The evolving role of interventional cytopathology from thyroid FNA to NGS: Lessons learned at Federico II University of Naples
Elena Vigliar
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorAnna Maria Carillo
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorMariantonia Nacchio
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorDomenico Cozzolino
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorGennaro Acanfora
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorMaria Salatiello
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorPasquale Pisapia
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorUmberto Malapelle
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorCorresponding Author
Giancarlo Troncone
Department of Public Health, University of Naples Federico II, Naples, Italy
Correspondence
Giancarlo Troncone, Department of Public Health, University of Naples Federico II, Naples, Italy.
Email: [email protected]
Search for more papers by this authorClaudio Bellevicine
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorElena Vigliar
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorAnna Maria Carillo
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorMariantonia Nacchio
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorDomenico Cozzolino
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorGennaro Acanfora
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorMaria Salatiello
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorPasquale Pisapia
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorUmberto Malapelle
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorCorresponding Author
Giancarlo Troncone
Department of Public Health, University of Naples Federico II, Naples, Italy
Correspondence
Giancarlo Troncone, Department of Public Health, University of Naples Federico II, Naples, Italy.
Email: [email protected]
Search for more papers by this authorClaudio Bellevicine
Department of Public Health, University of Naples Federico II, Naples, Italy
Search for more papers by this authorAbstract
Fine-needle aspiration (FNA) guided by ultrasound (US) has emerged as a highly precise diagnostic method for managing thyroid nodules, significantly diminishing unnecessary surgeries. The effectiveness of US-guided FNA is high when a single specialist performs the FNA procedure and the microscopy. This paradigm has paved the way for the evolution of interventional cytopathology, a specialist with a pivotal role in the preoperative diagnostic process, encompassing patient history review, clinical examination, FNA execution under US guidance, preparation, and microscopic interpretation of cytological samples. As the landscape of precision medicine unfolds, molecular testing assumes greater importance in thyroid cytopathology, particularly in refining the risk of malignancy for indeterminate nodules. The updated Bethesda classification system underscores the clinical significance of molecular tests, emphasizing their role in refining diagnostic accuracy. With this evolving landscape, interventional cytopathologists must adapt by acquiring expertise in molecular technologies and addressing ongoing challenges in workflow harmonization and optimization. This paper delves into our decade-long experience as interventional cytopathologists, focusing on recent endeavours to ensure adequate samples not only for microscopic diagnosis but also for molecular testing. Additionally, here we review the challenges of integrating next-generation sequencing (NGS) technology into clinical practice, highlighting the importance of integrating clinically meaningful molecular data into comprehensive molecular cytology reports.
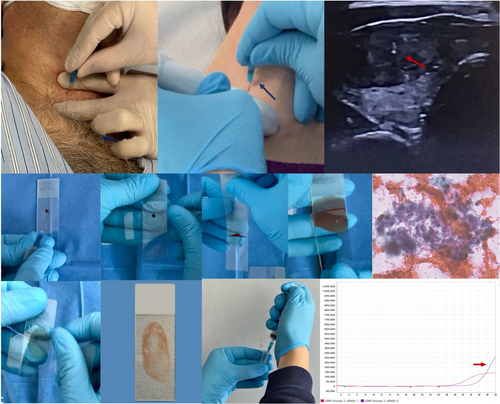
Graphical Abstract
Interventional cytopathologist procedure from the FNA to the molecular result.
This paper delves into decade-long experience of interventional cytopathologists focusing on recent endeavours to ensure adequate samples not only for microscopic diagnosis but also for molecular testing, integrating next-generation sequencing technology into clinical practice.
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Supporting Information
| Filename | Description |
|---|---|
| cyt13415-sup-0001-VideoS1.aviAVI video, 4.4 MB |
Video S1 |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Vohra P, Khanafshar E, Balassanian R. Interventional cytology benefits patients undergoing thyroid FNA. Cancer Cytopathol. 2023; 131(4): 214-216.
- 2Wu M. A comparative study of 200 head and neck FNAs performed by a cytopathologist with versus without ultrasound guidance: evidence for improved diagnostic value with ultrasound guidance. Diagn Cytopathol. 2011; 39(10): 743-751.
- 3Bellevicine C, Vigliar E, Malapelle U, et al. Cytopathologists can reliably perform ultrasound-guided thyroid fine needle aspiration: a 1-year audit on 3715 consecutive cases. Cytopathology. 2016; 27(2): 115-121.
- 4Wu M, Choi Y, Zhang Z, et al. Ultrasound guided FNA of thyroid performed by cytopathologists enhances Bethesda diagnostic value. Diagn Cytopathol. 2016; 44(10): 787-791.
- 5Pitman MB, Abele J, Ali SZ, et al. Techniques for thyroid FNA: a synopsis of the National Cancer Institute thyroid fine-needle aspiration state of the science conference. Diagn Cytopathol. 2008; 36(6): 407-424.
