Embryonal and pineal tumours
Abstract
Embryonal and pineal tumours represent a diverse group of central nervous system (CNS) neoplasms. While many of the small round blue cell tumours that make up the embryonal neoplasms share similar histologic qualities, there are several morphologic and cytologic characteristics that are useful in distinguishing different tumour types. Similarly, pineal parenchymal tumours represent clinically diverse tumours, ranging from benign to overtly malignant. The most recent iteration of the World Health Organization Classification of CNS Tumours expanded greatly on the significance of molecular alterations in brain tumour diagnostics. In this article, we summarize the salient cytologic and histologic features of CNS embryonal and pineal tumours, and highlight diagnostically relevant molecular alterations within each tumour type.
Abstract
In the article by Reznicek et al., the authors provide a comprehensive overview of the cytologic, histologic, and molecular features of embryonal tumours and pineal gland tumours of the central nervous system. Integrating high-quality photomicrographs with the most up-to-date knowledge of molecular diagnostics, the article will serve as an invaluable resource for practicing pathologists.
1 INTRODUCTION
The most recent 2021 World Health Organization (WHO) Classification of Central Nervous System (CNS) Tumours expanded greatly on the molecular characteristics and diagnostic classification of brain tumours, initially introduced in the prior 2016 edition. Basic and clinical research has revealed myriad molecular alterations that have significant importance in brain tumour diagnosis, prognostication and treatment. As such, practicing pathologists must be keenly aware not only of the morphologic features of brain tumours but also the growing list of clinically relevant molecular alterations associated with specific tumour entities.
Embryonal and pineal tumours of the CNS represent diverse and heterogenous groups of neoplasms with wide-ranging histologic, cytologic and molecular features. In some instances, histocytologic features between tumour entities can be essentially identical, thereby requiring molecular testing in order to arrive at the most specific diagnosis. This is especially true of medulloblastoma and other embryonal neoplasms. Alternatively, there is a wide range of morphologic features of pineal parenchymal tumours, ranging from the benign pineocytoma to the overtly malignant pineoblastoma.
In this review, we summarize and consolidate the most salient histopathologic features of CNS embryonal and pineal tumours. We provide specific discussion on the cytologic features of these tumours, thereby facilitating diagnosis of smear preparations during intraoperative consultation as well as cytologic preparations in other clinical contexts. We also describe important histologic patterns observed on permanent sections. Finally, we present the molecular features of these tumours and how molecular results are integrated with morphology to arrive at specific diagnostic entities.
2 EMBRYONAL TUMOURS
Central nervous system embryonal tumours are histologically and molecularly diverse. Many share relatively common cytologic and histologic morphologies, composed of poorly differentiated small round blue cell tumours. Others demonstrate more specific features that provide clues to the most appropriate diagnosis, such as the presence of rhabdoid cells in atypical teratoid/rhabdoid tumour (AT/RT) or multilayered rosettes in embryonal tumour with multilayered rosettes (ETMR). Prior to robust molecular profiling studies, many embryonal tumours were lumped together as ‘Central Nervous System Primitive Neuroepithelial Tumors (CNS PNET)’; however, the term CNS PNET is now generally discouraged as these tumours have been shown to represent one of several distinct diagnostic entities, with distinct clinical behaviour.1-4 Similarly, DNA methylation profiling has confirmed and expanded on the range of unique CNS embryonal neoplasms,3-5 with increasingly new entities emerging.6, 7
2.1 Medulloblastoma
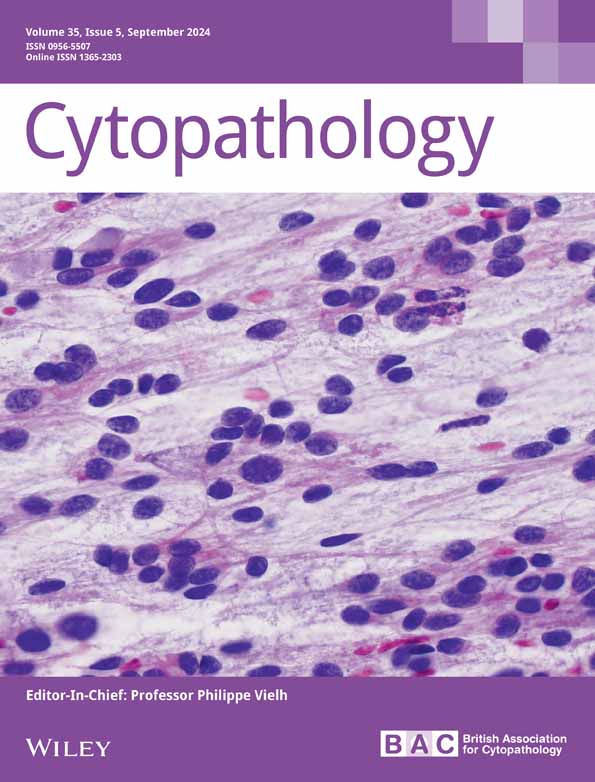
