Organ-on-chip to investigate host-pathogens interactions
Funding information: ASSU2000; PTR, Grant/Award Number: PTR232-2019; Institut Pasteur
Abstract
Infectious diseases remain the subject of intense research. This topic reaches a new era towards the study of host-pathogen interactions mechanisms at the tissue scale. The past few years have hence witnessed the emergence of new methods. Among them, organ-on-chip, which combines biomaterial technology, microfluidic and tissue engineering to recreate the organ physiology is very promising. This review summarises how this technology recapitulates the architecture, the mechanical stimulation and the interface of a tissue and how this particular microenvironment is critical to study host-pathogen interactions.
Take Away
- Organ-on-chip combines microfluidic and tissue engineering technologies
- OOC recapitulates 3D architecture, physical forces and interfaces of tissues
- Organ-on-chip sustains complex microbial communities growing in symbiosis
- OOC is key to address the role of tissue mechanics during infection processes
- OOC is highly relevant to study infections propagating through multiple tissues
- OOC uncovers unprecedented findings on host-pathogen interactions
1 INTRODUCTION
Biomedical research aims at understanding cellular and molecular mechanisms of diseases to provide a suitable diagnosis or a possible cure. Methods to study deregulated human physiology have evolved and got widely improved in the last 50 years. Every tissue in the body behaves differently, has its own microenvironment, thereby complexifying a lot the methods to fully recapitulate their physiology. Even though studies performed on individual cells plated on classical petri dishes have enabled great discoveries, they are no longer sufficient to explore deeply complex mechanisms, forcing biomedical research to find new approaches.
When it comes to host-pathogen interactions, some concepts about pathogenic mechanisms still remain unclear and not completely explored yet. In particular, the role of mechanical forces during infectious processes has been only recently investigated in few studies. Yet, these physical forces may modify the pathogen adhesion and virulence.
In that respect, our understanding of the role of the microenvironment and more specifically the biomechanical stimuli on host-pathogen interactions is still limited due to the lack of physiologically relevant organ models. The interface is considered as primordial since pathogens dynamics rely on mechanisms occurring at that precise location. The skin, the gastrointestinal tract, respiratory organs are examples of tissues which harbour interfaces with multiples microorganisms, some being pathogens. Therefore, deciphering host-pathogen interactions necessarily goes through the modelling of an accurate organ-like tissue as a working base. Tissue engineering has come in this context, as a huge breakthrough, offering broader perspectives with the design of organ-on-chip.
In this review, we will present different approaches to recapitulate the tissue physiology using organ-on-chip technology and how it can help to better investigate host-pathogen interactions at the tissue scale.
2 ORGAN-ON-CHIP: CONCEPTS AND DESIGNS
Organ-on-chip emerged from the combination of microfluidic engineering and tissue engineering. This recent state-of-the-art methodology does not aim to reconstitute the whole living organ but rather to synthesise the cell organisation and physical parameters of an organ. The number of organ-on-chip models has grown exponentially and has been listed in several recent reviews (Park et al., 2019; Zhang, Korolj, et al., 2018). There are many challenges associated to organ-on-chip technologies, depending to the system one study.
Organ-on-chip are microfluidic cell culture devices, engineered by microfabrication methods. The device made of clear and biocompatible polymers contains continuously perfused chambers, which can receive specific cell cultures. Today organ-on-chip microfluidic platforms are starting to reach the market and several companies such as Emulate™, Alevolix™, Mimetas™ and BiOND™ proposed such models. All chips have at least one channel providing various interfaces (air–liquid, liquid–liquid), mechanical stimulation (shear, stretch) and recapitulating 3D or 2D tissue organisation (examples are described in Figure 1). In most devices, microchannels are perfused with culture medium with flow rate leading to laminar fluid flows inside the channels. An advantage of these organ-on-chips is their ability to recover exit fluids and the possibility to add any drugs or microorganisms in real time. Moreover, organ-on-chips are often made of transparent material enabling live imaging (Grassart et al., 2019).
