A snapshot of injecting drug consumption from the analysis of used syringes within the Medically Supervised Injecting Centre in Sydney, Australia
Abstract
Introduction
The administration of illicit drugs by injection is associated with considerable harm, including an increased risk of overdose. The chemical analysis of used syringes can enhance knowledge on injecting drug consumption beyond traditional data sources (self-report surveys). This additional information may be useful during significant global events like the COVID-19 pandemic. This study aimed to examine a snapshot of the drugs injected at the Medically Supervised Injecting Centre (MSIC) in Sydney, Australia, in 2019–2020.
Methods
Used syringes were collected from MSIC across three periods throughout 2019 and 2020 (February 2019, March—April 2020 and June—September 2020). Drug residues were extracted from used syringes using methanol before detection by gas chromatography—mass spectrometry and ultra-performance liquid chromatography—tandem mass spectrometry. The chemical analysis results were compared to self-report data obtained from MSIC clients.
Results
Heroin (46–53%), methamphetamine (24–34%) and pharmaceutical opioids (15–27%) were the most common drug residues detected. The chemically detected drugs had declining coherence with the drugs self-reported by MSIC clients across the time periods examined.
Discussion and Conclusions
There was no significant change in the drugs injected (heroin, methamphetamine and pharmaceutical opioids) across the three periods collected throughout varying COVID-19 lockdown restrictions. Changes in the frequency of other drugs injected and discrepancies between chemical analysis and self-report were potentially related to regulatory changes, degradation or misinformed sales. Routine chemical analysis of used syringes has provided an alternative information source to promote awareness of current drug trends and aid harm reduction.
Key Points
- Heroin, methamphetamine and pharmaceutical opioid injection remain dominant at the Sydney supervised injecting facility throughout 2019–2020.
- The main pharmaceutical opioid injected changed throughout this period (from oxycodone/buprenorphine to methadone).
- There were some discrepancies between the drugs chemically detected and those self-reported.
- A declining number of people attended the Sydney supervised injecting facility that coincided with the onset of the COVID-19 pandemic.
1 INTRODUCTION
The administration of drugs by injection perseveres within Australia by a small but high-risk population of people who use drugs. In 2019/2020, the number of people who inject drugs (PWID) regularly in Australia was estimated to be 75,756 (no uncertainty interval reported) [1]. There is a high level of harm associated with injecting drug use from blood-borne infections [2] and a variety of accompanying morbidity, mortality and social impacts [1]. Within Australia, typically heroin or methamphetamine are the drugs most commonly injected, followed by pharmaceutical opioids (methadone, buprenorphine, morphine and oxycodone) and cocaine [3]. This is further reflected within New South Wales injecting trends which are co-dominated by heroin and methamphetamine, followed by methadone and cocaine [3].
Australia, under a harm minimisation approach to the problem of illicit drug use, employs a variety of harm reduction strategies that specifically target PWID [4]. Within Sydney, one of the fundamental harm reduction strategies for PWID has been opening Australia's first supervised injection facility, the Uniting Medically Supervised Injecting Centre (MSIC). Supervised injecting facilities provide clean injecting paraphernalia and supervision of injecting events, while also offering health and social services and pathways to treatment [5, 6]. Given the dynamic nature of the illicit drug market and consumption trends, the effectiveness of harm reduction strategies is susceptible to significant global events, for example, the COVID-19 pandemic. Correspondingly, the implementation of harm reduction strategies needs to be iterative and adaptive to provide relevant support during the onset of such a global event (e.g., [7]).