The prototypical embryonal tumour of the CNS, medulloblastoma is a high-grade neuroepithelial neoplasm derived from the progenitor cells involved in cerebellar development.8-11 Thus, by definition, medulloblastomas arise in the cerebellum or dorsal brainstem. The cerebellum is more cellular than most brain regions, due to the high density of internal granular cells; however, these can generally be easily identified as small, mature neurocytic cells with occasional larger Purkinje cells interspersed (Figure 1A). Medulloblastoma most commonly presents in children and is the second most common CNS malignancy in childhood, accounting for approximately one-fifth of all intracranial neoplasms in children.12 Classification of medulloblastoma must be undertaken carefully with both histomorphology and molecular studies playing key roles in integrating the final diagnosis. Cytology also plays an essential role in medulloblastomas, as cerebrospinal fluid assessment and evaluation is required for staging of affected patients. There are four established and well-recognized histologic subtypes of medulloblastoma: classic medulloblastoma, desmoplastic/nodular medulloblastoma (D/N), medulloblastoma with extensive nodularity (MBEN), and large cell/anaplastic medulloblastoma (LCA).
The classic subtype of medulloblastoma accounts for approximately 70%–80% of all medulloblastomas.13, 14 Tumours are characterized cytologically by small, poorly differentiated clusters of discohesive cells with scant cytoplasm and hyperchromatic nuclei with indistinct nucleoli (Figure 1B–D). Apoptotic bodies (Figure 1C) and mitotic figures (Figure 1D) are easily appreciable. Histologically, tumour cells grow in densely packed sheets. Homer-Wright pseudorosettes can be seen, where tumour cells form a ring around a neuropil core (Figure 1E). Foci of nodular growth can be encountered but lack associated desmoplasia. These tumours are typically centred in the midline cerebellum, most commonly in children but can be observed in any age group. Leptomeningeal spread at diagnosis is seen in almost half of cases.15
Desmoplastic/nodular medulloblastoma is characterized by biphasic histomorphology composed of mature appearing ‘pale nodules’ surrounded dense aggregates of immature tumour cells (Figure 1F). Homer-Wright pseudorosettes are observed less frequently. Reticulin stain produces a characteristic ‘reticulin-free zone’ within pale nodules, while the surrounding embryonal cells produce an extensive intercellular reticulin network. It is difficult to distinguish this histologic subtype by smear preparation alone, as the cytologic appearance is generally similar to that of classic tumours; however, sometimes cells from pale zones can be identified by more mature, neurocytic appearing chromatin pattern. Most medulloblastomas arising in the cerebellar hemispheres are D/N, and they account for approximately 20% of all medulloblastoma cases but are enriched in children less than 3 years old (40%–60%).15, 16 Leptomeningeal spread is present in 20% at initial diagnosis.15
Medulloblastoma with extensive nodularity is histologically related to D/N medulloblastoma but differs in several important ways. The nodules in MBEN are often larger with more extensive neuropil background and internodular regions lack significant desmoplasia (Figure 1G). Within the lobular nodules, mature neurocytic tumour cells can often be seen in linear arrays or streams. Cytologically, these tumours are generally indistinguishable from classic and D/N medulloblastoma; however, in some treated cases of MBEN, large, mature ganglion cells can be encountered. MBEN is more likely to occur in the cerebellar vermis and can extend bilaterally with large lobular tumour growth.17 MBENs account for <5% of all medulloblastomas but are enriched in young children less than 3 years old, in which they account for 20% of tumours.15, 18 CSF dissemination is uncommon and these tumours generally respond well to treatment.19
