Feasibility of ultrasound-assisted catheter-directed thrombolysis for submassive pulmonary embolism: A meta-analysis of case series
Funding information
National Key Laboratory of Respiratory Diseases, 2017 Self-determined Project (Youth Project) (No. SKLRD-QN-201707).
Abstract
Background
During the past few years, there has been a surge in the use of ultrasound-assisted catheter-directed thrombolysis (UACDT) for submassive pulmonary embolism (SPE). However, few studies evaluated the feasibility of UACDT for SPE.
Purpose
To evaluate the feasibility of UACDT in treating SPE.
Methods
A comprehensive search of online databases was performed. Search terms UACDT in SPE were entered into PubMed, Embase, Scopus and the Cochrane Library to identify related articles published until October 2018. A quality assessment and data extraction were performed by two researchers. Meta-analysis was performed using R statistical software.
Results
Twelve studies with 485 patients were included in this meta-analysis. The pooled right ventricular/left ventricular ratio decrease and pulmonary artery systolic pressure drop after treatment was −0.34 (95% CI: −0.43, −0.25) and −15.05 (95% CI: −18.10, −12.00) mm Hg, respectively. The pooled major bleeding rate was 1.0% (95% CI: 0.0%, 3.0%), and the in-hospital mortality was 0.0% (95% CI: 0.0%, 1.0%).
Conclusion
This up to data meta-analysis confirms that UACDT is a feasible treatment for SPE.
1 OBJECTIVES
Acute pulmonary embolism is a leading cause of in-hospital morbidity and mortality. It remains the most common preventable cause of in-hospital death.1, 2 In accordance with the 2014 European Society of Cardiology guideline on the diagnosis and management of acute pulmonary embolism, it can be divided into the following three categories: low, intermediate and high risk.3 While American Heart Association classifies it with massive, submassive, and minor pulmonary embolism.4 Submassive pulmonary embolism (SPE) defined as pulmonary embolism patient with right ventricular dysfunction (RVD) on imaging or biochemical assay.5 Currently, there are no uniformly accepted recommendation for systemic thrombolysis in the treatment of SPE6-8 for the growing evidence that systemic thrombolysis in SPE might increase bleeding complications.9
A novel ultrasound-assisted catheter-directed thrombolysis (UACDT) technology, EkoSonic Endovascular System (EKOS Corporation, Bothell, Washington) was reported to facilitate thrombolysis with reducing bleeding risk in treating pulmonary embolism series.10, 11 It combined conventional catheter-directed thrombolysis equipment with a catheter system that used high-frequency, low-power ultrasound.5 According to in vitro experiments, it caused reversible disaggregation of uncrosslinked fibrin fibres, thus might increase the thrombus permeability for thrombolytic drugs. Additionally, the penetration of thrombolytic drugs into the thrombus might be increased by ultrasound pressure waves. But whether UACDT was an alternative therapy for SPE was uncertain. The purpose of this retrospective study was to assess hemodynamic and clinical outcomes in patients with SPE treated by UACDT.
2 DATA SOURCE
A systematic and comprehensive literature search of online databases including PubMed, Embase, Scopus and the Cochrane Library were performed to identify observational studies and RCTs that reported the outcomes of UACDT in treating SPE. These keywords were used to identify relevant articles: ultrasound-assisted catheter-directed thrombolysis, ultrasound-assisted thrombolysis, ultrasound-accelerated thrombolysis, submassive pulmonary embolisms, submassive pulmonary thromboembolisms, intermediate-risk pulmonary embolisms, intermediate-risk pulmonary thromboembolisms. The Boolean logical operators OR and AND were used to maximize the number of hits.
3 STUDY SELECTION
Literature were independently evaluated by two investigators, and conflicts were adjudicated by a third investigator. The following inclusion criteria were applied: (a) submassive (intermediate-risk) pulmonary embolism; (b) use of computed tomography (CT), pulmonary angiography, or pulmonary perfusion scans to diagnose; (c) UACDT in the treatment; (d) reported outcomes of clinical efficiency and main complications. The following exclusion criteria were used: the treatment of SPE patients without UACDT, or patients with low-risk pulmonary embolism or massive (high-risk) pulmonary embolism.