The heterogenous repercussions of the COVID-19 pandemic on illicit drug consumption have already been identified [8]. Government travel restrictions had noticeable impacts on the illegal trade of drugs [9], which in Australia relies on air and sea cargo. These were significantly affected throughout the pandemic by widespread border closures and movement restrictions [10]. Some studies suggested a speedy recovery and adaption in operation among drug manufacturers, traffickers and street sellers after initial lockdown restrictions [11, 12], although this is drug and location-dependent [13]. Conversely, many countries have reported initial shortages of various drugs; consequently, people who use drugs have been substituting the substances they consume [14, 15]. There is limited published information on consumption trends of PWID within Sydney during changing lockdown restrictions. The Illicit Drug Reporting System (national survey relating the experience of Australian PWID) examined how COVID-19 impacted PWID. In March 2020, a third of PWID reported changing the frequency at which they consumed drugs. Furthermore, PWID reported that some substances, such as methamphetamine and morphine, were decreasingly available, while heroin and methamphetamine were perceived as less pure. Additionally, the price of heroin and methamphetamine increased [3]. Further examination of how PWID responded to a global crisis such as COVID-19 could provide insight into how they might be impacted by and respond to future global disruptions.
Drug consumption information on PWID has traditionally relied on self-report surveys [16]. Survey data provides valuable insights and has been shown to be a reliable source of information among PWID [17]. However, it depends on the consumer's perception of the drug they are using, which might be misinformed [17]. The chemical extraction of drug residues from used injecting paraphernalia can help address the limitations of self-report surveys and complement them [16].
The analysis of used injecting paraphernalia has been accomplished in a variety of locations in Europe [16, 18], the United States of America [19] and Australia [20, 21]. Analysis of used injecting paraphernalia has provided valuable insights into adulteration and emerging drug trends at the consumption level, such as further examining the growing fentanyl-laced opioid epidemic in the United States [22] and other new psychoactive substances emergence [19]. The analysis of used injecting paraphernalia has also provided insight into the prevalence of polydrug mixtures; for example, in Norway, 50% of specimens contained multiple drugs (the most common mixture being amphetamine and heroin; 31% of all syringes) [23]. In Washington, DC, a high prevalence of fentanyl polydrug mixtures was detected in heroin (60% of heroin specimens), tramadol (88% of tramadol specimens) and cocaine (61% of cocaine specimens) [19]. Used injecting paraphernalia analysis completed in Victoria, Australia, after the onset of COVID-19 detected a relatively high prevalence of β-U10 and etizolam, both of which are highly potent new psychoactive substances that present a significant public health risk [21].
This study aimed to examine and characterise the drugs that were injected at MSIC in Sydney in three time periods across 2019 and 2020. Furthermore, it assessed whether there was a correlation between the chemically detected drugs and those self-reported by the consumers (aggregate comparison). This will provide an overview of all drugs to be injected by clients at MSIC (at a group level) and if generally people were likely injecting what they planned to inject. Correlation at the individual level (i.e., direct comparison of a chemical identity aligns the expected substance) cannot be accomplished based upon ethical and logistical considerations.
2 METHODS
2.1 Sampling method
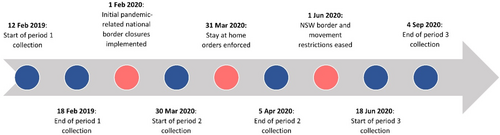
To capture a snapshot of injecting drug consumption in Sydney, used syringes were collected from the Uniting MSIC in Sydney, Australia across three time periods. The three time periods were chosen around COVID-19 lockdown restrictions (see Figure 1) from March 2020 until December 2021 in New South Wales [24, 25]. The first period (P1) was collected from 12 to 18 February 2019, and it has been published previously [20]. The second period (P2) was collected from 30 March to 5 April 2020, as major lockdown restrictions were first implemented across Australia. The third period (P3) was collected across 3 months from 18 June to 4 September 2020, as lockdown restrictions were initially eased in NSW. The first two samples collected one multi-user sharps disposal bin from a randomly selected MSIC injecting booth each day for a 1-week period. The third sample collected one multi-user sharps disposal bin from a randomly selected MSIC injecting booth across a single day (Thursday or Friday) per week over six non-consecutive weeks. Due to social distancing, the number of injecting spaces decreased from 16 in the first sample to 8 in the second and third samples [7]. Across all time periods, 1 day of sampling resulted in one multi-user sharps disposal bin filled with used syringes. Unless otherwise stated, the parameters relating to how specimens were processed were consistent across all time periods.