Finally, LCA medulloblastoma is remarkable for marked nuclear anaplasia, including large cells with nuclear pleomorphism, prominent nucleoli, nuclear pseudoinclusions and nuclear moulding with cell wrapping (‘cannibal cells’) (Figure 1H). These tumours have particularly high mitotic indices and necrosis is commonly encountered. LCA accounts for 10% of all medulloblastomas, occurs in any age group, and is often located in the midline cerebellum with involvement of adjacent structures, including the fourth ventricle and brainstem.20, 21 CSF dissemination is observed at primary diagnosis in up to 70% of cases, and tumour recurrence is frequent.14, 20
By definition, all medulloblastomas are defined as high-grade neuroepithelial tumours and are designated as CNS WHO grade 4. However, recent studies have demonstrated important differences in prognosis based on the molecular underpinnings of these tumours, with four primary groups of molecularly defined medulloblastoma subtypes: WNT-activated, SHH-activated, Group 3 and Group 4.21-23 WNT-activated tumours are characterized by somatic CTNNB1 alterations or germline APC mutations. WNT-activated tumours almost always display classic histomorphology, demonstrate aberrant nuclear beta-catenin immunoreactivity and have remarkably good long-term outcomes with overall survival approaching 100%.24 The SHH-activated group is divided into TP53-wildtype and TP53-mutant subgroups. SHH activation can be demonstrated with GAB1 or YAP1 immunostains. It should be noted that WNT-activated and SHH-activated tumours both express YAP1; however, SHH-activated tumours lack nuclear beta-catenin expression.25-27 Tumours with SHH activation and wildtype TP53 often demonstrate D/N or MBEN morphology18, 25 and are commonly seen in medulloblastomas from patients with nevoid basal cell carcinoma (Gorlin) syndrome.28 SHH-activated, TP53-mutant tumours often demonstrate LCA morphology or D/N with foci of anaplasia.29 These tumours have especially poor prognosis in children with co-occurring MYCN amplification.30-32 Unlike the previously described molecular subtypes, group 3 and group 4 tumours do not have a known molecular driver alteration. Instead, these tumours are defined by unique DNA methylation profiles. Together, these tumours account for the majority of medulloblastoma cases (25% groups 3, 40% group 4), though group 3 tumours are rare in adults.15, 33 Group 3 and group 4 can be categorized together as non-WNT/non-SHH medulloblastoma, particularly if DNA methylation profiling is not available. Most group 3 and group 4 tumours demonstrate classic morphology, though some tumours display LCA histology, and patients generally experience poor outcomes.25 DNA methylation can also be useful in rare cases with myogenic and/or melanotic differentiation, which can be diagnostically challenging and display unusual immunohistochemical staining profiles.34 It should be noted that even more granular, DNA methylation-based provisional subtypes exist for many of these molecular classes of medulloblastoma.33, 35
2.2 Atypical teratoid/rhabdoid tumour (AT/RT)
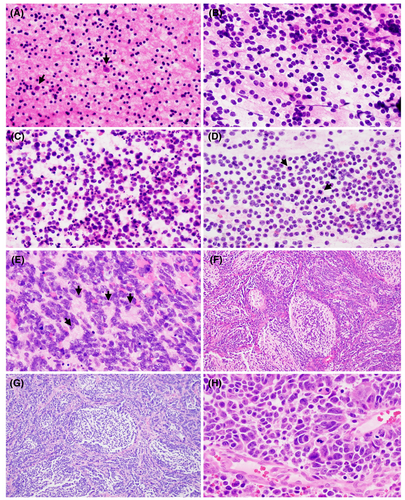
Atypical teratoid/rhabdoid tumour is a high-grade neuroepithelial malignancy (CNS WHO grade 4), typically encountered during infancy, and can arise anywhere along the neuraxis.36, 37 The cytology and histomorphology of AT/RT are heterogeneous. Cytologic smears are generally composed of immature, embryonal-appearing cells with high nuclear-to-cytoplasmic ratio and well-defined cell borders (Figure 2A). Nuclei are large and round with vesicular chromatin and prominent nucleoli (Figure 2A,B), the latter of which can be helpful to distinguish from most types of medulloblastoma. Rhabdoid cells are often larger than background embryonal cells and contain brightly eosinophilic cytoplasm which displaces the nucleus to the periphery. The presence of rhabdoid cells varies from numerous to focal to completely absent.38 Intracytoplasmic eosinophilic granules can be encountered in some cases. Histologically, tumours can display components of neuroectodermal, mesenchymal and/or epithelial features.39, 40 In tumours with mesenchymal features, cells may show a spindled morphology in a myxoid background. Brisk mitotic activity, apoptosis and necrosis are readily