Data were extracted independently by two investigators, and conflicts were adjudicated by a third investigator. For the selected studies, information on all available variables was extracted and entered into a Microsoft Excel database. The extracted information included the number of patients, the number of in-hospital deaths, the number of major and minor bleeding patients, the pulmonary artery systolic pressure (PASP) before and after treatment, the right ventricular/left ventricular (RV/LV) ratio before and after treatment, thrombolytic drugs and dose, the number of unilateral and bilateral intervention, the number of days of follow-up and the Miler index before and after treatment. Major bleeding was defined as bleeding in a vital organ or site, including retroperitoneal, intraocular, spinal, intraperitoneal and intracranial bleeding or required infusion of one or more units of red blood cells. Quality assessment of included studies was performed by the Newcastle-Ottawa Scale (NOS). It includes three metrics: patient selection, comparability of groups and the ascertainment of either the exposure or outcome of interest.
Mean difference (MD) with 95% confidence interval (95% CI) was calculated for continuous outcomes (decrease of RV/LV ratio and PASP after the treatment). Proportion with 95% confidence interval (95% CI) was calculated for categorical outcomes which includes the rate of major bleeding and in-hospital mortality. To evaluate the feasibility of UACDT in treating SPE, we conducted meta-analysis to get the pooled results which includes the rate of major bleeding and in-hospital mortality, decrease of RV/LV ratio and PASP after the treatment. We used Q test and I2 to examine the heterogeneity among effect estimates. Statistical heterogeneity among studies was defined as I2 statistic greater than 50%. Fixed effects model was preferred to random effects model when there was no statistically significant heterogeneity, and vice versa when there was significant heterogeneity. The possibility of publication bias was assessed using the Begg’s test and visual inspection of a funnel plot. We also performed the non-parametric “trim and fill” procedure to further assess the possible effect of publication bias in our meta-analysis. Statistical significance was taken as two-sided (P < .05). The analysis was conducted with R statistical software (version 3.5.1).
4 RESULTS
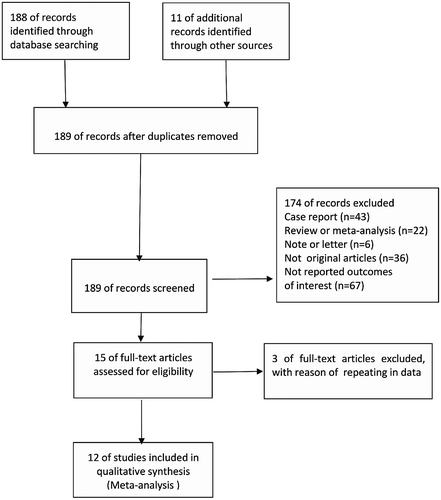
A total of 188 records were screened from four previously mentioned online databases up to October 10, 2018. A manual search and inspection of reference lists and previous reviewed articles identified 11 additional relevant studies. After removing duplicate records, a total of 189 studies remained. Additionally, 174 studies were excluded based on the title, abstract and content details. Finally, 12 full-text articles were included in this analysis (Figure 1). The results of the quality assessment of each study and the contextual details are summarized in Table 1. All 12 studies, within the period from October 2009 to December 2017, were conducted in five different countries, with the inclusion of 485 patients. Two studies were prospective observational studies, while other 10 were retrospective observational studies. All studies received 5, 6 or 7 stars in study quality assessment on a scale of 0-9. At the same time, the number of in-hospital death, the number of major and minor bleeding patient, the PASP before/after treatment and the RV/LV ratio before and after treatment were also shown in Table 1. The demographics of included studies and the interventions detail including thrombolytic drugs and doses, the number of unilateral and bilateral intervention were shown in Table 2. All thrombolytic drugs used in the included study were tissue plasminogen activator (tPA). For all the 12 observational studies, only 1 study had a control group which treated SPE by anticoagulation with heparin alone.
