MRE11 is essential for the long-term viability of undifferentiated spermatogonia
Zhenghui Tang, Zhongyang Liang, and Bin Zhang contributed equally to this study.
Abstract
In the meiotic prophase, programmed SPO11-linked DNA double-strand breaks (DSBs) are repaired by homologous recombination (HR). The MRE11-RAD50-NBS1 (MRN) complex is essential for initiating DNA end resection, the first step of HR. However, residual DNA end resection still occurs in Nbs1 knockout (KO) spermatocytes for unknown reasons. Here, we show that DNA end resection is completely abolished in Mre11 KO spermatocytes. In addition, Mre11 KO, but not Nbs1 KO, undifferentiated spermatogonia are rapidly exhausted due to DSB accumulation, proliferation defects, and elevated apoptosis. Cellular studies reveal that a small amount of MRE11 retained in the nucleus of Nbs1 KO cells likely underlies the differences between Mre11 and Nbs1 KO cells. Taken together, our study not only demonstrates an irreplaceable role of the MRE11 in DNA end resection at SPO11-linked DSBs but also unveils a unique function of MRE11 in maintaining the long-term viability of undifferentiated spermatogonia.
1 INTRODUCTION
As one of the most deleterious forms of DNA damage, DNA double-strand breaks (DSBs) must be repaired promptly to maintain genomic stability. Among multiple pathways of DSB repair, homologous recombination (HR) repairs DSBs with high fidelity when sister chromatids are available as templates in the S and G2 phases of the cell cycle.1, 2 HR starts with DNA end resection at DSB sites to generate single-stranded DNA, which is bound by recombinases to initiate the invasion of the DNA template strand and to promote subsequent steps of HR.3
In somatic cells, the MRE11-RAD50-NBS1 (MRN) complex initiates DNA end resection at DSB sites. As the catalytic subunit of the MRN complex, MRE11 harbours both endonuclease and exonuclease activity.4 MRE11 first uses its endonuclease activity to nick the DNA several hundred base pairs away from the DSB site and then uses its 3′-5′ exonuclease to digest DNA towards the DSB site, generating short single-stranded DNA. This creates the entry sites for EXO1 and BLM/DNA2, which continue to perform the long-range resection to generate longer single-stranded DNA.5 Due to the unique dual nuclease activities of MRE11, the MRN complex is essential for DNA end resection at DSBs with blocked ends, such as TOP2-linked DSBs.6
Hundreds of DSBs with blocked ends are generated in the meiotic prophase. After programmed DNA cutting, SPO11 is covalently linked to the 5′ end of DSBs to form SPO11-linked DSBs, which are repaired exclusively by HR to facilitate the obligate crossover between homologous chromosomes.7 The MRX complex, an orthologue of MRN complex in yeast, is essential for DNA end resection in yeast meiotic prophase.8 The MRN complex is also required for DNA end resection and HR repair of SPO11-linked DSBs in mammalian meiotic prophase. Loss of NBS1, a key component of the MRN complex, in spermatocytes results in meiotic arrest and male infertility.9
Interestingly, residual DNA end resection still occurs in Nbs1 knockout (KO) spermatocytes, raising the possibility that other proteins can function as backup nucleases to initiate DNA end resections at SPO11-linked DSBs when the MRN complex is absent. However, although EXO1 and BLM/DNA2 can initiate DNA end resection at DSBs with clean ends, no proteins are known to have dual nuclease activities similar to those of MRE11, which are essential for DNA end resection at DSBs with blocked ends.10 Alternatively, MRE11 is still responsible for the residual DNA end resection in Nbs1 KO spermatocytes. As NBS1 loss does not affect the stability of the MRE11-RAD50 complex but compromises its localization at DSBs,11, 12 it is possible that a small amount of MRE11-RAD50 complex still localises at DSBs and promotes limited DNA end resection in the absence of NBS1. These two possibilities can be tested by inactivating MRE11 in spermatocytes.
Like Nbs1 KO mice, both Mre11 KO and Mre11 nuclease-dead mice are lethal, preventing direct examination of meiotic prophase in these mice. Two viable Mre11 mutant mice are also generated, but none of them have defects in DNA end resection or HR repair in the meiotic prophase.13-15 In this study, we have generated germ cell-specific Mre11 KO mice and found that MRE11 is absolutely required for DNA end resection in meiotic prophase. In addition, we have discovered that Mre11 KO, but not Nbs1 KO, leads to complete loss of germ cells due to apoptosis in undifferentiated spermatogonia. Therefore, we have identified a unique role of MRE11 in maintaining the long-term viability of undifferentiated spermatogonia.
