New methods to decrypt emerging macropinosome functions during the host–pathogen crosstalk
Funding information: Agence Nationale de la Recherche, Grant/Award Numbers: AutoHostPath, HBPSensing, StopBugEntry; H2020: European Research Council, Grant/Award Number: CoG EndoSubvert; Fondation pour la Recherche Médicale, Grant/Award Numbers: FDT20170436843, SPF20160936275
Abstract
Large volumes of liquid and other materials from the extracellular environment are internalised by eukaryotic cells via an endocytic process called macropinocytosis. It is now recognised that this fundamental and evolutionarily conserved pathway is hijacked by numerous intracellular pathogens as an entry portal to the host cell interior. Yet, an increasing number of additional cellular functions of macropinosomes in pathologic processes have been reported beyond this role for fluid internalisation. It emerges that the identity of macropinosomes can vary hugely and change rapidly during their lifetime. A deeper understanding of this important multi-faceted compartment is based on novel methods for their investigation. These methods are either imaging-based for the tracking of macropinosome dynamics, or they provide the means to extract macropinosomes at high purity for comprehensive proteomic analyses. Here, we portray these new approaches for the investigation of macropinosomes. We document how these method developments have provided insights for a new understanding of the intracellular lifestyle of the bacterial pathogens Shigella and Salmonella. We suggest that a systematic complete characterisation of macropinosome subversion with these approaches during other infection processes and pathologies will be highly beneficial for our understanding of the underlying cellular and molecular processes.
Take Away
- Macropinocytosis serves as an entry door to numerous intracellular pathogens
- Infection-Associated Macropinosomes are formed besides the pathogen entry vacuole
- Novel method developments allow in-depth analysis of IAMs molecular identity
- IAMs are subverted to establish pathogenic replicative niches
- In-depth investigations of the IAM-pathogen interplay are promising research tracks
1 INTRODUCTION
1.1 Macropinocytosis – evolutionarily conserved and functionally multi-faceted
In eukaryotic cells, elements of the extracellular environment are constantly internalised through a variety of endocytic processes. Among them, macropinocytosis, named from Greek 'pino' meaning 'to drink', allows the cells to take up a big amount of extracellular fluids and soluble macromolecules. First described by Warren Lewis in 1931 (Lewis, 1931), this pathway is initiated through the formation of dynamic protrusions of actin-rich plasma membrane folds called ruffles. When the ruffles close at their distal margins, they enclose the extracellular content within membrane-bound organelles called macropinosomes (Swanson & Watts, 1995). These compartments present a heterogeneous size range and are on average significantly larger (>200 nm) than other endocytic compartments such as clathrin-coated vesicles, caveolae and other clathrin-independent carriers (Doherty & McMahon, 2009). The nascent macropinosomes mature and follow different paths for their recycling or degradation. These processes are tightly controlled by a plethora of molecular regulators, including growth factor receptors, kinases, small GTPases and phospholipids (Kerr & Teasdale, 2009). Substantially different and often partly redundant pathways govern the formation and maturation of macropinosomes depending on the cell type, stimulation and macropinosome function (reviewed in Lin et al., 2020; Buckley & King, 2017; Bloomfield & Kay, 2016; Amyere et al., 2001).
From an evolutionary point of view, macropinocytosis is thought to have first fulfilled a nutritive function, as it takes place in amoeba. Later in time, macropinocytosis is related to specialised roles in vertebrates. For example, it is crucial in immune cells for the sampling of environmental material processed for antigen presentation (Lanzavecchia, 1996; Norbury, 2006) and clearance of apoptotic cells (Henson et al., 2001; Krysko et al., 2006), and it eases dendritic cell migration (Moreau et al., 2019; Stow et al., 2020). In neurons, macropinocytosis allows bulk endocytosis during intense synaptic activity and enables modulation of synapse signalling by regulating the amounts of cell surface receptors (Clayton & Cousin, 2009). In addition to their physiological roles, macropinosomes have been associated with many pathologic processes, ranging from neurodegenerative disease and tumour growth to infections. Our knowledge on the overarching paradigms of this puzzling diversity has remained limited, underpinning the need for tools to scrutinise this diversity at the molecular level.