| Study | Year | Country | Study type | Study period | Quality score | Death (n) | Major bleeding (n) | Minor bleeding (n) | PASP (mm Hg) before/after | RV/LV before/after |
|---|---|---|---|---|---|---|---|---|---|---|
| Mohan [18] | 2018 | USA | Retro | 2012.10-2017.12 | 5 | 0 | 0 | 4 | NA | 1.48 ± 0.32/1.17 ± 0.26 |
| Fuller [19] | 2017 | USA | Retro | 2012.12-2015.7 | 6 | 0 | 0 | 4 | NA | NA |
| Kaymaz [20] | 2017 | Turkey | Retro | 2012.10-2015.6 | 6 | NA | NA | 2 | 55.8 ± 12.6/37.3 ± 8 | 1.18 + 0.19/0.91 + 0.10 |
| Ozmen [21] | 2016 | Turkey | Retro | 2012.10-2013.8 | 6 | 1 | 0 | 0 | 49/29 | 1.26 ± 0.58/0.91 ± 0.19 |
| Bagla [22] | 2015 | USA | Retro | 2012.6-2014.4 | 7 | 0 | 1 | 4 | 49.8 ± 13.76/31.1 ± 9.9 | 1.59 ± 0.54/0.93 ± 0.17 |
| Engelberger [23] | 2015 | Swit | Retro | 2010.4-2013.1 | 6 | 0 | 2 | 2 | 51.4 ± 15.5/40.7 ± 10.8 | 1.12 ± 0.30/0.98 ± 0.20 |
| McCabe [24] | 2015 | USA | Retro | 2010.11-2014.2 | 5 | 0 | 1 | 6 | 60 ± 15/42 ± 13 | 1.42 + 0.21/1.06 + 0.23 |
| Piazza [25] | 2015 | USA | Pro | 2012.6-2013.2 | 7 | 0 | 8 | NA | 51.4 ± 16 /37.5 ± 11.9 | 1.55 ± 0.39/1.13 ± 0.20 |
| Kucher [26] | 2014 | Swit | Pro | 2010.11-2013.1 | 7 | 0 | 0 | 2 | NA | 1.28 ± 0.19/0.99 ± 0.17 |
| Quintana [27] | 2014 | USA | Retro | 2010.3-2012.1 | 6 | 0 | 0 | 0 | NA | NA |
| Dumantepe [28] | 2014 | Turkey | Retro | 2010.11-2012.12 | 6 | 0 | 0 | 2 | NA | NA |
| Kennedy [29] | 2013 | Australia | Retro | 2009.10-2012.4 | 6 | 0 | 0 | 1 | 47 ± 15/37 ± 13 | NA |
- Abbreviations: NA, not available; Pro, prospective study; Retro, retrospective study.
| Study | No. of patients | Male/Female | Mean age (y) | Follow-up days | t-PA dose (mg) | Unilateral/Bilateral | Comparison groups | Miler index before/after |
|---|---|---|---|---|---|---|---|---|
| Mohan 2018 | 30 | 16/14 | 50 | NA | 23.34 ± 2.30 | 13/17 | NO | NA |
| Fuller 2017 | 27 | 11/16 | 54 ± 15.5 | NA | 24 | 4/21 | NO | NA |
| Kaymaz 2017 | 60 | NA | NA | 310 | 35.4 + 16.7 | NA | NO | NA |
| Ozmen 2016 | 10 | 6/4 | 53.2 ± 14.1 | 180 | 31.7 ± 3.22 | NA | NO | 21/8 |
| Bagla 2015 | 45 | 25/20 | 56.5 ± 13.6 | 30 | 24 | 3/42 | NO | 21/8 |
| McCabe 2015 | 53 | 27/26 | 57.6 ± 16.2 | NA | 24.6 ± 9 | NA | NO | NA |
| Engelberger 2015 | 38 | 26/12 | 69 ± 12 | 90 | 20.1 + 3.7 | 7/31 | NO | NA |
| Piazza 2015 | 119 | NA | 59 ± 16.1 | 30 | 23.7 ± 2.9 | NA | NO | 22.5 ± 5.7/15.8 ± 5.9 |
| Kucher 2014 | 30 | 11/19 | 64 ± 15 | 90 | 20 | 8/22 | Anticoagulation with heparin alone | NA |
| Quintana 2014 | 8 | NA | NA | 180 | 18 | 5/3 | NO | NA |
| Dumantepe 2014 | 17 | NA | NA | 180 | 21 | 12/5 | NO | NA |
| Kennedy 2013 | 48 | NA | NA | 90 | 35.1 ± 11.1 | NA | NO | 26 ± 3/17 ± 6 |
- Abbreviations: LV, left ventricle; NA, not available; PASP, pulmonary artery systolic pressure; RV, right ventricle.