- 6Linsk JA. Aspiration cytology in Sweden: the Karolinska group. Diagn Cytopathol. 1985; 1(4): 332-335.
- 7Grohs HK. The interventional cytopathologist. A new clinician/pathologist hybrid. Am J Clin Pathol. 1988; 90(3): 351-354.
- 8Nishino M, VanderLaan P, Troncone G, et al. Molecular and other ancillary tests. In: SZ Ali, PA VanderLaan, eds. The Bethesda System for Reporting Thyroid Cytopathology: definitions, Criteria, and Explanatory Notes. Springer International Publishing; 2023: 263-284.
10.1007/978-3-031-28046-7_14 Google Scholar
- 9Cibas ES. We are minimally invasive diagnosis. J Am Soc Cytopathol. 2021; 10(2): 113-114.
- 10Abate S, Palombini L, Ferulano GP, Vetrani A, Fresini A, Salvati AM. Our experience in diagnosis of cold nodules of the thyroid by means of thin-needle biopsy. Minerva Med. 1980; 71(23): 1633-1638.
- 11Zajdela A, de Maublanc MA, Schlienger P, Haye C. Cytologic diagnosis of orbital and periorbital palpable tumors using fine-needle sampling without aspiration. Diagn Cytopathol. 1986; 2(1): 17-20.
- 12Romitelli F, Di Stasio E, Santoro C, Iozzino M, Orsini A, Cesareo R. A comparative study of fine needle aspiration and fine needle non-aspiration biopsy on suspected thyroid nodules. Endocr Pathol. 2009; 20(2): 108-113.
- 13Abele JS, Miller TR, King EB, Lowhagen T. Smearing techniques for the concentration of particles from fine needle aspiration biopsy. Diagn Cytopathol. 1985; 1(1): 59-65.
- 14Mayall F, Cormack A, Slater S, McAnulty K. The utility of assessing the gross appearances of FNA specimens. Cytopathol off J Br Soc Clin Cytol. 2010; 21(6): 395-397.
- 15Tetikkurt US, Oz Puyan F, Oz F, Erdogan N, Ceylan S, Yakupoglu A. Diagnostic value of liquid-based (Liqui-PREP) preparations and interobserver reproducibility in fine needle aspiration cytology of the nodular thyroid lesions. Diagn Cytopathol. 2012; 40(5): 388-393.
- 16Padmanabhan V, Marshall CB, Akdas Barkan G, et al. Reproducibility of atypia of undetermined significance/follicular lesion of undetermined significance category using the bethesda system for reporting thyroid cytology when reviewing slides from different institutions: a study of interobserver variability: reproducibility of atypia of undetermined significance/follicular lesion. Diagn Cytopathol. 2017; 45(5): 399-405.
- 17Kuzan TY, Güzelbey B, Turan Güzel N, Kuzan BN, Çakır MS, Canbey C. Analysis of intra-observer and inter-observer variability of pathologists for non-benign thyroid fine needle aspiration cytology according to Bethesda system categories. Diagn Cytopathol. 2021; 49(7): 850-855.
- 18Kocjan G, Chandra A, Cross PA, et al. The interobserver reproducibility of thyroid fine-needle aspiration using the UK Royal College of Pathologists' classification system. Am J Clin Pathol. 2011; 135(6): 852-859.
- 19Crescenzi A, Trimboli P, Basolo F, et al. Exploring the inter-observer agreement among the members of the Italian consensus for the classification and reporting of thyroid cytology. Endocr Pathol. 2020; 31(3): 301-306.
- 20 SZ Ali, PA VanderLaan, eds. The Bethesda System for Reporting Thyroid Cytopathology: definitions, Criteria, and Explanatory Notes. Springer International Publishing; 2023.
10.1007/978-3-031-28046-7 Google Scholar
- 21Salvatore G, Giannini R, Faviana P, et al. Analysis of BRAF point mutation and RET/PTC rearrangement refines the fine-needle aspiration diagnosis of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2004; 89(10): 5175-5180.
- 22Troncone G, Cozzolino I, Fedele M, Malapelle U, Palombini L. Preparation of thyroid FNA material for routine cytology and BRAF testing: a validation study. Diagn Cytopathol. 2010; 38(3): 172-176.
- 23Nishino M. Less is more meets do more with less: exploring differences in thyroid FNA molecular testing between Asian and Western practices. Cancer Cytopathol. 2023; 131(7): 421-423.
- 24Bellevicine C, Migliatico I, Sgariglia R, et al. Evaluation of BRAF, RAS, RET/PTC, and PAX8/PPARg alterations in different Bethesda diagnostic categories: a multicentric prospective study on the validity of the 7-gene panel test in 1172 thyroid FNAs deriving from different hospitals in South Italy. Cancer Cytopathol. 2020; 128(2): 107-118.