1.2 Naissance, maturation and recycling of a macropinosome
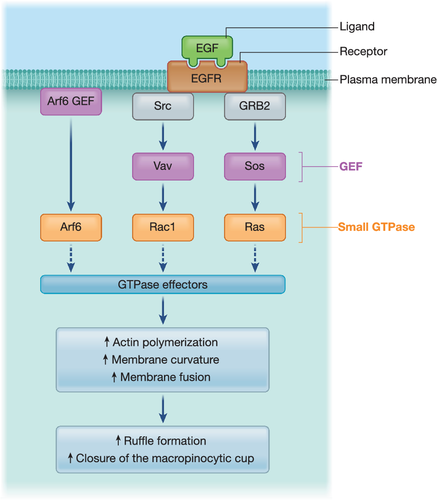
Macropinocytosis starts with actin-driven membrane ruffles (Quinn et al., 2020). These membrane protrusions can take the cup-shaped form of circular dorsal ruffles in a large number of cell types such as fibroblasts, epithelial cells, macrophages or glial cells (Bernitt et al., 2015). Otherwise, flat sheet-like projections called lamellipodia can be formed at protrusive regions of motile cells (Swanson, 2008). These processes either occur constitutively – as observed in dendritic cells – or they are induced by extracellular signals such as the binding of growth factors (e.g. epidermal growth factor [EGF]) to their specific receptor tyrosine kinases (RTK) (as detailed in Box 1). Alternatively, macropinocytosis is triggered by signals generated inside the cell that activate the signalling cascade downstream of RTK activation. This is the case for the oncogenic v-Src and K-Ras (Hobbs & Der, 2020; Veithen et al., 1996). Similarly, a variety of particles including apoptotic bodies, necrotic cells, bacteria and viruses bypass RTK activation and induce ruffle formations independent of growth factors to trigger their internalisation (Hoffmann et al., 2001; Mercer & Helenius, 2009). Following the induction signal, actin polymerisation is initiated by the activated small GTPases acting in concert with the phosphatidylinositol phosphates (PIPs) on the plasma membrane (reviewed by Buckley & King, 2017). Together, they activate actin nucleation–promoting factors allowing the formation and growth of new actin branches. As the actin microfilaments grow, the plasma membrane locally extends up to dozen micrometre-long protruding ruffles. The need of additional membrane for the expanding ruffles is fuelled to varying degrees by different intracellular organelles (Huynh et al., 2007). The membrane protrusions usually recede spontaneously. However, they sometimes curve at their base into open crater-like cups, while the protrusions apex can either fuse together (circular dorsal ruffle) or fold back and fuse with the flat cell-surface membrane (lamellipodia). Following these fusion events, the crater-like cups (called macropinocytic cups) turn into intracellular organelles called macropinosomes and encapsulate a large volume of extracellular fluid into their lumen. Some molecular players such as the actin-associated motor myosin (Buss et al., 1998; Jiang et al., 2010), and the CtBP1/BARS-dependent fission machinery (Liberali et al., 2008) have been reported to be involved in macropinosome fission.
After closure of the macropinocytic cup and fission from the plasma membrane, the newly formed macropinosomes obtain a specific identity. The small GTPase Rab5 is recruited to the closing macropinosomes and recruits the PI-3-kinase Vps34 that converts PI to PI(3)P (Bohdanowicz & Grinstein, 2013; Porat-Shliom et al., 2008). This shift in the macropinosome lipid composition allows the organelle maturation through the temporally dependent recruitment of a suite of membrane tethering and coat proteins (Feliciano et al., 2011; Schnatwinkel et al., 2004). In addition, these early macropinosomes strip off their dense actin coat and escape from the cortical actin meshwork (Schink et al., 2017). They are further partly deflated by the extrusion of ions and the osmotically coupled release of H2O (Freeman et al., 2020). Concomitantly, BAR-domain containing sorting nexin (SNX) proteins are recruited to discrete subdomains of the membrane (Kerr et al., 2006). This leads to the formation of extensive tubules for membrane removal. After scission, these tubulations communicate via the retromer protein complex with the Golgi network allowing the recycling of key surface proteins (Seaman, 2012).