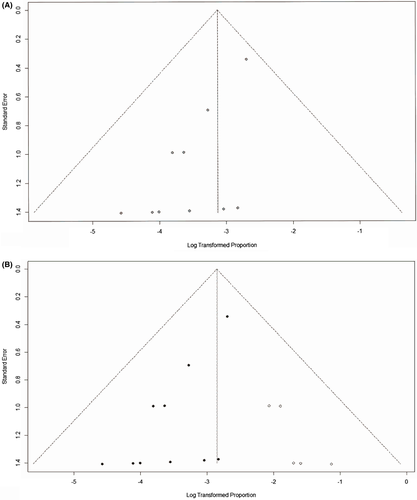
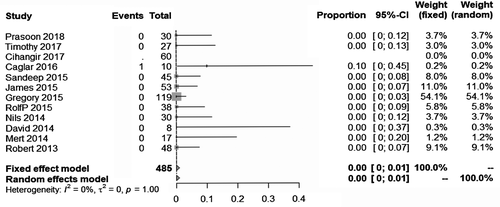
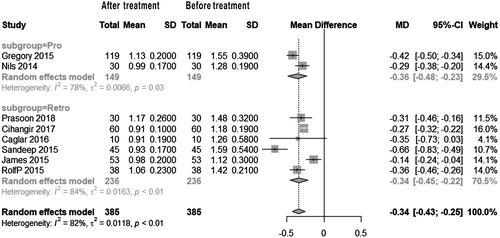
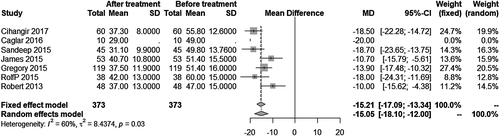
The pooled major bleeding rate was 1.0% (95% CI: 0.0%, 3.0%) (Figure 2). Visual inspection of the Begg’s funnel plot revealed asymmetry (P = .0013) (Figure 3A). This might present the possibility of publication bias. Because of this, we undertook a sensitivity analysis using the trim and fill method, which conservatively imputes hypothetical negative unpublished studies to mirror the positive studies that cause funnel plot asymmetry. The imputed studies produce a symmetrical funnel plot (Figure 3B). The pooled analysis incorporating the hypothetical studies continued to show low major bleeding rate of SPE patients treating by UACDT (4.8% (95% CI: 3.0%, 7.8%)), suggesting the low possibility of publication bias. The pooled in-hospital mortality was 0.0% (95% CI: 0.0%, 1.0%) (Figure 4). The pooled rate of decompensation (with circulatory shock defined by cardiac arrest, persistent hypotension (systolic blood pressure <90 mm Hg), requirement of vasoactive medications or evidence of end-organ hypoperfusion) was 0.0% (95% CI 0.00, 0.79%). The pooled estimate for decrease in RV/LV ration after treatment was −0.34 (95% CI: −0.43, −0.25). The I2 statistic showed high heterogeneity. To determine the source of the heterogeneity, a subgroup analysis was performed based on study type (Figure 5). The pooled difference of PASP after treatment was −15.05 (95% CI: −18.10, −12.00) mm Hg (Figure 6).