2 RESULTS
2.1 MRE11 is required for male fertility in mice
To investigate if MRE11 is essential for DNA end resection and HR repair of SPO11-linked DSBs in mammalian meiotic prophase, we generated germ cell-specific Mre11 KO (Mre11 vKO) mice using Vasa-Cre (Figure 1A). Successful depletion of MRE11 protein in Mre11 vKO testes was verified by western blotting (Figure 1B). Similar to Nbs1 vKO male mice that we generated previously, Mre11 vKO male mice were infertile (Figure 1C). No mature sperm was found in the epididymides of adult Mre11 vKO male mice (Figure 1D). The testes of Mre11 vKO male mice were smaller (Figure 1E,F). In the testis sections of Mre11 vKO male mice, no haploid cells were found by H/E staining (Figure 1G,H), and no cells with acrosomes were identified by peanut agglutinin (PNA) staining (Figure 1I). These observations were identical to those in Nbs1 vKO male mice, suggesting that both MRE11 and NBS1 are essential for the completion of meiosis in male mice.
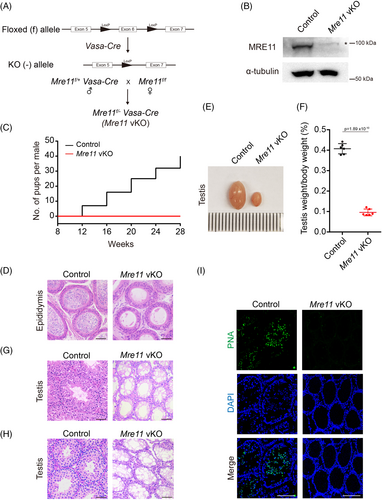
2.2 MRE11 is required for DNA end resection at SPO11-linked DSBs
To dissect the meiotic defects in Mre11 vKO male mice, we first analysed the meiotic prophase in these mice (Figure 2A). The intensity of γH2AX signals in leptotene-stage spermatocytes of Mre11 vKO mice was indistinguishable from that in control mice, suggesting that there is no defect in the formation of programed SPO11-linked DSBs in Mre11 vKO mice. However, diminished γH2AX signals could not be observed in any spermatocytes at meiotic prophase, suggesting that DSB repair was severely compromised in Mre11 vKO male mice. Concomitantly, meiotic progression was arrested at the zygotene stage in these mice (Figure 2B). The HR repair of SPO11-linked DSBs in the meiotic prophase requires two meiotic recombinases, RAD51 and DMC1. In the zygotene-stage spermatocytes of Mre11 vKO male mice, both recombinases were undetectable at DSB sites (Figure 2C,D). The recruitment of recombinases to DSBs is mediated by single-stranded DNA, a product of DNA end resection. The single-stranded binding proteins, RPA2 and MEIOB, were also undetectable at DSB sites (Figure 2E,F). These observations suggest that DNA end resection is dramatically decreased below our detection limit in the meiotic prophase in Mre11 vKO male mice, which is the reason for DSB repair defects in these mice. Notably, unlike Nbs1 vKO spermatocytes, no residual DNA end resection could be observed in Mre11 vKO spermatocytes. Therefore, MRE11 is likely the only nuclease that initiates DNA end resection at SPO11-linked DSBs in meiotic prophase, which is responsible for the residual DNA end resection in Nbs1 KO spermatocytes.