Upon admission to MSIC, clients were asked a number of questions as a part of the regular admission process, including the drug intended to be consumed. All clients were informed that participation in this study was voluntary and anonymous. Non-consenting clients were directed to one of the non-study booths. For each time period and study booth, the chemical analysis results and the self-report data were aggregated and anonymised based solely on sampling day and booth number. It was not possible to link an individual syringe back to a specific client since no identifying information was sought. Human research ethics approval was provided by the Human Research Ethics Committee at the University of Technology Sydney: ETH18-2295 and ETH21-6777.
2.2 Chemicals
Methanol, ethyl acetate, hexane and acetonitrile were obtained at liquid chromatography–mass spectrometry (LC–MS) grade from Chem Supply (Adelaide, Australia). N-methyl-N-(trimethylsilyl)trifluoroacetamide (synthesis grade) and ammonium formate (LC–MS grade) were obtained from Merck (Sydney, Australia). The following certified reference materials of representative drugs were purchased from Novachem (Melbourne, Australia) at 1000 ppm methanolic solutions, unless otherwise stated: morphine, cocaine (obtained in acetonitrile), methamphetamine, fentanyl, codeine, caffeine, diazepam, methadone and acetaminophen. Heroin and α-pyrrolidinopentiophenone hydrochloride were obtained as solids from the National Measurement Institute (Sydney, Australia) and prepared as 1000 ppm methanolic solutions. These standards were subsequently prepared in a 50 ppm standard mixture (except for methamphetamine and caffeine, which were prepared at 100 ppm) fresh for each analysis by gas chromatography–mass spectrometry (GC–MS). A 250 ppb standard mixture of all reference standards was also prepared fresh for analysis on ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS).
2.3 Sample preparation
Sample preparation was conducted according to previously published studies [16, 20, 26]. Although alternative injecting paraphernalia such as needles, spoons and concealment bags are captured within sampled sharps bins and have been proven to provide valuable information, they were omitted in this study in order to avoid the inflation of results (i.e., multiple paraphernalia used for one injecting episode) [20, 23]. In short, 1 mL of methanol was pumped in and out of each individual syringe five times. The methanol extract was then divided into three aliquots: (i) direct detection by GC–MS; (ii) silyl derivatisation before detection by GC–MS; and (iii) direct detection by UPLC–MS/MS. Aliquot (ii) was evaporated to dryness under nitrogen and reconstituted in N-methyl-N-(trimethylsilyl)trifluoroacetamide (100 μL), incubated for 30 min (80°C) before being evaporated again and reconstituted in N-methyl-N-(trimethylsilyl)trifluoroacetamide: acetonitrile (1:1; 100 μL). Each aliquot was filtered by wheel filtration (nylon, 13 mm, 0.45 μm) at the final step before instrumentation detection. Various sample preparation methods were employed across different time periods due to varying resource availability; however, collation of different data sources has been previously employed in this area [18]. The method for aliquot (i) was used for all three time periods, aliquot (ii) was used for only P1 and P3, and aliquot (iii) was only employed within P3.
2.4 Instrumentation
Methanol extracts were analysed using GC–MS and UPLC–MS/MS. Both methods were previously validated by [16, 27], respectively. The GC–MS method was implemented via an Agilent 7890 series gas chromatographic system coupled with an Agilent 5975C Network Mass Selective detector. The UPLC–MS/MS method was conducted using an Agilent Infinity 1290 liquid chromatography system coupled to an Agilent 6490 Triple Quadrupole Mass Spectrometer. Optimised multiple-reaction monitoring parameters are provided in Table S1, Supporting Information.