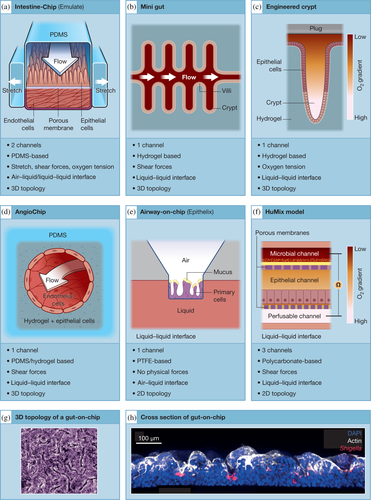
Several organ-on-chip designs differ from each other's by the material used, their ability to modulate physical forces or to recapitulate the 3D architecture of the organ.
2.1 Materials
The choice of the material is highly important in the design process of organ-on-chip technologies. Biocompatible, non-toxic, optically clear for microscopic imaging are often preferred approaches. The stiffness of the material is decisive for cellular growth. One main challenge is to find the right balance between stiffer materials with more precise mechanical properties and softer materials (hydrogels) closer to physiological tissue properties, but with less opportunities to modulate mechanical stimulation.
Polymers show many advantages since they can be shaped and designed with several techniques (photolithography, multiphoton excitation leading to local degradation, electrospinning…). Polydimethylsiloxane (PDMS) is the most common polymer used to build microfluidic organ-on-chip (Figure 1a,h. Kim & Ingber, 2013), classified as elastomer for its elasticity, with a high stiffness (800 kPa ~ 10 MPa, [Seghir & Arscott, 2015]). In this context, a microengineered organ-on-chip incorporating a built-in perfusable vascular network has been described (Zhang, Lai, et al., 2018; Zhang & Radisic, 2017). This multi-material scaffolding (poly (octamethylene maleate [anhydride] citrate) and PDMS) named AngioChip is being cultivated with endothelial and parenchymal cells to model vascular systems (Figure 1d). Thanks to tunable mechanical properties and great biocompatibility, fabricated vascular networks demonstrate applications for human myocardium engineering.
Hydrogels are being investigated nowadays since they are highly biocompatible and many features like porosity, mechanical stiffness, or elasticity can be modulated (Ding et al., 2020). They are 3D polymers soft networks containing mostly water (Seliktar, 2012), with a lower but tunable stiffness (0.5 kPa ~ 100 kPa, [Zhao et al., 2019]) and can be classified into natural, synthetic and hybrid materials. Natural hydrogels exhibit closer to native tissues matrix but do not provide reproducible mechanical properties. Further considerations lead to finding the best choice between synthetic or hybrid hydrogels, guaranteeing a sufficient control on mechanical properties and a scaffold presenting interesting cell-binding sites. As an example, two hydrogel-based models mimicking intestinal crypts are shown Figure 1b (Nikolaev et al., 2020) and Figure 1c(Kim et al., 2019). Some advanced techniques like stereolithographic high-resolution printing have enabled the development of such devices achieving precise localization and patterning of cells. In light of their modular composition, hydrogels offer the possibility to create internal molecular gradients such as oxygen, which can be useful in particular for anaerobic microorganisms. In addition, hydrogels stiffness can be modulated in a reversible manner by crosslinking reactions following light stimulation (Rosales et al., 2017), thereby offering a remote spatio-temporal control useful to address mechanobiology issues.
2.2 Physical forces in microfluidic devices
Organ and tissue inside the body experience physical changes, constraints within their own microenvironment and depending on their functions. Indeed, a blood vessel or the urinary tract will experience strong shear forces due to not only pulsatile blood and urinary flow rate but also some stretching forces during diastolic and systolic cycles in cardiac tissues. Intuitively, same forces can be considered in respiratory tissues (lung, pulmonary alveoli) since air is cyclically renewed during respiration. Some tissues such as the intestine are subjected to slower flows but experience similarly a cyclic stretch during peristalsis-like motions.
