window for KI. Another advantage is that the homology arms of the MMEJ-based donor vector are shorter, at 40 bp, making it easier to construct than the HR-based donor vector (Sakuma et al., 2016). However, further investigation is needed to clarify which method is more efficient and precise in rats.
We also performed freezing and thawing of zygotes to preserve established strains. Although those methods have been reported previously (Taketsuru & Kaneko, 2013), the strain preservation techniques were revisited because of their importance in facilitating the distribution of genetically engineered animals to a wide range of researchers. In this study, we were able to collect a sufficient number of zygotes for strain preservation through the natural mating of rats after hormone treatments (an average of 42.7 to 82.5 zygotes was obtained from each line per pregnant female rat). However, it is known that the genetic background affects the response to hormone treatment (Honda et al., 2019), so this issue should be considered when using other rat strains.
The reproducible KI rat production technique reported in this paper will contribute to the advancement of various studies, taking advantage of the superiority of rats as human disease models, which cannot be replicated in mice. Further advancements in reproductive technologies for rats, including studies on the cryopreservation of zygotes after IVF and the effects of hormones in individual strains, will not only improve the production efficiency of genetically engineered rats but should also contribute to the research community by enabling better strain selection for research purposes, improving animal distribution, and facilitating microbiological cleaning, and other related areas.
AUTHOR CONTRIBUTIONS
T.A. conceived and designed the experiments. T.A. and K.I. performed the majority of the experiments under the supervision of H.K. Data interpretation was conducted by T.A., K.I., and H.K. The manuscript was written by T.A., and H.K.
ACKNOWLEDGMENTS
We thank Drs. Dorus Gadella, and Atsushi Miyawaki for the gifts of vectors and Dr. Takehito Kaneko for advice on reproductive techniques. This work was supported by intramural grants from RIKEN.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