5 DISCUSSION
During the past few years, there has been a surge in the use of UACDT for SPE.12-14 EkoSonic (EKOS, Bothell, Washington) UACDT system was adopted for treating pulmonary embolism. It consists of a 5.2 Fr conventional infusion catheter which contains an internal cable that transmits high-frequency, low-power ultrasound to release the fibrin chain and to enhance the ability of thrombolysis. But its feasibility has not been fully understood because of the lack of meaningful large-scale studies.
A meta-analysis performed by Avgerinos et al30 mainly compared the clinical success (in-hospital survival with stabilization of hemodynamics, without decompensation or any major complication) and major bleeding in patients with massive and SPE. In their study, the pooled estimate was 94.5% (95% CI, 91.7%-96.9%) for UACDT clinical success without stratifying for PE type, and clinical success for UACDT in SPE was 97.5% (95% CI, 95.0%-99.4%). However, they did not report the pooled RV/LV ratio decrease and PASP drop of UACDT in treating SPE. Another meta-analysis performed by Kaymaz et al15 included 11 studies of UACDT to treat acute pulmonary embolism. The results showed that UACDT significantly reduced PASP and RV/LV ratio, and the pooled incidence of major bleeding was 5.5%. However, they did not report the major bleeding rate of massive and SPE under the same condition. The submassive (intermediate-risk) PE was defined as cardiopulmonary deterioration without hypotension,16, 17 whereas massive pulmonary embolism was defined as hemodynamically unstable patients with shock or hypotension.3 Consequently, in order to accurately evaluate the feasibility of UACDT in treating SPE without considering massive pulmonary embolism patients, we conducted the meta-analysis. This meta-analysis included 12 contemporary studies with 485 SPE patients who were treated by UACDT. All those 12 studies, conducted from October 2009 to December 2017, got 5, 6 or 7 stars in study quality assessment on a scale of 0-9.
To evaluate the feasibility of UACDT for SPE, we collected information including the number of in-hospital deaths, the number of major and minor bleeding patients, the PASP before/after treatment and the RV/LV ratio before/after treatment. In our meta-analysis, in-hospital mortality was 0.0% (95% CI: 0.0%, 1.0%). Specifically, 9 out of the 12 studies reported follow-up results, ranging from 30 to 180 days, with 3 studies reporting deaths during follow-up. In Mohan’s study, mortality at 30 days was 4 (14.33%) out of 33 patients. These patients all had extensive, life-limiting comorbidities and none of the deaths were related to major bleeding complications. One fatality was a 52-year-old woman with acquired immunodeficiency syndrome, dementia and end-stage renal disease who developed extended spectrum b-lactamase sepsis after undergoing an amputation. The second fatality was a 38-year-old female undergoing a treatment of gastric lymphoma who was arrested the morning after completion of UACDT. The third fatality was a 67-year-old male with a medical history of metastatic colon cancer as well as pancreatic cancer status post-Whipple procedure. Three days after UACDT, he developed unstable atrial fibrillation and expired. The fourth fatality was a 31-year-old African American male with a history of complex congenital heart disease as well as recurrent PE. He developed renal failure and required pressors and extracorporeal membrane oxygenation. Patient expired 9 days after UACDT. In Piazza’s study, 100% (48 of 48 patients) of the patients survived to hospital discharge with 98% (47 of 48 patients) alive on 90-day follow-up, but there was no specific information about the dead patient was reported. In Dumantepe’s study, 100% (14 of 14 patients) of the patients survived to hospital discharge with 92.8% of patients being alive at 180-day follow-up. At the time of follow-up, one patient died from cancer.