The remaining macropinosomes presenting early endosome markers mature and participate in homo- and heterotypic fusions (Kerr & Teasdale, 2009). While some macropinosomes undergo acidification, acquire late endosome markers such as Rab7 and Rab9, migrate to the centre of the cells and eventually fuse with the lysosome (Racoosin & Swanson, 1993), others recycle back to the plasma membrane via a fast-recycling process involving Rab4 and Rab35 or through a slow recycling process involving Rab8 and Rab11.
2 MACROPINOCYTOSIS AND INFECTION
2.1 Viral induction of macropinocytosis
As viruses need to enter host cells for their replication and propagation, macropinocytosis has been known to be subverted by viruses for a long time. When infecting non-phagocytic cells, viruses must mimic a physiological extracellular stimulus to induce macropinocytosis. Extracellular viral particles interact with receptors on the cell surface for the triggering of membrane ruffling. Usually, this is followed by the formation of macropinosomes and virus internalisation. The virus or their capsids can subsequently penetrate into the cytosol breaching the macropinosome membrane. Even though many viruses hijack the macropinocytosis process, the subverted molecular mechanisms differ profoundly among them (reviewed by Mercer & Helenius, 2009, 2012).
Vaccinia virus, influenza A virus or reovirus induce macropinocytosis by activating RTK (Aravamudhan et al., 2020). This activation is triggered either through direct binding or via glycans acting as bridging molecules. Alternatively, some adenoviruses, echovirus and herpes virus bind to integrin receptors to induce macropinocytosis through a molecular mechanism similar to RTK-induced macropinocytosis. Finally, apoptotic mimicry is a typical strategy of enveloped viruses to be taken up into macrophages (reviewed by Amara & Mercer, 2015). Here, viruses such as vaccinia virus, dengue virus, Ebola virus and pseudotyped lentiviruses expose the apoptosis marker phosphatidylserine (PS) on their surface to mimic apoptotic bodies. As they bind to PS receptors on the surface of macrophages, they elicit a macropinocytic response similar to necroptosis.
2.2 Bacterial induction of macropinocytosis
2.2.1 Macropinocytosis in non-phagocytic cells
Intracellular bacteria have developed many mechanisms for their internalisation, one of the foremost being the hijacking of macropinocytosis. Before entering their host, many bacteria employ different secretion systems (SS) as molecular weapons to communicate from the extracellular environment with the host cytosol (Wagner et al., 2018). The injected effectors induce host cell ruffling and trigger macropinocytosis either within phagocytic or non-phagocytic cells (see Table 1).
| Type of Induction | Bacteria | Effector | Enzymatic Activity | Functional Implication | References |
|---|---|---|---|---|---|
| spi1-encoded T3SS | Salmonella | SopE/E2 | Mimic Rho GEFs and activate Cdc42 and Rac1 | Activation of Rac1 and Cdc42 downstream cascades for macropinocytosis induction | Friebel et al. (2001) |
| SipA/SipC | Bind directly to actin | Actin filament nucleation, polymerisation and bundling | Zhou et al. (1999) Hayward and Koronakis (1999) |
||
| SipC | Interacts directly with Exo70, a component of the exocyst complex | Exocytosis of vesicles increases the available membrane for ruffle formation | Nichols and Casanova (2010) | ||
| SopB | Dephosphorylates PI(3,4,5)P3 into PI(3,4)P2, and PI(3,4)P2 into PI(3)P leading to local depletion of PI(4,5)P2 from the plasma membrane | Weakening of the interactions between the plasma membrane and the actin cortex promoting ruffle extension Recruitment of proteins involved in actin-modulation and membrane ruffling |
Piscatelli et al. (2016) Mason et al. (2007) Terebiznik et al. (2002), Patel and Galán (2006) |
||
| SptP | Inactivates Rac1 and Cdc42 via its GAP activity | Restoration of the cytoskeleton architecture | Fu and Galán (1999) | ||
| T3SS | Shigella | IpaA | Interacts with vinculin to promote capping of actin barbed ends Targets beta1-integrin- >loss of actin fiber |
Altered actin polymerisation dynamics | Ramarao et al. (2007) DeMali et al. (2006) |