appreciated. All embryonal neoplasms, especially in infants, should be screened with INI1/BAF47 immunohistochemistry, which demonstrates loss of nuclear expression in tumour cells of AT/RT (retained nuclear expression in non-neoplastic background cells) and corresponds to biallelic molecular loss of SMARCB1 on chromosome 22q11.2,41, 42 though this is not a feature unique to AT/RT and is seen in other neoplasms with SMARCB1 deficiency.43, 44 Rare cases of AT/RT will demonstrate loss of SMARCA4, corresponding to loss of BRG1 expression by IHC.45-47 Correct identification of AT/RT at the time of intraoperative consultation is important as, in some practices, a small portion of tumour is not resected in order to gauge treatment response. Similar to medulloblastoma, cytology of CSF is required for staging. AT/RT forms a unique cluster on DNA methylation profiling, with recent studies suggesting at least three distinct AT/RT subgroups.48 AT/RT is a highly aggressive malignancy with poor long-term outcome.49-51
2.3 Cribriform neuroepithelial tumour (CRINET)
Cribriform neuroepithelial tumour is a provisional entity in the 2021 CNS WHO. This extremely rare tumour is characterized as neuroectodermal tumour with INI1 loss and displaying unique cribriform/ribbon-like morphology but lacking rhabdoid features.52, 53 CRINET is densely cellular and composed of round cells with scant cytoplasm and immature chromatin. True rosettes can be encountered but rhabdoid cells are absent. Like AT/RT, CRINET generally demonstrates loss of nuclear INI1/BAF47 expression, corresponding to biallelic loss of SMARCB1.53 Given their unusual cribriform morphology, the differential diagnosis can include choroid plexus carcinoma; however, CRINET lacks expression by choroid plexus marker Kir7.1.52 CRINETs are typically located in the peri-ventricular regions in infants and young children. Chromosome 22q loss, affecting the SMARCB1 region, is the only recurrent chromosomal alterations described thus far. While molecularly similar to AT/RT (including DNA methylation profile, interestingly), patients with CRINET seem to have more favourable long-term outcome.53
2.4 Embryonal tumour with multilayered rosettes (ETMR)
Embryonal tumour with multilayered rosettes are embryonal neoplasms harbouring distinct yet varied histological patterns and unified molecularly by C19MC structural alterations or (rarely) DICER1 mutations.54, 55 ETMR now includes the formerly disparate entities of embryonal tumour with abundant neuropil and true rosettes (ETANTR), medulloepithelioma and ependymoblastoma.56 These rare tumours occur predominantly in young children, most commonly in the cerebral hemispheres but other neuraxial locations can be encountered.57, 58 Cytologically, these tumours are similar to other embryonal malignancies, composed of tumour cells with large nuclei, immature chromatin and scant cytoplasm (Figure 2C). Rosette formations are characteristic, often composed of elongated, pseudostratified cells in multiple layers, with nuclei oriented away from the core (Figure 2C,D). Brisk mitotic activity and numerous apoptotic bodies are common. ETMR is uniquely immunopositive for LIN28,59 though this is not entirely specific.60 Clinical staging includes CSF cytology and the prognosis of ETMR is poor.
The ETANTR pattern of ETMR is characterized by a biphasic morphology, composed of areas with malignant embryonal cells with scant cytoplasm and other areas with neuropil and paucicellular mature neurocytic appearing tumour cells. Multilayering can be seen among the embryonal component.61, 62 The ependymoblastoma pattern consists of sheets of embryonal cells with numerous multilayered rosettes and lacks areas of mature neuropil.63 Finally, the medulloepithelioma pattern (not to be confused with the ciliary body derived neoplasm that occurs within the eye) is composed of multilayered rosettes forming tubules, papillary structures or trabeculae.64 Again, these three histologic patterns have been unified under the diagnostic entity ETMR due to shared molecular characteristics and thought to represent divergent differentiation of the same entity.