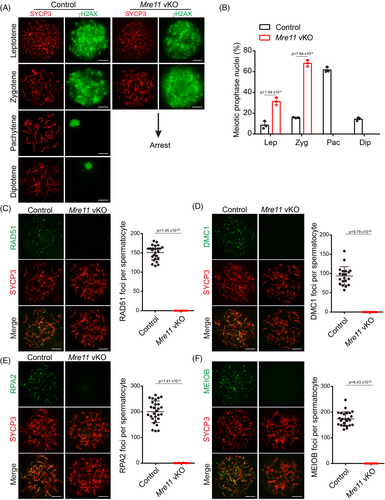
2.3 MRE11 is essential for the long-term viability of undifferentiated spermatogonia
During the analysis of DNA end resection, we noticed that much fewer spermatocytes at meiotic prophase could be obtained from the testes of Mre11 vKO male mice than from control mice at postnatal day (PD) 21. Examination of testis sections of Mre11 vKO male mice after H/E staining also revealed a dramatic reduction of spermatocytes at meiotic prophase at PD21 (Figure 1H). These observations suggest that Mre11 vKO male mice have additional defects in germ cells besides meiotic prophase arrest in spermatocytes. To characterise the germ cell defects, we analysed the germ cells in control and Mre11 vKO male mice of different ages by immunofluorescent (IF) staining of MVH (Figure 3A), which is expressed in germ cells at all stages from primordial germ cells to round spermatids.16 The numbers of germ cells were comparable between control and Mre11 vKO male mice at PD7 but were decreased in Mre11 vKO male mice at PD14. Consistent with our initial findings, a dramatic reduction of germ cells in Mre11 vKO male mice was observed at PD21. Three weeks later, germ cells could be barely observed in Mre11 vKO male mice at PD42 (Figure 3A,B).
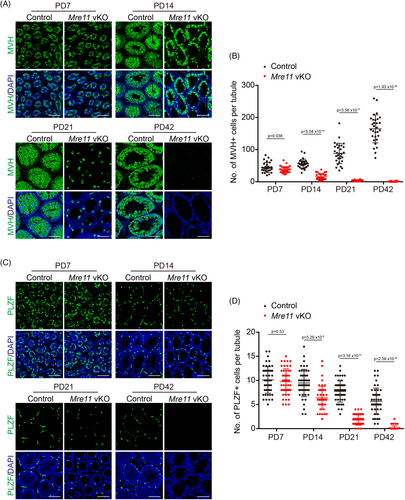
The near complete absence of germ cells at PD42 indicates that the undifferentiated spermatogonia, which maintain long-term spermatogenesis, are rapidly exhausted in Mre11 vKO male mice. Therefore, we analysed the undifferentiated spermatogonia in control and Mre11 vKO male mice of different ages by IF staining of PLZF (Figure 3C), a marker of undifferentiated spermatogonia. The undifferentiated spermatogonia were similar between control and Mre11 vKO male mice at PD7, suggesting that the establishment of undifferentiated spermatogonia was normal. The decrease of undifferentiated spermatogonia in Mre11 vKO male mice was first observed at PD14 and was more dramatic at PD21. The undifferentiated spermatogonia were almost undetectable in Mre11 vKO male mice at PD42 (Figure 3C,D). These observations suggest that MRE11 is essential for the long-term viability of undifferentiated spermatogonia.
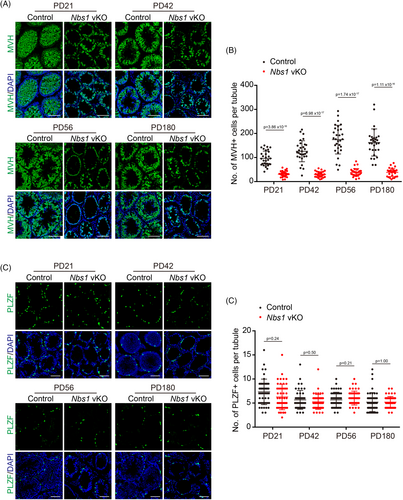
2.4 NBS1 is dispensable for the long-term viability of undifferentiated spermatogonia
In most scenarios, MRE11 and NBS1 function together as an MRN complex, and their deficiency leads to similar phenotypes in most studies. Although Mre11 vKO and Nbs1 vKO male mice have subtle differences in DNA end resection defects, the meiotic prophase defects are similar. However, unlike Mre11 vKO male mice, our previous study did not notice a dramatic reduction of undifferentiated spermatogonia in Nbs1 vKO male mice. In case any defects in Nbs1 vKO male mice were missed previously, we revisited whether NBS1 is required for the long-term viability of undifferentiated spermatogonia. Like Mre11 vKO male mice, germ cells were dramatically reduced in Nbs1 vKO male mice at PD21. However, unlike Mre11 vKO male mice, germ cells were still abundant at PD42 and were present at PD180 in Nbs1 vKO male mice (Figure 4A,B). Interestingly, the numbers of undifferentiated spermatogonia were similar between control and Nbs1 vKO male mice at all ages analysed, including PD180, suggesting that undifferentiated spermatogonia were not exhausted in Nbs1 vKO male mice (Figure 4C,D). The reduction of germ cells in Nbs1 vKO male mice is likely due to meiotic prophase arrest only. These observations suggest that, unlike MRE11, NBS1 is dispensable for the long-term viability of undifferentiated spermatogonia.