| IpaC | Recruits and activates Src | Activation of the downstream Src cascade of macropinocytosis induction | Mounier et al. (2009) | ||
| IpgB1 | Activates Rac1 and Cdc42 | Activation of the downstream Rac1 and Cdc42 cascades of macropinocytosis induction (similar to SopE/E2) | Ohya et al. (2005) | ||
| IpgB2 | Binds to mDia1 and ROCK | Actin nucleation and stress fibre formation | Alto et al. (2006) | ||
| IpgD | Dephosphorylates PI(4,5)P2 into PI(5)P | Weakening of the interactions between the plasma membrane and the actin cortex promoting the ruffle extension (similar to SopB) | Niebuhr et al. (2002) | ||
| T3SS | Chlamydia | aTARP | Binds to actin Activates Rac1 |
Actin nucleation and polymerisation No direct proof of role during macropinocytosis |
Lane et al. (2008) Jewett et al. (2006) |
| aCT116 | Induces Rac1 glycosylation | Actin reorganisation No direct proof of role during macropinocytosis |
Thalmann et al. (2010) | ||
| aTepP | Activates PI3K | No direct proof of role during macropinocytosis | Carpenter et al. (2017) | ||
| aPotentially T3SS or T6SS- mediated | Edwardsiella piscicida | aPotentially T3/6SS effectors-mediated | Unknown | Macropinocytosis-like internalisation in non-phagocytic cells | Hu et al. (2019) |
| aPotentially receptor-mediated | Escherichia coli K1 | N/A | Activates Cdc42, Rac1, RhoA | Internalisation via macropinocytosis in brain microvascular endothelial cells | Loh et al. (2017) |
| Unknown | Mycobacterium | Unknown | Unknown | Internalisation via macropinocytosis in pneumocytes and B cells | García-Pérez et al. (2003, 2012) |
| Unknown | Neisseria gonorrhoeae | Unknown | Unknown | Internalisation by macropinocytosis in primary human urethral epithelial cells | Zenni et al. (2000) |
| Unknown | Haemophilus influenzae | Unknown | Unknown | Internalisation by macropinocytosis in primary human bronchial epithelial cells | Ketterer et al. (1999) |
| icm/dot encoded T4SS | Legionella | Unknown | Unknown | Internalisation by bacteria-induced macropinocytosis in macrophages | Watarai et al. (2001) |
| virB encoded T4SS | Brucella | Unknown | Unknown | Generalised ruffling Internalisation within macropinosome |
Watarai et al. (2002) |
- a Suggested molecular players.
The Gram-negative bacterium Salmonella is among the first reported bacterium to induce macropinosome formation during its internalisation within epithelial cells (Garcia-del-Portillo & Finlay, 1994). Salmonella injects a set of effectors within its host cytosol using a type 3 SS (T3SS) leading to membrane ruffling and bacterial entry (reviewed by LaRock et al., 2015). Briefly, the effectors SopE and SopE2 mimic Rho GEFs and activate the Rho GTPase Cdc42 and Rac1 triggering the recruitment of actin regulatory complexes to the plasma membrane. In addition, SipA and SipC bind directly to actin promoting microfilament nucleation, polymerisation and bundling. Additionally, SipC induces the exocytosis of vesicles to increase the source of membrane needed for the ruffle formation by interacting directly with the exocyst complex (Nichols & Casanova, 2010). SopB modulates the membrane PI composition and weakens the interactions between the plasma membrane and the actin cortex, promoting the ruffle extension (Piscatelli et al., 2016). In addition, the local change in membrane PI composition leads to the recruitment of proteins involved in actin-modulating pathways and membrane ruffling (Brooks et al., 2017). Following bacterial entry, SptP inactivates both Rac1 and Cdc42 via its GAP activity promoting the restoration of the cytoskeleton architecture (Fu & Galán, 1999).
The triggering of macropinocytosis in non-phagocytic cells by the Gram-negative bacterium Shigella is highly similar to Salmonella. Using its T3SS, Shigella injects about 25 effectors within the host cell (reviewed by Pizarro-Cerdá et al., 2016): IpaA and IpgB2 control the actin dynamics at the bacterial contact sites through direct binding with actin-interacting proteins. IpgB1 and IpaC recruit and activate Src, Rac1 and Cdc42, stimulating the downstream macropinocytosis induction cascade. IpgD, similarly to SopB, modulates the PI membrane composition and weakens the connection between cortical actin and the plasma membrane to facilitate ruffling.