2.5 CNS neuroblastoma, FOXR2-activated
A new entity in the 2021 CNS WHO, CNS neuroblastoma, FOXR2-activated, is a rare embryonal neoplasm with variable neurocytic differentiation, including the presence of ganglioid cells and neuropil matrix. Perivascular pseudorosettes are common, which can resemble ependymoma; however, CNS neuroblastoma lacks widespread GFAP expression and is often strongly Olig2 positive.65, 66 Nuclear palisading, Homer-Wright pseudorosettes, and invasion of brain tissue have also been described.1 Immature elements of the tumour are composed of cells with high N:C ratio, round to angulated nuclei, and hyperchromatic chromatin (Figure 2E,F). Mature elements of the tumour are composed of large ganglioid cells with open chromatin, variable prominent nucleoli and often organized in clusters (Figure 2F, inset). This tumour is characterized by activation of the transcription factor FOXR2 by way of various structural rearrangements on chromosome Xp11.21.1, 67 CNS neuroblastoma, FOXR2-activated is generally located in the cerebral hemispheres adjacent to the lateral ventricles of children.68 Cytologic examination of the CSF for staging purposes.
2.6 CNS tumour with BCOR internal tandem duplication
Another new entity in the 2021 CNS WHO, CNS tumour with BCOR internal tandem duplication (ITD) is a rare embryonal malignancy predominantly affecting children and located in the cerebral or cerebellar hemispheres.69 Tumour cells are round to oval with open chromatin, faintly eosinophilic cytoplasm and often with delicate glial-like fibrillary processes (Figure 2G). Tumour growth is generally compact with a defined interface with the adjacent brain, though focal infiltration may be observed. Perivascular pseudorosettes, myxoid background, glomeruloid microvascular proliferation and pseudopalisading necrosis can all be observed (Figure 2H). These tumours have high mitotic activity. It is defined by the presence of ITD of exon 15 of the BCOR gene,1, 69 molecularly identical to that observed in clear cell sarcoma of the kidney, primitive myxoid mesenchymal tumour of infancy and undifferentiated round cell sarcoma in infants.70-72 CNS tumour with BCOR ITD forms a unique cluster by DNA methylation analysis.73 CSF cytology is required for staging and, due to the rarity of this tumour, clinical outcome data are limited though limited case series suggest poor overall prognosis.69
2.7 CNS embryonal tumour not elsewhere classified (NEC)/not otherwise specified (NOS)
Like other tumour types, the 2021 CNS WHO allows for designation of embryonal tumours as not elsewhere classified (NEC) or not otherwise specified (NOS). These tumours demonstrate histologic and immunophenotypic evidence of an embryonal malignancy, but molecular testing does not place a tumour in a known diagnostic category (in which the NEC designation is used), or if molecular testing was not possible or failed to yield results (in which case NOS designation is most appropriate).74 These tumours can share histologic features with other tumour types, such as high-grade glioma, and care should be taken to rule out other diagnostic entities. Rare embryonal tumours have been reported with structural alterations including BRD4-CREBBP fusion, MYO5A-NTRK3 fusion, CIC-LEUTX fusion and others.75-77
3 PINEAL TUMOURS
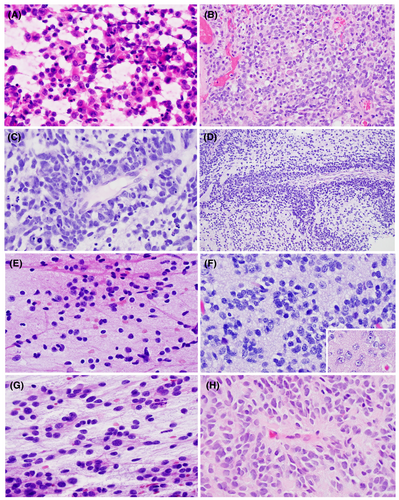
The pineal gland is a small midline brain structure composed primarily of pinealocytes, oval cells with speckled chromatin organized in vague lobules (Figure 3A) often with admixed background calcifications. These cells have both photosensory and neuroendocrine functions. Moreover, the gland plays an important role in the production and secretion of melatonin.78 Many tumours of the pineal region are thought to be derived from the pinealocytes themselves. Unlike the CNS embryonal tumours, which are composed entirely of CNS WHO grade 4 neoplasms, tumours of the pineal parenchyma are heterogeneous, including the benign pineocytoma (CNS WHO grade 1) to the malignant pineoblastoma (CNS WHO grade 4). Additionally, tumours of the pineal region are rare, accounting for less than 1% of all intracranial neoplasms, and affect many different age groups. Due to the gland's location near the posterior wall of the third ventricle, these tumours often cause patients to present clinically with signs and symptoms of CSF obstruction.79 Finally, non-neoplastic pineal gland (Figure 3A) is occasionally sampled during surgical biopsy of pineal region masses and can create diagnostic confusion, especially with other low-grade neoplastic processes, due to its cellularity and variable histologic appearance that changes with age. Helpful distinguishing features include presence of corpora arenacea (microcalcifications, sometimes referred to as ‘brain sand’), intracytoplasmic lipofuscin pigment and lobular architecture.80, 81