Moreover, mechanical forces stimulate cell differentiation and morphological polarisation. In the gut, fluid shear stress induces for instance cell organisation into 3D crypt and villi-like structure (Figure 1g). It has been shown thanks to a kidney model that fluid shear stress not only results in a morphological polarisation triggering actin cytoskeleton reorganisation but also led to the expression of differentiated epithelial cell functions (Jang & Suh, 2010; Theobald et al., 2018). Therefore, it appears capital to reproduce mechanical forces in 3D existing models. One of the main advantages that offer organ-on-chip is that tissue grown on a porous membrane can experience multiple physical stresses at the same time (Bhatia & Ingber, 2014). Furthermore, microorganisms have been also reported to sense physical cues such as the shear stress, reinforcing the needs to recapitulate physical forces to study host-pathogen interactions (Mordue et al., 2021).
2.2.1 Stretching
Organ-on-chip containing a porous polymer-based membrane can be easily stretched, but so far only few devices provide this mechanical stimulation. In the Emulate chip (Figure 1a), stretching is generated thanks to vacuum applied in two chambers located on both sides of the channels and can be applied either cyclically or continuously with big range of frequency and intensity. A negative pressure will apply stretching forces on the tissue whereas increasing the pressure inside the chambers will compress it. As an example, a cyclic stretch of 10% at 0.15 Hz on a gut-on-chip recapitulates the peristaltic motion found in the human colon (Kim & Ingber, 2013).
2.2.2 Shear forces
Microfluidic devices are particularly relevant platforms to expose cells to fluid shear forces. In fact, laminar flow circulating in organ-on-chip naturally produces such forces on engineered tissue. As an example, primary kidney epithelial cells loaded into the apical side of a two-channels device and exposed to a fluid shear stress of 0.2 dyne/cm2, mimic the actual forces found in living kidney tubules and enhanced polarisation and function of the cells (Jang et al., 2013).
2.2.3 Electric field
Some models include electrodes around the channels allowing the application of an electrical field within the tissue. The electrical stimulation of contractile cells helps reproducing electrophysiological and mechanical features of cardiac muscles for instance (Agarwal et al., 2013).
2.2.4 Biochemical tension
Organ-on-chip are suitable platforms to simulate and engineer a relevant chemical microenvironment. Gradients of nutrients or oxygen can be often observed in multiple human tissues but pathological tissues usually exhibit chemotaxis as well, in tumour cells for instance (Huh et al., 2012), or in heart ischemia characterized by a strong lack of oxygen inside the tissue (Khanal et al., 2011). Tumour-stromal interaction was engineered into a microfluidic chip designed to generate a biochemical tension, physiological chemotactic gradients influencing cancer cells migrations (Torisawa et al., 2010). In addition, the gut microbiota should differ upon aerobic or anaerobic conditions. Therefore, several groups could recreate an oxygen gradient in gut-on-chips supporting anaerobes co-culture (Figure 1c,f, [Kim et al., 2019; Shah et al., 2016]).
2.3 Representing specific 3D topology, interface and cellular richness
One of the main challenges of tissue engineering is to recapitulate the cellular and molecular composition of the organ. In that respect, stem cells such as induced-pluripotent stem cells (iPS) or even embryonic stem cells has been used (Wan et al., 2011). Many studies related to organ-on-chip have considered cell patterning as an efficient method to create precise cell environment with specific arrangement inside the chip. Surface treatments or even 3D printing guarantee precise patterns or complex hydrogels scaffold which can be eventually grown with cells. A recent study uses cell patterning to develop a hydrogel-based mini-gut model shaped with microcavities that recapitulate the 3D geometry and cell diversity (by loading organoids) of the native crypt and villi structure of the human gut (Figure 1b, (Nikolaev et al., 2020). Similarly, a collagen-based array of crypt-like invaginations could closely mimic the human intestinal epithelium monolayer with a gradient of proliferative to differentiated cells along crypts (Figure 1c), (Kim et al., 2019; Wang et al., 2018). These two devices enable the maintenance and spatial organisation of both stem and differentiated cells and hence represent good models to investigate gut regeneration.