2.5 Data analysis
Given the screening nature of this approach, the total ion chromatograms from the GC–MS were reviewed on MSD Enhanced ChemStation Software. Peaks of interest were identified by the libraries available on the GC–MS (National Institute of Science and Technology; NIST14, Scientific Working Group for the Analysis of Seized Drugs v.3.11, and Cayman Spectral Library v.21022022). The relevant standard mixture (all drug standards at variable concentration; GC–MS: 50/100 ppm or UPLC–MS/MS: 250 ppb) was run in every sequence and used to validate the identity of these drugs when detected in specimens, as well as to monitor instrument performance. Only the main drug identified within a syringe was reported in this work (assessed as the analyte with the most abundant peak area detected within GC–MS or UPLC–MS/MS and thus likely the intended drug identity). Similarly, the self-reported consumption of polydrug mixtures were also omitted within this analysis (but included in Data S1). Specimens where 6-monoacetylmorphine was detected and heroin was absent were reclassified as heroin syringes. The UPLC–MS/MS results (aliquot (iii)) were included when GC–MS detected no drugs within syringe extracts.
The correlation between chemical detections and self-reported drugs was calculated using the Kendall rank correlation test, as previously described by [20]. The hypothesis test determines whether two variables are statistically dependent or not, considering the significance level (5% herein), which is represented as a value between −1 and +1 (the tau coefficient). Within this work, the Kendall rank correlation was used to measure the correlation between overall categories of self-reported drugs and chemically analysed drugs in the three time periods (correlation between individuals is not possible based on aggregated sample collection parameters that were unavoidable). That is, if a higher proportion of a drug was chemically detected, the same drug would have also been similarly highly self-reported (i.e., a tau at or close to 1). The Kendall rank correlation test was also used herein to assess the similarity of drugs chemically detected across different time periods. Frequency information, statistical tests and other trend analysis were accomplished using Microsoft Excel (v.2207), Tableau (v.2022.4.0) and R/RStudio (v.1.4.1717) [28].
3 RESULTS
3.1 General results
Throughout all sampling, a total of 587 syringes were analysed within this study, in which a range of 75–77% tested positive for at least one drug across the three time points (see Table 1). Across all sampling, no clients chose to ‘opt out’ of providing their used syringes to this research. The largest sample was P2, followed by P1 and P3 (P1 = 118, P2 = 166, P3 = 77). The average daily number of clients attending MSIC across all injecting booths within the 30 days prior to booth sampling decreased across the time periods. There was a noticeable decrease in P3 in comparison to the other two samples (P1 = 153, P2 = 150, P3 = 109).
| Sample number | Collection dates | Number of sharps disposal bins | Number of syringes | Positive syringes (%) | Total number of client visits (sample booths only) | Average number of clients (daily)a |
|---|---|---|---|---|---|---|
| P1 | 12–18 February (2019) | 7 | 147 | 76 | 118 | 153 |
| P2 | 30 March–5 April (2020) | 7 | 208 | 75 | 166 | 150 |
| P3 | 18 June, 25 June, 9 July, 23 July, 6 August and 4 September (2020) | 6 | 232 | 77 | 77 | 109 |
- a This includes all injecting booths at MSIC, averaged across the 30 days prior to the start of each sample.
A comprehensive summary of the self-reported drug frequencies across all sample periods (except the P1 frequencies, which were reported elsewhere [20]) can be found in Tables S2 and S3.
3.2 Main psychoactive substances
Across the three sampling periods, psychoactive substances were detected within 446 of the 587 syringes analysed (see Figure 2). In terms of the main drug detected within each syringe, heroin was the most frequent (P1 = 53%, P2 = 48%, P3 = 46%), followed by methamphetamine (P1 = 28%, P2 = 34%, P3 = 24%). Pharmaceutical opioids were also commonly detected, with fluctuating preference across the time periods: morphine (P1 = 2%, P2 = 2%, P3 = 13%), methadone (P1 = 2%, P2 = 2%, P3 = 9%), oxycodone (P1 = 11%, P2 = 6%, P3 = 4%) and buprenorphine (P1 = 4%, P2 = 3%, P3 = 1%).