Looking at other bacterial pathogens, it was reported that the intracellular obligate bacterium Chlamydia uses macropinocytosis induction to facilitate its capture and internalisation within non-phagocytic cells (Ford et al., 2018). While it is known that Chlamydia injects T3SS effectors during its entry process, their contributions to Chlamydia induction of macropinocytosis are not precisely understood. Likewise, very recently, Hu and colleagues proposed that the fish pathogen Edwardsiella piscicida utilises the host macropinocytosis pathway to enter into non-phagocytic cells (Hu et al., 2019). As E. piscicida uses a T3SS and a type 6 SS (T6SS) to inject effectors into target cells, a future direction is to examine their roles in bacterial entry. In contrast, Escherichia coli K1 was proposed to enter endothelial cells through receptor-mediated induction of macropinocytosis (Loh et al., 2017). Finally, older studies have shown the capacity of Mycobacterium (García-Pérez et al., 2003, 2012), Neisseria gonorrhoeae (Zenni et al., 2000) and Hemophilus influenzae (Ketterer et al., 1999) to enter non-phagocytic cells through macropinocytosis, but the underlying mechanisms remain mostly unexplored. These older studies combined several microscopic techniques and inhibitor treatments to demonstrate the entry of bacteria into macropinosomes. Yet, these investigations have not resulted in a detailed follow-up work, and the techniques used are now considered suboptimal in terms of temporal and spatial resolution. Opportunely, a mechanistic investigation of the relevance of these processes is now achievable taking advantage of the recent methodology developments.
2.2.2 Macropinocytosis in phagocytic cells
Macropinocytosis induction in phagocytic cells has been less investigated, partially due to the technical difficulties distinguishing between bacteria- and host-driven macropinocytosis induction. Legionella is a Gram-negative bacterium that mainly invades phagocytic cells such as amoeba and macrophages. Using a Dot/Icm type 4 SS (T4SS, encoded by the dot/icm genes), Legionella translocates more than 330 bacterial effectors into host cells allowing the formation of a permissive macrophage vacuole. It was reported that the T4SS promotes Legionella entry into phagocytic cells via a macropinocytic uptake pathway (Watarai et al., 2001), but the molecular mechanism of Legionella macropinocytosis induction remains to be clarified. Less canonically, the Gram-negative bacterium Brucella displays an original mechanism of macropinocytosis induction mediated by its virB-encoded T4SS. After initial contact with macrophages, Brucella swims on the cell surface for several minutes which results in generalised plasma membrane ruffling, after which the bacteria are enclosed within macropinosomes (Watarai et al., 2002). These observations relied on the use of fixed and time-lapse microscopic techniques that were challenging at the time of the study due to analytical limitations. Nowadays, such investigations could be performed at a larger scale with high-throughput time-lapse microscopy combined with automatic image analyses to consolidate the proposed model and decipher the overall contribution of macropinocytosis to Brucella entry.
3 METHODS FOR MACROPINOCYTOSIS ANALYSIS
Classically, subversion of macropinocytosis by intracellular pathogens was perceived as purely entry-related. Yet, while methodological innovations for macropinosome studies untangled the physical and molecular changing identities of this compartment, it has emerged that macropinosomes also play non-entry-related roles at the centre of the host–pathogen crosstalk. This paradigm shift was only possible thanks to the use of an increasingly sophisticated toolbox combining candidate-based and comprehensive unbiased approach.
3.1 Candidate-based approaches to trace macropinosomes
For candidate-based approaches, specific proteins known to be present on macropinosomes are labelled, for example, by genetically encoded fluorescent probes to monitor them by standard fluorescence microscopy (Buckley et al., 2016; Morishita et al., 2019). Commonly employed macropinosome markers include regulatory proteins, such as GTPases Rab5 and Rab11 or certain lipids, such as PI3P that is recognised by the FYVE domain (reviewed by Kühn et al., 2017). Besides, introduction of advanced microscopic techniques, such as lattice light sheet microscopy (LLSM), structured illumination microscopy (SIM) and correlative light electron microscopy (CLEM) have contributed to the broadening of knowledge on macropinosome dynamics and identity (Condon et al., 2018;Fredlund et al., 2018; Weiner et al., 2016). CLEM, coupled with fluorescent markers, such as labelled dextran or probes recognising PI3P, has been instrumental for investigating macropinosomes during Salmonella and Shigella invasion. Performing focused ion beam milling combined with scanning electron microscopy (FIB-SEM) revealed that the nascent bacteria-containing vacuoles (BCVs) and the surrounding macropinosomes are discrete compartments, which acquired strikingly different molecular identities (Fredlund et al., 2018; Weiner et al., 2016). For the sake of clarity, we named these latter compartments 'infection-associated macropinosomes' (IAMs). These studies also substantiated that Salmonella and Shigella engulfment within enterocytes is distinct from and does not rely on macropinocytosis. Consequently, this finding calls for revisiting the trigger entry paradigm and for scrutinising the identities of the nascent compartments of other ruffle-inducing bacteria.