A tissue exhibits specific interface and needs a precise cell organisation to be fully functional and to interact with the rest of the body. Human body displays several of them in many organs: lungs, intestine, vessels, bladder, skin, vagina are the most important. Pathogens enter the body through the airflow or through contaminated food and fluids. Organ-on-chips having two adjacent channels separated by a porous membrane offer many options in terms of cellular seeding and medium type (air, liquid or gas). Indeed, different kind of cells can be cultured on both sides of the porous membrane with their respective physiological media, allowing hence exchanges between several types of tissues/cells (Figure 1). Lung is an appropriate example of tissue interface presenting an air–liquid boundary with relevant alveolar barrier that has been recapitulated in several chips designed by Emulate, Alevolix or Epithelix (Figure 1a,e, Stucki et al., 2018). The intestinal lumen is another example of tissue liquid–liquid interface (Figure 1a–c). In addition, several devices allow at recapitulating several tissue-tissue interface as exemplified in Figure 1a,d,f.
3 ORGAN-ON-CHIP TO STUDY HOST-PATHOGEN/COMMENSAL INTERACTIONS
Respiratory tissues, gastrointestinal tract, skin or even mucosal surfaces are tissues that most likely encounter pathogens since they are in direct contact with outside the body. They are precisely considered as the first barriers against pathogens. Respiratory pathogens have to cross several barriers (mediators and bactericidal peptides) before reaching and infecting their target cells (Grubor et al., 2006). Concerning enteric pathogens, they experience strong pH changes and oxygen gradient tensions once ingested. Plasma membrane is the precise location where host and pathogens meet and is composed of several mechano-sensitive structures: caveola, lipid rafts, cytoskeleton components (actin fibres, tubulin transducing signals to mechanosensitive proteins). Indeed, Caco-2 cells grown in gut-on-chip or in Transwell device have a totally different profile of gene expression, reinforcing the impact of mechanical stimulation on tissue molecular organisation (Kasendra et al., 2020). Given the great importance of mechanosensing in eukaryotic cells, it is not surprising that pathogens might experience such phenomenon as well.
3.1 Mechanical forces trigger pathogen virulence induction
In this part, we will briefly describe several studies reporting the induction of virulent genes by mechanical stimulation that were obtained with microfluidic devices (not truly organ-on-chip). Bacteria often experience mechanical forces when they encounter cell surfaces. They can be triggered by a fluid shear stress generated by a flow or be stimulated by the host surface stiffness and topology. It has been shown that bacteria could actually feel such signals and induce the upregulation or downregulation of their virulence factors.
Enterohemorrhagic Escherichia coli (EHEC), a foodborne pathogen that can colonise the gastrointestinal tract, was capable to adapt the expression of its virulence genes depending on the mechanical constraints of its host (Alsharif et al., 2015). More precisely, the locus of enterocyte effacement containing a range of virulence factors, notably the transcriptional activator GrlA, was shown highly expressed and localized into the cytoplasm in response to physiological relevant shear stress (Sirisaengtaksin et al., 2020).