2.5 MRE11 loss leads to DSB accumulation and apoptosis in undifferentiated spermatogonia
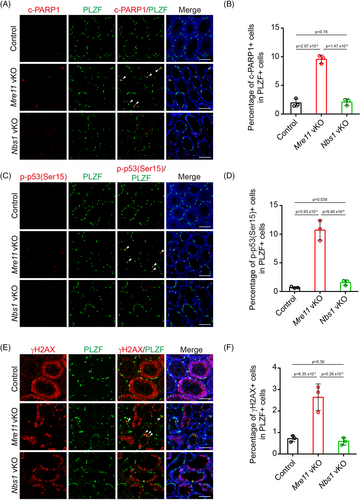
To investigate why undifferentiated spermatogonia are exhausted in Mre11 vKO but not Nbs1 vKO male mice, we examined undifferentiated spermatogonia in these mice at PD14, when the decrease of undifferentiated spermatogonia was first observed in Mre11 vKO male mice. Compared with control mice, the number of undifferentiated spermatogonia positive for cleaved PARP1 was significantly increased in Mre11 vKO male mice (Figure 5A,B). The signal of p-p53(S15), a phosphorylation event associated with p53 activation, was also dramatically increased in Mre11 KO undifferentiated spermatogonia. These observations suggest that many Mre11 KO undifferentiated spermatogonia are undergoing apoptosis at PD14 (Figure 5C,D). p53 can be robustly activated by DNA damage.17 As MRE11 is essential for DSB repair, the elevated apoptosis in Mre11 KO undifferentiated spermatogonia may be caused by DSB repair deficiency. To test this idea, we examined the status of γH2AX, a marker of DSBs, in Mre11 vKO male mice. In control mice, most γH2AX signals were found in spermatocytes when programmed DSBs were generated in meiotic prophase, and γH2AX signals could hardly be identified in undifferentiated spermatogonia (Figure 5E). On the contrary, the number of undifferentiated spermatogonia positive for γH2AX was significantly increased in Mre11 vKO male mice (Figure 5E,F). Therefore, DSBs were indeed present in Mre11 KO undifferentiated spermatogonia, which could be the cause for the elevated apoptosis in these cells. In sharp contrast to the observations in Mre11 vKO male mice, the number of undifferentiated spermatogonia positive for cleaved PARP1, p-p53(S15), or γH2AX in Nbs1 vKO male mice was comparable with those in control mice (Figure 5). The different levels of persistent DSBs likely account for the different fates of Mre11 KO and Nbs1 KO undifferentiated spermatogonia.
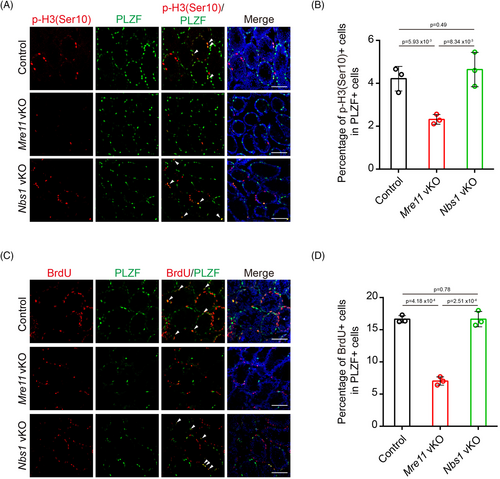
2.6 MRE11 is required for the proliferation of undifferentiated spermatogonia
Besides triggering apoptosis, persistent DSBs can also activate cell cycle checkpoints to cease cell proliferation. In agreement with this idea, the signal of p-H3(S10), a phosphorylation event associated with mitotic entry, was significantly decreased in Mre11 KO undifferentiated spermatogonia (Figure 6A,B). On the contrary, p-H3(S10) signals were comparable between control and Nbs1 KO undifferentiated spermatogonia. Similarly, the percentage of cells undergoing DNA replication, as determined by BrdU incorporation, was significantly decreased in Mre11 KO but not in Nbs1 KO undifferentiated spermatogonia (Figure 6C,D). Therefore, proliferation defects were present in Mre11 KO but not in Nbs1 KO undifferentiated spermatogonia. It is likely that the proliferation defects and apoptosis in Mre11 KO undifferentiated spermatogonia eventually lead to the exhaustion of these cells in older mice.