3.2 Toward an unbiased understanding of macropinosomes
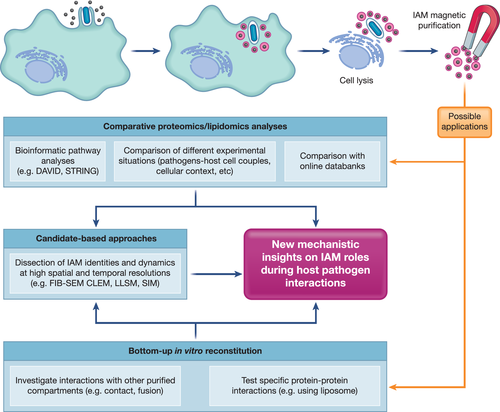
While candidate-based approaches illuminated IAMs as novel distinct pathogenic compartments, unbiased approaches such as proteomic analysis provides the means to address their whole molecular identity. Conventional cell fractionation of vesicular compartments is markedly dependent on the equilibrium states of the density separation, whereas IAMs, by nature, are very similar to other endosomal compartments (Walker et al., 2016). Due to the rapid maturation of macropinosomes and dynamics of host–pathogen interactions, methodologies were thus under-equipped to elucidate the molecular players on IAMs. These hurdles have been overcome by a novel method to purify IAMs inspired by a reported magnetic cell fractionation method (Chang et al., 2020; Steinhäuser et al., 2014; Stévenin et al., 2019). This magnetic purification utilises magnetic beads of moderate size (100 nm in diameter), which are readily englobed within IAMs during Salmonella and Shigella invasion when administered to the extracellular medium (Figure 1). Infected cells are then lysed and the magnetic bead-containing IAMs are immobilised on a table-top magnet. The non-magnetic material is eluted, whereas the purified IAMs are recovered within the magnetic fraction. The isolated IAMs are of high purity and can be analysed by mass spectrometry without any additional purification procedure. This method thus minimises the contamination of other endosomal compartments and specifically isolates IAMs. The proteome of the magnetic and non-magnetic fractions extracted from both infected and non-infected samples can be comparatively analysed to determine protein candidates that are significantly enriched on the IAMs.
4 EXPLOITING THE NEW METHODS – GOING BEYOND THE ENDOCYTOSIS–MACROPINOCYTOSIS LINK
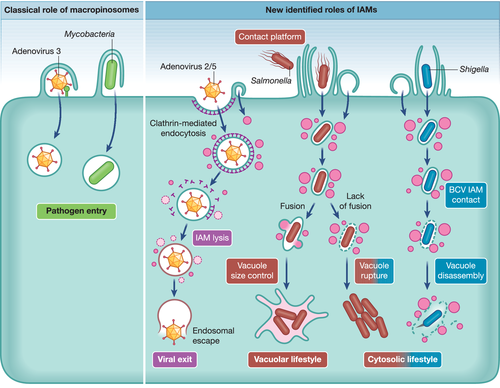
The described method innovations have been crucial to discover the central role of IAMs on intracellular niche formation of two bacterial pathogens, Salmonella and Shigella. Combining CLEM and rapid time-lapse microscopy, Shigella was found to induce the clustering of IAM around its BCV at the moment of vacuolar rupture (Chang et al., 2020; Weiner et al., 2016) (Figure 2). By applying the above-mentioned magnetic purification, we mined the proteome of Shigella IAMs and uncovered the molecular players that mediate the BCV–IAM interaction. More specifically, statistical analysis of the large datasets identified enrichment of the components of the exocyst complex on the IAMs, a multi-subunit tethering complex that tethers opposing membranes for interaction (Chang et al., 2020). We further validated that the exocyst complex is manipulated by Shigella via the action of its T3SS effector IpgD to hijack Rab8 and Rab11 trafficking. The exocyst-mediated IAM clustering thus enhances BCV–IAM contact that leads to translocation of vacuolar membrane remnants away from the bacteria and enables the naked bacteria to spread to neighbouring cells via actin-based motility (Figure 2). Although the host–pathogen interplay involved in membrane translocation is still under investigation, the Shigella IAM proteome implicates a sketch of the efficient vacuolar escape of this cytosolic-dwelling pathogen.