Type IV pili are long and thin dynamic filaments based at the surface of bacteria and often involved in pathogenic processes. These surface appendages can polymerise and depolymerise from pilin molecules in a short period of time (Craig et al., 2019). Biomechanical considerations have revealed that type IV pili had an influence on not only how bacteria interact with host surfaces but also how they can be modulated by external mechanical stresses. They are actually considered by the literature as mechano-sensitive structure. The case of Neisseria meningitides, a pathogenic bacterium infecting the human nasopharynx is worth noting. When this pathogen reaches the blood–brain barrier and integrates the bloodstream, it can cause severe inflammation named meningitis. Several studies have shown that despite the strong mechanical forces generated by the blood flow, Neisseria succeed to reach the bloodstream, bind to the epithelial vessel structure and aggregate, confirming their striking abilities of adaptation (Dos Santos Souza et al., 2020). N. meningitides aggregate in microcolonies, very stable and flow-resistant which induced host cell surface reorganisation and protrusion formation enhancing the adhesion (Charles-Orszag et al., 2016). Interestingly, in vivo data showed that nesserial adhesion was mainly observed in capillaries, having heterogeneous flow, compared to normal-sized vessels and this would favour the initial adhesion of the bacterium (Mairey et al., 2006).
Biofilms are communities of bacteria held together with a specific extracellular matrix. The biofilm is created when a bacterium adheres to a surface. Interestingly, pathogens developing within a biofilm are phenotypically different form their free-living counterparts. It has been demonstrated that intracellular levels of cyclic-di-GMP participate to the initiation of Pseudomonas aeruginosa biofilms, such signals inducing a change in genes expression (Rodesney et al., 2017). In this context, the role of mechanical forces such as shear forces would trigger bacterial behaviour, enhancing the surface adhesion of P. aeruginosa, activating, via PilY1 and type IV pili, the cyclic-di-GMP pathway decisive for biofilm development (Rodesney et al., 2017).
The direct role of mechanical inputs of tissues on pathogenic gene expression has not yet been clearly investigated using organ-on-chip, but will surely be addressed in the future.
3.2 Organ-on-chip to model host-microbiota crosstalk
Microbial community is a major part of the cellular richness of some tissues, especially in the gut where the number of bacteria can reach ~1014 bacteria (Qin et al., 2010), (Turnbaugh et al., 2007), (Lozupone et al., 2012). The intestinal tract composition varies according to space and time. The interaction between microbiota and the organ participate to tissue homeostasis and function, but also turns out to be a strong protection against pathogens. Indeed, several protective mechanisms such as mucin secretion, or the triggering of bacteria sensors are controlled by certain entities of the microbiota (Ashida et al., 2012). Classical devices exhibit several drawbacks to co-cultivate eukaryotic cell monolayers with microorganisms. In fact, they fail to recapitulate the microbial diversity, the long-term microbiota interaction and the complexity of the underlying tissue structure. In this context, several studies have highlighted that organ-on-chip devices can sustain complex microbial communities growing in symbiosis with human intestinal cells, relevant to host-microorganism interaction investigations.
The lack of fluid-flow in classical devices was thought to be the main missing cue leading to bacterial overgrowth. However, peristaltic motion is another important parameter, since cyclic stretching (peristalsis-like deformations) greatly reduces bacterial overgrowth in a gut-on-chip already maintained under luminal flow (Kim et al., 2016). This suggests that modulation of mechanical deformation is decisive to prevent bacterial overgrowth (Bures et al., 2010), and that gut-on-chip are relatable models to co-culture commensal microorganisms over an extended period of time. As an example, viable bacterial microcolonies from a commercial probiotic formulation containing several strains (Bifidobacterium breve, B. longum, B. infantis, Lactobacillus acidophilus, L. plantarum, L. paracasei, L. bulgaricus and Streptococcus thermophiles) have been successfully maintained for several days (Kim et al., 2016).
In addition, hypoxia gradient in gut-on-chip greatly increase the microbial diversity since it increases the growth of facultative and obligate anaerobic bacteria like Bacteroides fragilis (Jalili-Firoozinezhad et al., 2019) or B. adolescentis and L. rhamnosus, (Kim et al., 2019). In particular, co-cultivating in anaerobic conditions L. rhamnosus with human colon cells in HuMiX device was able to modify the transcriptional, metabolic and immunological host responses as reported previously by in vivo studies (Shah et al., 2016).