2.7 NBS1 loss compromises but not fully abolishes the nuclear localization of MRE11-RAD50 complex
Previous studies have shown that NBS1 is dispensable for the stability of the MRE11-RAD50 complex, but it is required for the localization of the MRE11-RAD50 complex in the nucleus. Interestingly, unlike the absence of MRE11 signals in the nucleus of Mre11 KO undifferentiated spermatogonia, weak MRE11 signals could still be detected in the nucleus of Nbs1 KO undifferentiated spermatogonia (Figure 7A,B). Although the status of RAD50 could not be directly analysed due to the unavailability of RAD50 antibody for IF staining, it is likely that NBS1 loss compromises but not fully abolishes the nuclear localization of MRE11-RAD50 complex. It is possible that complete loss of MRE11 may be detrimental to cell proliferation and viability of Mre11 KO undifferentiated spermatogonia, but a small amount of intact MRE11-RAD50 complex in the nucleus is sufficient for the proliferation and viability of Nbs1 KO undifferentiated spermatogonia.
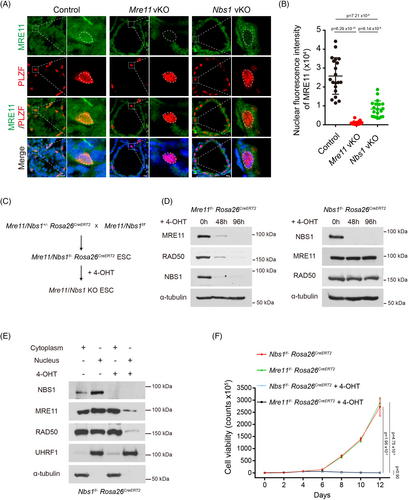
Multiple studies have shown that both MRE11 and NBS1 are essential for cell proliferation and long-term cell viability. Interestingly, our study reveals that MRE11, but not NBS1, is required for cell proliferation and the long-term viability of undifferentiated spermatogonia in vivo. Therefore, we continued to examine if loss of MRE11 and NBS1 have different impact on the proliferation of cells cultured in vitro. As undifferentiated spermatogonia are challenging to culture in vitro, we utilised embryonic stem cells (ESCs). We generated inducible Mre11 KO (Mre11f/− Rosa26CreERT2) and inducible Nbs1 KO (Nbs1f/− Rosa26CreERT2) ESCs, which proliferated well in vitro (Figure 7C). Upon 4-hydroxytamoxifen (4-OHT) treatment, complete Mre11 KO and Nbs1 KO ESCs were obtained within 4 days (Figure 7D). In agreement with previous studies, the protein levels of RAD50 and NBS1 were dramatically decreased in Mre11 KO ESCs, but the protein levels of both MRE11 and RAD50 were unaltered in Nbs1 KO ESCs (Figure 7D). Consistent with our observation in Nbs1 KO undifferentiated spermatogonia and a previous study in Purkinje cells,18 the nuclear localization of MRE11 and RAD50 were severely compromised but still detectable in Nbs1 KO ESCs (Figure 7E). Interestingly, unlike undifferentiated spermatogonia in vivo, both Mre11 KO and Nbs1 KO ESCs were deficient in proliferation after 4 days and died eventually (Figure 7F), suggesting that the amount of MRE11-RAD50 complex is not sufficient for supporting the proliferation and viability of Nbs1 KO ESCs in vitro. Further investigation is required to uncover the mechanism underlying this observation.
3 DISCUSSION
MRE11 is the catalytic subunit of the MRN complex that initiates DNA end resection at DSBs. Besides the MRN complex, other nucleases can also initiate DNA end resection at DSBs with clean ends.19-21 In this study, we have demonstrated that DNA end resection is dramatically decreased below our detection limit in Mre11 KO spermatocytes, suggesting that other nucleases cannot substitute the MRN complex to initiate DNA end resection at SPO11-linked DSBs. Most nucleases promote DNA end resection in a 5′-3′ direction, requiring a free 5′ end to initiate.22, 23 However, the 5′ ends of all DSBs in the meiotic prophase are covalently linked with SPO11, which blocks the access of these nucleases.24 This unique endonuclease and 3′-5′ exonuclease activities of MRE11 enable the MRN complex to nick the DNA several hundred base pairs away from DSBs and digest the DNA in a 3′-5′ direction towards SPO11-linked DSBs to remove SPO11 from DSBs, creating the entry sites for other nuclease to continue the DNA end resection in a 5′-3′ direction.5 No enzyme with similar activities as MRE11 has ever been identified, and our study suggests that such an enzyme does not exist, at least in the meiotic prophase.