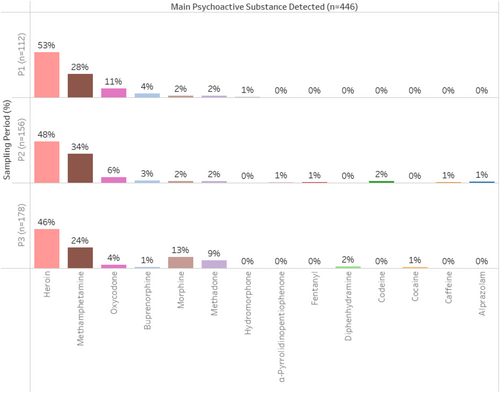
Some drugs were not consistently observed across different time periods; hydromorphone (P1 = 1%), alprazolam (P2 = 1%), α-pyrrolidinopentiophenone (P2 = 1%), caffeine (P2 = 1%; no other drug detected within this syringe and also never self-reported), fentanyl (P2 = 1%; also self-reported), diphenhydramine (P3 = 2%), cocaine (P3 = 1%).
The Kendall rank correlation test comparing the drugs chemically detected found that all three time periods were positively correlated with each other to varying degrees of strength. The strongest agreement was seen in the comparison between the first and second period (P1 vs. P2: tau = 0.7), followed by the first and third period (P1 vs. P3: tau = 0.7). The weakest agreement was observed between the second and third periods (P2 vs. P3: tau = 0.5). For all the three comparisons the correlation was found to be statistically significant at the 0.05 level (P1 vs. P2: p-value = 0.0008, P1 vs. P3: p-value = 0.001, P2 vs. P3: p-value = 0.02).
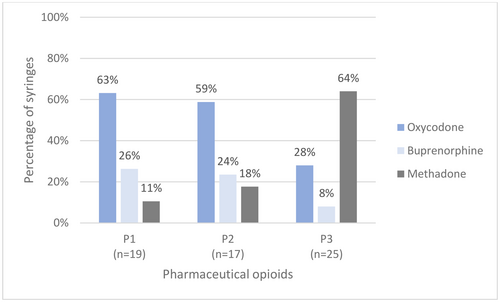
Across the three time periods, there appeared to have been a switch in the main pharmaceutical opioid consumed (Figure 3). In P1 and P2, of the most commonly detected pharmaceutical opioids, oxycodone (P1 = 63%, P2 = 59%, P3 = 28%) and buprenorphine (P1 = 26%, P2 = 24%, P3 = 8%) were the most frequent. However, within P3, both of these drugs were detected less frequently, with methadone being more common (P1 = 11%, P2 = 18%, P3 = 64%). There was also a large increase in the number of syringes containing morphine within P3 but unlike for methadone, this is not thought to be an intentional choice by MSIC clients and is further examined in the Discussion section.
3.2.1 Chemical analysis and self-report comparison
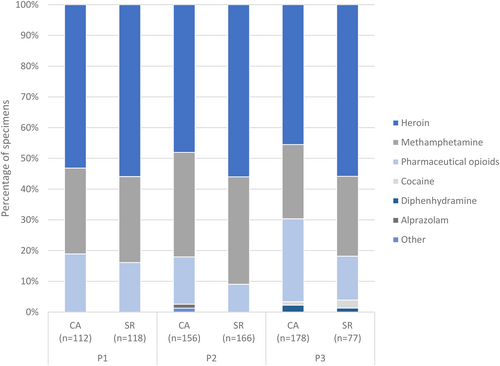
A more diverse picture is obtained through the comparison of the chemical analysis of used syringes to the drugs self-reported by MSIC clients (Figure 4). Chemical analysis and self-report data indicated a similar amount of methamphetamine use across the three time points (chemical analysis vs. self-report: P1 = 28% vs. 28%, P2 = 34% vs. 35%, P3 = 24% vs. 26%). As the time points progressed, there was declining coherence between the chemical analysis and self-reported drug frequencies for heroin (chemical analysis vs. self-report: P1 = 53% vs. 56%, P2 = 48% vs. 56%, P3 = 46% vs. 56%) and pharmaceutical opioids (chemical analysis vs. self-report: P1 = 19% vs. 16%, P2 = 15% vs. 9%, P3 = 27% vs. 14%). The large discrepancy within P3 was caused by a large increase in morphine syringes that were chemically detected but never self-reported (chemical analysis vs. self-report: P3 = 13% vs. 0%).