- 25Nacchio M, Palladino R, Vigliar E, et al. Evaluating local thyroid cytopathology practices by molecular quality metrics: a multi-institutional study on 4651 FNAs with a focus on the role of the interventional cytopathologist. Cancer Cytopathol. 2023; 131(12): 772-780.
- 26Chong Y, Ji SJ, Kang CS, Lee EJ. Can liquid-based preparation substitute for conventional smear in thyroid fine-needle aspiration? A systematic review based on meta-analysis. Endocr Connect. 2017; 6(8): 817-829.
- 27Bellevicine C, Sgariglia R, Nacchio M, et al. Molecular testing of thyroid fine-needle aspiration: local issues and solutions. An interventional Cytopathologist perspective. J Mol Pathol. 2021; 2(3): 233-240.
- 28Clark DP. Seize the opportunity: underutilization of fine-needle aspiration biopsy to inform targeted cancer therapy decisions. Cancer. 2009; 117(5): 289-297.
- 29Maxwell P, Salto-Tellez M. Training in molecular cytopathology testing. Cytopathol Off J Br Soc Clin Cytol. 2018; 29(1): 5-9.
- 30De Las Casas LE, Hicks DG. Pathologists at the leading edge of optimizing the tumor tissue journey for diagnostic accuracy and molecular testing. Am J Clin Pathol. 2021; 155(6): 781-792.
- 31Ferris RL, Baloch Z, Bernet V, et al. American Thyroid Association statement on surgical application of molecular profiling for thyroid nodules: current impact on perioperative decision making. Thyroid Off J Am Thyroid Assoc. 2015; 25(7): 760-768.
- 32Silver Karcioglu A, Iwata AJ, Pusztaszeri M, Abdelhamid Ahmed AH, Randolph GW. The American Thyroid Association (ATA) integrates molecular testing into its framework for managing patients with anaplastic thyroid carcinoma (ATC): update on the 2021 ATA ATC guidelines. Cancer Cytopathol. 2022; 130(3): 174-180.
- 33De Luca C, Sgariglia R, Nacchio M, et al. Rapid on-site molecular evaluation in thyroid cytopathology: a same-day cytological and molecular diagnosis. Diagn Cytopathol. 2020; 48(4): 300-307.
- 34Durante C, Hegedüs L, Czarniecka A, et al. 2023 European Thyroid Association Clinical Practice Guidelines for thyroid nodule management. Eur Thyroid J. 2023; 12(5):e230067.
- 35Andrioli M, Carocci S, Alessandrini S, et al. Testing for Afirma in thyroid nodules with high-risk indeterminate cytology (TIR3B): first Italian experience. Endocr Pathol. 2020; 31(1): 46-51.
- 36Aydemirli MD, Snel M, van Wezel T, et al. Yield and costs of molecular diagnostics on thyroid cytology slides in the Netherlands, adapting the Bethesda classification. Endocrinol Diabetes Metab. 2021; 4(4):e00293.
- 37Ye W, Hannigan B, Zalles S, et al. Centrifuged supernatants from FNA provide a liquid biopsy option for clinical next-generation sequencing of thyroid nodules. Cancer Cytopathol. 2019; 127(3): 146-160.
- 38Le Mercier M, D'Haene N, De Nève N, et al. Next-generation sequencing improves the diagnosis of thyroid FNA specimens with indeterminate cytology. Histopathology. 2015; 66(2): 215-224.
- 39Song Y, Xu G, Ma T, et al. Utility of a multigene testing for preoperative evaluation of indeterminate thyroid nodules: a prospective blinded single center study in China. Cancer Med. 2020; 9(22): 8397-8405.
- 40Sponziello M, Brunelli C, Verrienti A, et al. Performance of a dual-component molecular assay in cytologically indeterminate thyroid nodules. Endocrine. 2020; 68(2): 458-465.
- 41Ren M, Yao Q, Bao L, et al. Diagnostic performance of next-generation sequencing and genetic profiling in thyroid nodules from a single center in China. Eur Thyroid J. 2022; 11(3):e210124.
- 42Zhou Y, Wu X, Zhang Y, et al. Performance of multigene testing in cytologically indeterminate thyroid nodules and molecular risk stratification. PeerJ. 2023; 11:e16054.
- 43Bellevicine C, Sgariglia R, Malapelle U, et al. Young investigator challenge: can the ion AmpliSeq cancer hotspot panel v2 be used for next-generation sequencing of thyroid FNA samples? Cancer Cytopathol. 2016; 124(11): 776-784.
- 44Sgariglia R, Nacchio M, Migliatico I, et al. Moving towards a local testing solution for undetermined thyroid fine-needle aspirates: validation of a novel custom DNA-based NGS panel. J Clin Pathol. 2022; 75(7): 465-471.