As a critical component of the MRN complex, NBS1 is required for DNA end resection at SPO11-linked DSBs in meiotic prophase, but residual DNA end resection still occurs in Nbs1 KO spermatocytes. Our study using Mre11 vKO male mice suggests that MRE11, but not other nucleases, is responsible for the residual DNA end resection when NBS1 is lost. In Nbs1 KO cells, the MRE11-RAD50 complex is intact, and a small amount of it can be observed in the nucleus, which is likely functional to promote residual DNA end resection in the absence of NBS1. However, such residual DNA end resection is insufficient for meiotic recombination at all DSBs. Therefore, the meiotic prophase progression arrests at similar stages in Mre11 KO and Nbs1 KO spermatocytes (Figure 8).
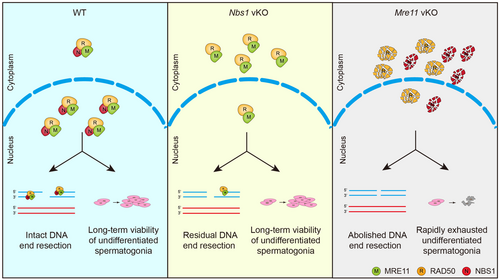
Unlike in meiotic prophase, loss of MRE11 and NBS1 leads to dramatically different outcomes in undifferentiated spermatogonia. While Nbs1 KO undifferentiated spermatogonia are indistinguishable from those in WT mice, Mre11 KO undifferentiated spermatogonia are rapidly exhausted. Before exhaustion, Mre11 KO undifferentiated spermatogonia display defects in cell proliferation, accompanied by the accumulation of unrepaired DSBs and elevated apoptosis. Therefore, MRE11, but not NBS1, is required for cell proliferation and long-term viability of undifferentiated spermatogonia (Figure 8). As Mre11 vKO male mice have no defects in establishing undifferentiated spermatogonia at PD7, it is likely that MRE11 is dispensable for DNA replication per se. However, the MRN complex has an active role in fixing endogenous replication errors by repairing collapsed replication forks and resolving replication intermediates, the defect of which leads to DSB accumulation.25, 26 In the absence of replication stress induced by exogenous DNA damage, replication errors occur infrequently in vivo. A small amount of intact MRE11-RAD50 complex in Nbs1 KO undifferentiated spermatogonia may be sufficient for fixing these replication errors. On the contrary, replication errors cannot be fixed in Mre11 KO undifferentiated spermatogonia, and DSBs gradually accumulate, eventually leading to proliferation arrest, apoptosis, and cell exhaustion. While weak MRE11 signals can be observed in Nbs1 KO undifferentiated spermatogonia, the status of RAD50 could not be analysed due to technical constraint of RAD50 antibodies. Therefore, this idea needs to be further interrogated using a suitable RAD50 antibody in future.
Our observations in undifferentiated spermatogonia suggest that loss of MRE11 and NBS1 can lead to different outcomes in vivo. Interestingly, loss of MRE11 and NBS1 in Purkinje cells leads to similar phenotypes, both of with are compatible with cerebellar development.18 It is possible that the similar or different phenotypes upon loss of MRE11 and NBS1 depend on cell types and their proliferation status in vivo. Consistently, human germline mutations in MRE11 and NBS1 genes predispose to A-T like disease (ATLD) and Nijmegen breakage syndrome (NBS), respectively, which are similar but not identical syndromes.11, 27, 28 Both syndromes are characterised by immunodeficiency and radiosensitivity. While microcephaly is a common feature of NBS but not ATLD, neurodegeneration is more frequently identified in ATLD than in NBS.29 In addition, similar but also different phenotypes are observed in mouse models of ATLD and NBS.13, 30 Therefore, although MRE11, RAD50, and NBS1 functions as a complex, loss or mutation of individual components of this complex can lead to similar or different outcomes in vivo, depending on the cell types and their proliferation status.