The nascent Salmonella-containing vacuole undergoes a dramatic resizing just after its formation. Rapid time-lapse microscopy indicated simultaneous Salmonella BCV expansion through IAM fusion and shrinkage through the formation of membrane tubules emanating from the vacuole. These antagonistic events are utilised by Salmonella to equilibrate its vacuolar niche settlement with BCV rupture leading to Salmonella cytosolic escape. Thus, fusions between nascent Salmonella BCV and IAM at early Salmonella infection stage prevent vacuolar rupture and favour Salmonella vacuolar lifestyle (Stévenin et al., 2019). By exploring the Salmonella IAM proteomes, we found a substantial enrichment of the soluble N-ethylmaleimide–sensitive factor attachment receptor (SNARE) proteins, which are well-characterised proteins that are required for membrane fusion (Stévenin et al., 2019). More importantly, integrating the proteome of Salmonella BCV (Santos et al., 2015) in our analysis, the Salmonella IAMs proteome showed the cognate SNARE pair that is present on the Salmonella BCV (Stx4) and the surrounding IAMs (SNAP25). This confirmed SNAREs are subverted to mediate the fusion between the Salmonella BCV and the proximal IAMs for Salmonella BCV expansion and bacterial proliferation. This proteomic study of IAMs thus expands the list of functions of macropinosomes for vesicular compartment size control during pathogen infection processes (Figure 2). Later on, Kehl et al. (2020) analysed the recruitment of trafficking-associated proteins on the Salmonella BCV–associated tubules at late time points of the infection (6–9 hr post infection). During this work, the authors showed also the enrichment of the SNAREs VAMP2, VAMP3 and VAMP8 that we found significantly enriched in the IAM proteome along these tubules. Besides, performing a trafficome-wide RNAi screen, they showed that siRNA depletion of many proteins significantly enriched on IAMs (e.g., SNAP23, Sec22b, Bet1, Rab35, among others) caused a defect in the establishment of the late Salmonella BCV tubular network. These results suggest a potential contribution of IAMs in the membrane composition of the late BCV and its associated tubules. Further integration of the results would thus be advantageous to understand the role of IAMs in the establishment of the late Salmonella tubular network.
Consistently, as pairwise comparative analysis of the proteomes of Salmonella and Shigella IAMs has the potential to reveal distinct molecular factors that correlate with the different lifestyles of the two pathogens, it has opened new exciting tracks of currently ongoing research. The magnetic extraction of macropinosomes during pathogen infections, therefore, sheds light on the determining roles of IAMs in establishing the diverse intracellular niches of bacterial pathogens.
In addition to their intracellular roles, macropinosome formation may also be considered to be involved in extracellular processes. As bacteria-induced ruffles stand-out from the cellular surface, it is conceivable that they act as a trap favouring bacterial attachment instead of simply englobing the surrounding pathogens (Figure 2). This was observed during Salmonella near-surface swimming on the epithelial surface during which salmonellae bump into ruffles that were formed by previously internalised Salmonella (Misselwitz et al., 2012). Hence, macropinocytosis induction during Salmonella invasion facilitates the entry of additional bacteria in a cooperative manner. Similarly, cryo-electron tomography of Chlamydia infection revealed that bacterial entry is facilitated by filopodial capture following a macropinocytosis-like pathway (Ford et al., 2018). These precocious steps of bacterial intracellular colonisation are particularly relevant for therapeutic targeting and should be further investigated at the molecular level.