Altogether, these studies demonstrate the huge benefit of using gut-on-chip under mechanical stimulation and oxygen gradient to provide a microbiota-intestine-on-chip relevant model.
3.3 Pathogenic mechanisms deciphered by organ-on-chip devices
Over the last few years, host-pathogen interactions have been increasingly investigated using organ-on-chip technology, some non-exhaustive examples are described below.
3.3.1 Digestive system infections
The gut is often targeted by pathogens and thus several studies use organ-on-chip devices. Gut-on-chip model (Figure 1a) was used to monitor enterohemorrhagic E. coli (EHEC) infection in the presence of human-derived microbiome metabolites. The study highlighted that some metabolites were associated to a greater injury following EHEC infection, by inducing flagellin expression in the bacterium genome (Tovaglieri et al., 2019). Thus, OOC technology enables to investigate complex host-microbiome-pathogen relationships. Human enterovirus infections have also been explored with organ-on-chip. For instance, the enterovirus coxsackievirus B1 injected to the lumen of gut-on-chip was able to enter the epithelium, and infectious virions and inflammatory cytokines were released in the apical exit fluid (Villenave et al., 2017). This work also reveals the importance of applying a continuous flow during infection that here enhances the viral replication, enables the detection of gradients of cytopathic effects and villus destruction and thus could model secondary infections and disease propagation. Another example is Shigella, a human-restricted bacterium leading to severe intestinal damages. S. flexneri apical invasion was 10,000-fold more efficient in a gut-on-chip than in Transwell classical models, recapitulating for the first time the high infectivity of this bacterium as seen in human patients, highlighting once again the benefits of organ-on-chip devices in modelling human infection (Grassart et al., 2019). Furthermore, the study showed that the 3D architecture of the colon recapitulated in the gut-on-chip provided crypt-like structures where S. flexneri were highly enriched, creating new infection outbreak points (Figure 1h). In addition, Shigella invasion and propagation through the epithelial monolayer were greatly enhanced by cyclic stretching and continuous flow. Altogether, this study reveals that Shigella takes advantage of the intestinal microarchitecture and mechanical forces found in the human colon to invade with great effectiveness the intestinal barrier (Grassart et al., 2019). How the physiological stretch enhances Shigella invasion is not yet known. Mechanical stimulation may induce the reorganisation of the actin cytoskeleton and/or plasma membrane cholesterol, two known host cell factors needed for bacterial uptake, these forces may also directly induce virulent genes of Shigella.
Liver can be also recapitulated in organ-on-chip and hence allowing hepatitis B virus (HBV) infection. Ortega-Prieto et al. designed a 3D microfluidic system made of collagen-coated polystyrene scaffold continuously perfused with oxygenated medium, allowing the culture of primary human hepatocytes in fully polarised manner. This platform could recapitulate the innate immune and cytokine responses mimicking the HBV-infected patient's response (Ortega-Prieto et al., 2018). Future investigations using this platform could help identifying pathways implicated in the host-HBV interaction.