Interestingly, Nbs1 KO ESCs have cell proliferation defects similar to Mre11 KO ESCs and fail to survive the long-term culture in vitro, which differs from Nbs1 KO undifferentiated spermatogonia in vivo. This observation is consistent with the conclusion from previous studies that the loss of either component of the MRN complex is incompatible with cell viability.31-33 The cell proliferation defects of in vitro cultured Nbs1 KO ESCs can be explained by the higher replication stress, which can potentially be induced by any environmental factors that are different from those in vivo, such as culture medium, stiffness of the culture plate, monolayer culture, higher oxygen level, etc. It is likely that the small amount of intact MRE11-RAD50 complex in Nbs1 KO cells is not sufficient for fixing higher numbers of replication errors that are induced by higher replication stress in vitro.
Both Mre11 KO and Nbs1 KO mice die at early embryonic stages,31, 33 but the reasons are unclear. Based on the cellular studies in vitro, it is believed that loss of either component of the MRN complex is incompatible with cell viability, and both Mre11 KO and Nbs1 KO embryos die because of cell proliferation defects.34, 35 However, our study suggests that the observation in vitro might not fully reflect the situations in vivo. Instead, our study shows that MRE11, but not NBS1, is required for long-term cell viability of undifferentiated spermatogonia in vivo. It is possible that NBS1 is dispensable for cell proliferation but required for certain critical events during embryonic development. It will be interesting to examine if different mechanisms underlie the embryonic lethality of Mre11 KO and Nbs1 KO mice.
4 MATERIALS AND METHODS
4.1 Mice
Mre1f/f mice were kind gifts from David Ferguson (University of Michigan). Nbs1f/f mice were kind gifts from Zhao-Qi Wang (Fritz Lipmann Institute). Vasa-Cre transgenic mice (The Jackson Laboratory, 006954) and Rosa26CreERT2 mice (The Jackson Laboratory, 008463) were used to generate Mre11 or Nbs1 conditional KO mice. All mice experiments were approved by Zhejiang University Animal Care and Use Committee.
4.2 Reagents and antibodies
The following reagents and antibodies were purchased: PNA (Sigma, C7381), 4-OHT (Sigma, H17), BrdU (Sigma, B5002), anti-MRE11 (CST, 4859S), anti-MRE11 (NOVUS, NB100-142), anti-NBS1 (Abcam, ab32074), anti-RAD50 (ABclonal, A3078), anti-UHRF1 (Santa Cruz, sc-373,750), anti-α-tubulin (Genscript, A01410-100), anti-γH2AX (Millipore, 05–636), mouse anti-SYCP3 (Santa Cruz, sc-74,569), rabbit anti-SYCP3 (Proteintech, 23,024-1-AP), anti-RPA2 (Proteintech, 10,412-1-AP), anti-RAD51 (Abcam, ab176458), anti-DMC1 (Santa Cruz, sc-22,768), anti-PLZF (Santa Cruz, sc-22,839), anti-cleaved PARP1 (CST, 9544), anti-MVH (Abcam, ab13840), anti-p-p53(Ser15) (CST, 9284S), anti-p-H3(Ser10) (CST, 9701S), and anti-BrdU (Abcam, ab6326). Anti-MEIOB antibody was a kind gift from Mengcheng Luo (Wuhan University).
4.3 Histological analysis
Mouse testis and epididymis were fixed in Bouin's solution (Sigma) and embedded in paraffin after dehydration in a series of increasing concentrations of ethanol. After section, the slides were deparaffinised, stained with haematoxylin and eosin (H/E) and mounted. The images were captured by an upright microscope (Leica DM4000).
4.4 Cell culture and cell viability assay
Mouse ESCs were cultured in DMEM medium supplemented with 15% FBS, 1% penicillin and streptomycin, 1× non-essential amino acids (NEAA), 1× l-glutamine, 10 ng/mL LIF (Santa Cruz), and 0.1 mM β-mercaptoethanol. Cells were grown in humidified incubator (37°C) with 5% CO2. For cell viability assay, ESCs were seeded in 6-well plates (1 × 105 cells per well) and 1 μM 4-OHT was added to the cell culture medium. Cells were passaged and cell numbers were counted every 2 days.