3.1 Pineocytoma
Pineocytoma is a rare, well-differentiated pineal parenchymal neoplasm (CNS WHO grade 1) composed of small, round to oval, monotonous cells with low-grade nuclear features, often closely resembling non-neoplastic pinealocytes (Figure 3A). Tumours grow in moderately cellular sheets and, in contrast to the non-neoplastic pineal gland and pineal cysts, form large pineocytomatous rosettes composed of eosinophilic cellular processes which resembles neuropil (Figure 3B). In some pineocytomas, pleomorphism may be encountered, with large, occasionally multinucleated, ganglioid cells.82 Mitotic activity is absent. There are no recurrent genetic alterations in pineocytoma, though they form a unique cluster on DNA methylation profiling.83 Pineocytoma is often encountered in adult patients and is associated with excellent long-term outcome, with extent of surgical resection being the primary prognostic factor.84, 85
3.2 Pineal parenchymal tumour of intermediate differentiation (PPTID)
Pineal parenchymal tumour of intermediate differentiation, as its name suggests, is a pineal parenchymal tumour that is intermediate in malignancy (CNS WHO grade 2–3) between pineocytoma and pineoblastoma, which tends to present in young adults. The tumour is composed of diffuse sheets or large lobules of monomorphic round cells with neuroendocrine-like ‘salt-and-pepper’ chromatin (Figure 3C,D). Some degree of cytoplasm should be appreciable. Overall, the tumour cells appear to be more differentiated than those observed in pineoblastoma (Figure 3E). Molecular alterations involving the KBTBD4 gene are highly characteristic and if lacking, alternative diagnoses should be considered.86, 87 PPTID displays a unique DNA methylation profile with two distinct groups, the significance of which is unclear.83 Prognosis is intermediate between pineocytoma and pineoblastoma, and PPTID is capable of local recurrence and CSF dissemination.88 Though precise grading criteria have not been established, some studies suggest that low-grade PPTID demonstrates little to no mitotic activity, low Ki67 proliferation index (<5%), and expression of neurofilament in numerous cells; whereas high-grade PPTID show elevated Ki67 (≥5%) proliferation index and focal to absent neurofilament immunoreactivity.84, 89
3.3 Pineoblastoma
Pineoblastoma is a malignant embryonal neoplasm (CNS WHO grade 4) arising from the pineal parenchyma. Pineoblastoma typically occurs in children but may present at any age and can present as part of the retinoblastoma and DICER1 tumour predisposition syndromes.90, 91 Like other CNS embryonal neoplasms, tumour cells of pineoblastoma show high nuclear-to-cytoplasmic ratio, scant cytoplasm, hyperchromasia and numerous mitoses (Figure 3E,F). Tumour cells grow in sheets with occasional Homer-Wright pseudorosettes; pineocytomatous rosettes are absent. Necrosis is a common feature. In addition to the neuroanatomic location of the tumour, molecular profiling is important to distinguish pineoblastoma from other embryonal neoplasms. DNA methylation profiling has demonstrated four subtypes of clinically and prognostically relevant groups: (1) pineoblastoma, miRNA processing-altered_1, which arises in younger children and has intermediate outcome; (2) pineoblastoma, miRNAprocessing-altered_2, which arises in older children and has excellent outcome; (3) pineoblastoma, RB1-altered, which arises in infants, resembles retinoblastoma and has terrible long-term outcome with frequent CSF dissemination; (4) pineoblastoma, MYC/FOXR2-altered, which also arise in infants and have poor outcome.83, 92, 93 The tumours with miRNA processing defects show mutually exclusive alterations in DICER1, DROSHA, or DGCR8.91 CSF cytology is utilized for staging in patients with pineoblastoma.