The result of the Kendall rank correlation test further demonstrates the relationship between the chemical analysis and self-report results across the time periods. The highest agreement was observed within P1 (tau = 1.0) and this correlation was found to be statistically significant at the 0.05 level (p-value = 0.001). A weaker positive correlation was seen in P2 (tau = 0.5). This was also statistically significant at the 0.05 level (p-value = 0.04). Lastly, for P3, there was insufficient evidence to reject the null hypothesis of no correlation at 0.05 level (p-value = 0.09) and hence the correlation coefficient is not reported. The weaker or lack of statistically significant correlation in P2 and P3 were potentially related to differences in frequencies and ranks that were compared.
4 DISCUSSION
Three samples of used syringes and self-reported information were collected from PWID attending the Sydney supervised injecting facility within 2 years before and during COVID-19 restrictions and were analysed to examine a snapshot of drugs injected by this population. During the collection of the second and third samples, there was a noticeable decrease in the number of clients attending MSIC, which coincided with the onset of the COVID-19 pandemic. The yearly average number of clients also decreased in 2020 in comparison to the preceding 5 years (see Figure A1). During the early stages of the pandemic, Australian PWID had concerns regarding drug supply (availability, price and purity). Other deterrents, such as heightened policing and fears of contracting COVID-19, might have contributed to a decrease in clients injecting at MSIC [29]. Furthermore, this was likely exacerbated by public health measures restricting movement and leading to supervised injecting facilities operating at reduced capacity [15]. At MSIC the number of injecting spaces was reduced by half in response to the pandemic [7], which might have influenced the number of PWID attending MSIC for injection.
The ranking of the most frequently injected drugs at MSIC did not change throughout the three time periods examined. All three time periods were statistically correlated with each other, suggesting that there was no significant change to the drugs being injected at MSIC in the time points examined here. However, the incomplete tau agreement did suggest that changes did occur but at a smaller scale.
Heroin followed by methamphetamine continued to dominate injecting drug trends within MSIC, with pharmaceutical opioids (namely methadone) occurring less frequently. Since 2015, the dominance of these drugs at MSIC preceding the pandemic has been established based on longitudinal MSIC client self-report data (see Figure A1). More extensive population self-report surveys also found that after initial pandemic-related disruptions, heroin, methamphetamine and pharmaceutical opioids consumption remained relatively stable; the differences that did occur were often minor and geographically specific [3, 30]. Conversely, wastewater drug monitoring in Sydney found methamphetamine consumption to be highest within the first month of the pandemic-related lockdown restrictions, as seen within the used syringes analysed within this study at the same time (P2) [31].
Pharmaceutical opioid injection perseveres in Sydney due to access from licit sources [32]. In particular, methadone and buprenorphine are commonly available through opioid agonist treatment programs [33, 34] which can be subsequently injected [35-37]. As Sydney began to ease its lockdown restrictions, the prevalence of oxycodone and buprenorphine injections decreased while methadone injection frequency increased at MSIC, which might have been linked to changes in access to opioid agonist treatment programs hindering their subsequent injection. Specifically, these changes related to new regulations changing the pack sizes available (oxycodone), prescribing guidelines (buprenorphine-naloxone over buprenorphine only) and an increase in the availability of takeaway doses for methadone [34, 38, 39]. It is important to note that opioid agonist treatment programs are just one point of access for pharmaceutical opioids for injection but changes therein likely had some influence on the trends observed within this study. COVID-19 has been related to a variety of impacts on health services accessed by PWID (including disruption to treatment), which might have further impacted which pharmaceutical opioid was injected by MSIC clients [3].