4.5 Western blotting analysis
Proteins were extracted using NETN300 lysis buffer (300 mM NaCl, 0.5 mM EDTA, 0.5% NP-40, 20 mM Tris–HCl pH 8.0). Cell lysates were incubated on ice for 10 min. After centrifuged at 12,000g for 10 min at 4°C, the supernatants were collected as protein extracts. The protein extracts were separated by SDS-PAGE and then transferred onto PVDF membrane (Millipore). The membranes were blocked by 5% non-fat milk in PBST buffer (PBS with 20% Triton X-100), incubated with primary antibodies overnight at 4°C, washed with PBST, and incubated with HRP-conjugated secondary antibodies (Jackson ImmunoResearch). Proteins of interest were detected by chemiluminescence system with ECL reagents.
4.6 Surface spread of spermatocytes
Meiotic chromosome spreads of spermatocytes were prepared as described previously.9 Briefly, testes were dissected from 3-week-old mice and digested by 1 mg/mL type IV collagenase for 30 min at 37°C. The cell pellets were resuspended in hypotonic buffer (30 mM Tris–HCl pH 8.2, 50 mM sucrose, 17 mM sodium citrate) and were kept for 10 min. After centrifugation, cells were resuspended in 100 mM sucrose for 5 min. The cell suspension was mixed with equal volume of fixation buffer (1% PFA, 0.15% Triton X-100, 10 mM sodium borate, pH 9.2) and was spread onto slides and air dried.
4.7 IF staining
For IF staining of meiotic spreads, the slides were treated with 0.5% Triton X-100 for 10 min and were incubated with primary antibodies for 3 h. After washing in 1× PBS for three times, the slides were incubated with Alexa Fluor 488 or 594-conjugated secondary antibodies for 30 min. Finally, the slides were stained with Hoechst 33342 and mounted with anti-fade reagents. The images were captured by an inverted fluorescence microscope (ECLIPSE Ti2-E, Nikon).
For IF staining of frozen sections, the slides were incubated with primary antibodies overnight and secondary antibodies for 1 h, respectively. The images were taken by a laser scanning confocal microscope (STELLARIS 5, Leica Microsystems). The fluorescence intensity was quantified by ImageJ.
4.8 Separation of nuclear and cytoplasmic proteins
Cell pellets were treated with lysis buffer 1 (10 mM Tris–HCl pH 8.0, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA) and were kept on ice for 20 min. 0.15% NP40 was added to the lysates and incubated for 2 min. After centrifugation, the supernatant was collected as cytoplasmic protein. The insoluble fraction was washed with ice-cold 1× PBS for three times and treated with lysis buffer 2 (10 mM Tris–HCl pH 8.0, 1.5 mM MgCl2, 400 mM NaCl, 1 mM EDTA) for 15 min. After centrifugation, the supernatant was collected as nuclear protein.
4.9 Germ cell enrichment
Germ cell enrichment was performed as described previously.9 Mouse testes were isolated and digested in 1 mg/mL type IV collagenase for 10 min at 37°C. After centrifugation, cell pellets were further digested with trypsin for 10 min. The reaction was then stopped by adding foetal bovine serum and cells were then centrifugated at 400 g for 2 min. The cell pellets were resuspended in DMEM medium supplemented with 10% FBS and 1% penicillin and streptomycin, and then cultured in dishes at 37°C with 5% CO2. One hour later, the supernatant cells were collected for subsequent Western blotting analysis.
4.10 Statistical analysis
The statistical significance between two groups was evaluated using two-tailed unpaired Student's t-test.
AUTHOR CONTRIBUTIONS
Lejun Li, Lin-Yu Lu, and Yidan Liu designed research. Zhenghui Tang, Zhongyang Liang, Bin Zhang, and Xiaohui Xu performed research. Zhenghui Tang, Zhongyang Liang, Bin Zhang, Xiaohui Xu, Peng Li, and Lejun Li analysed data. Zhenghui Tang, Zhongyang Liang, Lin-Yu Lu, and Yidan Liu wrote the article.
ACKNOWLEDGMENT
We thank David Ferguson and Zhao-Qi Wang for sharing mouse strains and Mengcheng Luo for sharing antibodies. We thank Zhong-Wei Zhou and Tangliang Li for helping with mice shipping. We thank Quanfeng Zhu and Shenghui Hong for helping with mouse strain rederivation. We thank Chao Bi and Xiaoli Hong from the Core Facilities, Zhejiang University School of Medicine for their technical support.
FUNDING INFORMATION
This work is funded by Zhejiang Provincial Natural Science Foundation (LQ21C120001 and LZ21H040001) and National Natural Science Foundation of China (32070829, 32100676, and 32370900).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
All data supporting the findings of this study are available within the paper.