Pineal anlage tumour is an extremely rare tumour, thought to represent a histologic pattern of pineoblastoma. These tumours demonstrate a combination of neuroectodermal and mesenchymal components.94 The former is characterized by sheets of malignant embryonal cells, occasionally with melanotic pigment or ganglioid differentiation. The heterologous mesenchymal elements can include cells with skeletal muscle differentiation and elements of cartilaginous differentiation. DNA methylation studies in limited cases have aligned with pineoblastoma and other tumour types.91, 95
3.4 Papillary tumour of the pineal region (PTPR)
Papillary tumour of the pineal region (CNS WHO grade 2–3) is a unique neuroepithelial tumour thought to be derived from the ependymal cells of the vestigial subcommisural organ remnant.96 It occurs in adults and children with a wide age range of affected patients.97 PTPR is characterized by distinct epithelial appearing tumour cells with a mixture of papillary and solid architecture. Fibrovascular cores lined by large eosinophilic cells are typical (Figure 3G). Tumour cells are round (solid areas) to columnar (papillary areas) with stippled chromatin and occasional nuclear pleomorphism (Figure 3H). Additionally, cytologic smears may show a tigroid background.98 PTPR demonstrate unique immunoreactivity for CK18.99 Molecularly, PTPR forms a unique cluster by DNA methylation profiling and recurrent copy number changes include loss of chromosome 10 and gains of chromosomes 4 and 9.99, 100 Prognosis is intermediate and variable (CNS WHO grade 2–3). Local recurrence is a common complication, and factors associated with outcome are unclear97; however, Ki67 proliferation index (≥10% by one study) may be associated with increased recurrence and shorter overall survival.101
3.5 Desmoplastic myxoid tumour of the pineal region, SMARCB1-mutant (DMTPR)
Desmoplastic myxoid tumour of the pineal region is a new entity in the 2021 CNS, with only limited case series and case reports. Limited data suggest that DMTPR occurs in a wide age range of patients, including both teenagers and adults.102-105 Tumour cells are epithelioid to spindled, embedded within a prominent collagenized background with variable amounts of myxoid material (Figure 3I,J). Whorling and fascicular growth patterns may be encountered and tumours often show hyalinized blood vessels. Mitotic activity is rare. Tumours show loss of INI1 (BAF47) immunoreactivity, consistent with genetic alterations of SMARCB1 on chromosome 22q11. DNA methylation profiling reveals a unique cluster of DMTPR that lies in close proximity to both AT/RT and poorly differentiated chordoma.102 Due to the rarity of cases, prognostic features and CNS WHO grading criteria have not been established for DMTPR.
4 CONCLUSIONS
In summary, we have described the salient clinicopathologic, cytologic, and molecular features of CNS embryonal and pineal tumours. While many embryonal tumours have histocytologic features of small round blue cell tumours, there are several features that can be identified enabling proper diagnosis. Similarly, the pineal parenchymal tumours are clinically diverse, and there are numerous unique cytologic and histologic characteristics allowing for proper identification. Finally, integrating morphologic information with a complete molecular profile allows the pathologist to provide not only a specific diagnosis but also prognostic and potential targetable therapeutic information as well.
AUTHOR CONTRIBUTIONS
Study conception, design, and supervision: JTA. Data collection and manuscript review and editing: JR, NM, PJ, NW, JTA. Original draft preparation: JR, JTA.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data are available by request to the corresponding author.