The discrepancy between chemical analysis and self-report data in the second and third samples might have been related to pandemic lockdown restrictions. For both of these time periods, this was primarily associated with the differences in frequencies across heroin and pharmaceutical opioids that were chemically detected and self-reported. Within P3, morphine was detected within 23 syringes (reported within the pharmaceutical opioids sub-category) but was never self-reported (i.e., no client ever chose to inject morphine). There are two explanations: (i) heroin degraded into morphine; and (ii) morphine was sold as heroin. Heroin is known to rapidly hydrolyse into morphine via 6-monoacetylmorphine [40], which could have occurred prior to injection by the client or during the long storage period of the specimens before chemical analysis (1.5 years spent at 4°C before extraction [related to logistic issues]). As to the second explanation, the implementation of widespread border restrictions initially impacted the heroin supply in Australia [41], which is reliant upon natural products sourced outside of Australia [10]. This might have resulted in increasing adulteration or substitution of heroin injected by MSIC clients to supplement the lack of supply. However, this was not formally tested within this study and the change might be explained by some other unknown factor.
There were a number of limitations to be considered in this work, most of which have been explored elsewhere but are briefly revisited here [18, 42]. In the analysis of used syringes, there is an increased risk of contamination (from blood residues and surface contact between syringes within sharps disposal bins). Given the anonymised nature of this work, there was no method to link chemical analysis results to the drug self-reported by the MSIC clients (i.e., it was not possible to ascertain if self-report was accurate at an individual level). It is also important to note that the injecting drug trends observed from MSIC clients might not be representative of all PWID within Sydney (i.e., people who do not attend supervised injecting facilities). Differences in the number of specimens for the chemical analysis and self-report data within the time points impacted the comparison of these two variables, which was related to the potential of multiple syringes being used within one injecting episode [36, 43]. Other sample collection and selection parameter limitations were related to logistical issues caused by the pandemic. The use of varying sample preparation/detection methods, limited sample sizes and only three time points sampled hindered the ability to report on significant differences in injecting drug consumption that could be attributed to the COVID-19 pandemic. Hence the results herein should be collated with information from other data sources to more comprehensively characterise the influence of COVID-19 on injecting drug trends.
Nonetheless, while there are a number of limitations associated with the analysis of used syringes, the information provided can have far-reaching impacts across drug policy. Obtaining objective analytical information on the drug market, directly at the consumption level and examining how these trends change overtime can enable more targeted policy directions by decision-makers that more effectively reduce harm (e.g., implementing drug checking services onsite at MSIC or better targeted health directives to PWID). The continuous monitoring of injecting drug trends through the chemical analysis of used syringes is an insightful harm reduction strategy that supports a harm minimisation approach to the consumption of drugs.
5 CONCLUSION
Within this study, the analysis of used syringes collected from MSIC has demonstrated its capability to complement self-report surveys in providing information on injecting drug consumption during the onset of the COVID-19 pandemic. Heroin and methamphetamine continued to be the most consumed drugs throughout the time periods examined here, with further consumption composed mainly of pharmaceutical opioids or other less frequent drugs. The main pharmaceutical opioid injected also changed throughout the onset of the pandemic from buprenorphine/oxycodone to methadone, related to changes in prescribing frameworks impeding access from licit sources.
The analysis of used syringes completed within this study has highlighted how continual monitoring via this method can complement traditional data sources by providing unique objective information. Further upscaling of this work would assist in detecting a more detailed picture of emerging drug trends more efficiently and facilitate disseminating health warnings more quickly to achieve harm reduction outcomes.
AUTHOR CONTRIBUTIONS
Each author certifies that their contribution to this work meets the standards of the International Committee of Medical Journal Editors.
ACKNOWLEDGEMENTS
The authors would like to acknowledge and thank MSIC for their continued support throughout this study. Specifically, this includes Dr Marianne Jauncey and the Consumer Action Group, who approved this project, the clients who took part and the staff who facilitated this work and provided valuable insight into different aspects of this research. The authors would also like to thank Dr Ronald Shimmon and Susan Shimmon for their support across sample collection and analysis. This research was supported by an Australian Government Research Training Program Scholarship awarded to H. Fursman. Financial support was also provided to conduct this research by the Australian Research Council via the Linkage Projects scheme (LP160100352). Open access publishing facilitated by University of Technology Sydney, as part of the Wiley - University of Technology Sydney agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
None to declare.
APPENDIX