Similar to bacterial pathogens, some viruses may require macropinocytosis for processes (e.g. efficient cell penetration) independent of host cell entry. For instance, despite its major internalisation route by clathrin-mediated endocytosis, binding of adenovirus 2/5 particles to cell-surface receptors concurrently induces macropinosome formation (Meier et al., 2002). Though the mechanism remains unclear, virus-dependent lysis of these macropinosomes contributes to the efficient viral exit to the cytosol and spreading of the infection (Figure 2). In addition, two coronaviruses, namely MHV and SARS, have been reported to induce continuous macropinocytosis at late infection stage, which implies a process not associated with viral entry. Inhibition of macropinocytosis resulted in lower extracellular but not intracellular viral titers, indicating that macropinocytosis possibly facilitates coronavirus infection through enhanced cell-to-cell spreading (Freeman et al., 2014). While viruses and bacteria profoundly differ by nature, these studies suggest that IAM hijacking could be another analogic feature reached via distinct evolution roads. The robust methodology to magnetically purify IAMs and extract their molecular compositions may thus encourage elucidation of IAM subversion by viruses.
5 PERSPECTIVES
A lack of methodologies to identify the molecular factors involved in macropinocytic pathway has remained challenging until recently. Purification of macropinosomes during different infection processes in different cell types provides a powerful toolset for IAM investigations (Figure 1). Due to the biocompatibility of the magnetic beads, the magnetic purification method can not only be applied during pathogen invasion but is also readily adaptable to study the macropinocytic behaviours of different cellular contexts. Here, it is important to carefully examine the microenvironment (e.g. level of cell surface receptors, cell morphology, etc.) as it was reported to influence macropinocytic activity (Lee et al., 2019) and pathogen invasion (Snijder et al., 2009; Voznica et al., 2017). It will be interesting to mine and compare the proteomes or lipidomes of macropinosomes of various origins (e.g. cell types, physiologic or pathologic inductions, etc.) to expand our knowledge on the regulatory machinery of macropinosomes linked with their versatile biological functions. The purified macropinosomes may also be used for bottom-up in vitro studies on the interaction with other compartments.
Having obtained the molecular composition of macropinosomes, it is now possible to characterise the specific interactions of the identified factors to unravel the formation and maturation of macropinosomes that have been originally perceived as indistinguishable from other endosomal vesicles in high temporal and spatial resolution. One prominent progress is to combine super-resolution light microscopic techniques, such as stimulated emission depletion (STED) microscopy and single-molecule localisation microscopy (SMLM) (Schermelleh et al., 2019) that may achieve sub-100 nm resolution, with electron microscopy in CLEM approaches. Besides, cryo-ET will continue to offer progress in improved sample preservation for investigating the IAM-compartment contact sites at near-atomic resolution. Apart from that, advancement in proximity-labelling techniques, particularly biotinylation conjugation, enables the study of the identified factors on IAMs with their interacting partners (Cho et al., 2020; Nguyen et al., 2020; Liu et al., 2020). In some cases, the protein of interest is conjugated to a biotin ligase, which enzymatically incorporates a biotin moiety to mine any proteins that are once in close proximity to the candidate protein during the course of incubation (Cho et al., 2020; Lam et al., 2015). This target-oriented proteomic technique may be applied to complement the unlabelled approach to investigate the specific protein–protein interaction in spatial manner.
The breakthrough in tool development to study macropinosomes during infection processes that we reviewed here will increasingly benefit research linking macropinocytosis and other pathologies. Future studies will foster our understanding of macropinocytosis-mediated nutrient uptake in cancerous cells (Commisso et al., 2013) and the implication of macropinosome in membrane recycling during metastasis-associated cancer cell migration (Li et al., 2020). Likewise, research interests have emerged aiming to understand how macropinocytosis is involved in the uptake and propagation of protein aggregates in neurodegenerative diseases (Zeineddine & Yerbury, 2015). Taken together, we are just at the beginning to decrypt the diverse roles of macropinosomes in pathogen infections and pathologies offering a perspective with many exciting discoveries in the near future.
ACKNOWLEDGMENTS
Y-Y.C. and V.S. were supported by grants from the Fondation pour la Recherche Médicale (FRM; SPF20160936275 and FDT20170436843). J.E. is a member of the LabEx consortia IBEID and MilieuInterieur. J.E. also acknowledges support from the ANR (grant StopBugEntry, AutoHostPath and HBPSensing) and the ERC (CoG EndoSubvert).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.