Simultaneously, in our study 12 patients suffered from major bleeding after UACDT with a rate of 1.0% (95% CI: 0.0%, 3.0%). Except for the complication of major bleeding, the article we included reported minor bleeding events, but the related complications of interventional therapy for PE, such as pericardial tamponade, pulmonary hemorrhage, distal embolization, arrhythmia, contrast nephropathy, anaphylaxis, pseudoaneurysm or AV fistula were not observed. In Mohan’s study, four patients experienced mild bleeding events during thrombolysis or in the period immediately after thrombolysis. One patient had minor oral mucosal bleeding which responded to topical tranexamic acid. One patient had bleeding from her right femoral catheter sheath which was successfully managed with compression and halving of her tPA dose. One patient experienced epistaxis because his tPA elution was stopped. One patient with a history of menorrhagia and dysmenorrhea complained about some vaginal bleeding the day after thrombolysis was completed. She was found to have uterine fibroid. In Fuller’s study, patients with hemoptysis, vaginal bleeding and multiple other co-morbidities, including multiple sclerosis and prior diagnosed menorrhagia, led to the cessation of the lytic therapy at 10 hours with resolution of bleeding without further treatment. The other three minor events included a patient with a hematoma at the access site, and two patients with minor oozing from access sites which do not require cessation of intervention or transfusion of blood products. In Kaymaz’s study, minor bleeding episodes at puncture sites were noted in two patients. In Bagla’s study, there were four minor venous access site haemorrhagic complications which did not require additional therapy.
SPE is a type of pulmonary embolism observed in patients with RVD. However, the uniform standard of how to diagnose RVD is missing currently. The right ventricular enlargement ratio (the ratio of right ventricular diameter to left ventricular diameter) greater than 0.9 in a colour Doppler ultrasound, cardiac four-chamber view or computer tomography imaging shows right ventricular systolic dysfunction. In our study, the pooled decrease in RV/LV ration after treatment was −0.34 (95% CI: −0.43, −0.25). This result confirmed that right ventricular enlargement was significantly improved in patients with SPE after UACDT. However, the RV/LV ratios were still greater than 0.9. The forest plot showed high heterogeneity (I2 = 82%, P < .01). The heterogeneity mainly came from the study of Bagla and Ozmen. By reading their original literature in detail, we found patients’ Miler index before treatment were significantly lower than that of others, thus this would improve right ventricular enlargement much more dramatically with a better prognosis. In addition, for Ozmen’s research, the number of patients included was 10 which might be another source of heterogeneity. Also, a subgroup analysis was performed based on the type of study to seek other heterogeneity sources. According to the result, the subgroup of prospective study had lower heterogeneity (I2 = 78%, P = .03) than the pooled heterogeneity. While the heterogeneity (I2 = 82%, P < .01) of retrospective study subgroup was higher than the pooled heterogeneity. This result also imply that the pooled heterogeneity mainly comes from Bagla and Ozmen’s research.
Additionally, we collected the PASP. The pooled decrease in PASP after treatment was −15.05 (95% CI: −18.10, −12.00) mm Hg. This result showed that UACDT significantly reduced PASP in SPE patient.
Our meta-analysis has several limitations. First of all, the data are collected from published literature, thus the inclusion of these reports might represent a little publication bias. Second, we could not completely avoid selection bias. Third, most of the included studies were performed at a single medical centre, and their results may not be entirely representative of the outcomes in other medical centres. Finally, the most important limitation of this study is the short of adequately randomized and controlled studies.
In conclusion, this meta-analysis reveals that UACDT is a feasible treatment for SPE. UACDT in treating SPE has low in-hospital mortality and major bleeding rate and significantly reduces the RV/LV ratio and PASP. Even so, further large-scale prospective studies are encouraged to evaluate the feasibility of UACDT in treating SPE.
ACKNOWLEDGMENTS
We would like to express our special thanks to Weipeng Zheng (Guangzhou First People's Hospital, Guangzhou, China) for helping us learn R software.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Conception and design: JW, CH
Analysis and interpretation: JW, HL, YY, LP, JL, CH
Data collection: JW, HL, LP
Writing the article: JW, HL, YY, LP, JL, CH
Critical revision of the article: HL, MB, HR
Final approval of the article: JW, HC, YY, LP, JL, HL, MB, HR, CH
Statistical analysis: JW, HL
Overall responsibility: CH