3.3.2 Lung infections
The lung is also a target organ for pathogens and in particular bacteria. A sophisticated organotypic bronchiole model was engineered to provide multiple interfaces embedded in a 3D matrix of collagen and pulmonary fibroblasts. The center lumen, lined with primary human bronchial epithelial cells, filled with air to form an air–liquid interface, is flanked with two vascular compartments filled with primary human lung microvascular cells, that can be additionally loaded with polymorphonuclear leukocyte (PMN). This complex model enabled for the first time to perform volatile administrations of Aspergillus fumigatus and/or Pseudomonas aeruginosa, analyse the inflammatory response of host tissues and PMN extravasation, hence providing advance insights into the multikingdom (bacterial–fungal–human) complexities (Barkal et al., 2017). Another work describes the use of an alveolus-on-chip to perform co-infection by Staphylococcus aureus and influenza virus. Thanks to organ-on-chip device, the authors could mimic the superinfection seen in severe case of pneumonia (Deinhardt-Emmer et al., 2020). Pulmonary surfactant was proposed to play a host-protective role during the early interaction with Mycobacterium tuberculosis, however no animal model could accurately demonstrate this due the lethality of surfactant-deficient animals. Thanks to a lung-on-chip device, with air–liquid interface, having on the top channel alveolar epithelial cells and macrophages and at the bottom channel, endothelial cells, the authors investigated early steps of M. tuberculosis invasion and revealed the protective role of surfactants in tuberculosis (Thacker, Dhar, et al., 2020). Another study used a microfluidic lung-on-chip with a constant fluid-flow to explore the role of bacteriophage-host interactions in mucosal immunity. Thanks to this mucus producing lung interface, the authors uncovered the sub-diffusive motion of T4 bacteriophage that was determinant to efficiently reduce E. coli colonisation (Barr et al., 2015).
Virus can also infect respiratory tissues, several studies assessed their invasion with organ-on-chip. The example of influenza virus is relevant since engineered lung-on-chip offers a reliable model of respiratory tissues including alveolus, small airway and alveolar-capillary for viral infection and drug development (Gkatzis et al., 2018). Indeed, a study employed such platforms (Figure 1a) culturing primary human lung airway epithelial cells and inoculating different influenza virus strains (H1N1, H3N2, H5N1) (Si et al., 2019). The model reproduces faithfully the airborne influenza infection demonstrating high virulence accompanied by a barrier disruption and a loss of overlying cilia. Thus, the virulence of influenza virus and its possible mutations under antiviral drugs, through patient–patient transmission, could be also recapitulated using a lung-on-chip. Finally, the great adaptability of organ-on-chip devices allows investigations on emerging viral infections such as SARS-CoV-2 virus. Several works described the use of organ-on-chip to investigate SARS-CoV-2 infection. One study used the MucilAir platform (Epithelix) to observe the virus effect on primary human bronchial epithelial cells upon air–liquid interface. The authors observed a profound damage of the motile cilia function resulting in impaired mucociliary clearance (Robinot et al., 2020). Another study, using alveolar-on-chip reproduces key features of alveolar-capillary barrier by co-cultivating human alveolar epithelium, microvascular endothelium and circulating immune cells under fluid-flow. The authors reported that high dose of SARS-CoV-2 leads to an efficient viral production in epithelial cells in contrast to endothelial cells, triggers immune cells recruitment, endothelium detachment and inflammatory cytokines storm (Zhang, Korolj, et al., 2018). A similar alveolar-capillary barrier model was used with low dose of SARS-CoV-2, and revealed that even low viral replication still lead to an inflammatory response, and damage in endothelial layer integrity (Thacker, Sharma, et al., 2020). While these two works have some discrepancy, they both highlight the differential response of epithelial and endothelial cells to SARS-CoV-2.
3.3.3 Blood and bladder infections
A simple microvessel-on-chip using OrganoPlate (Mimetas) with bidirectional flow was used to address hemorrhagic shock syndrome with Ebola (Junaid et al., 2020) and Lassa (Tang et al., 2021) viruses. The luminal infusion of Lassa or Ebola virus caused a dramatic effect on vascular permeability.
The urinary tract is another tissue often targeted by pathogens, Uropathogenic E. coli (UPEC) is one such example. Using a bladder-chip (same design than Figure 1a) having bladder epithelial cells and microvascular endothelial cells under flow in urine and nutritive media, and recapitulating bladder filling and voiding cycles, they investigated the formation of intracellular bacterial communities (IBCs) by time-lapse microscopy. Their results suggest that, IBCs may play a role in reseeding sites of infection for a long period after antibiotic treatment initiation (Sharma et al., 2021). Once again, recapitulating tissue physical forces such as the mechanics of bladder filling/voiding cycles was determinant to revisit the role of IBCs as harbors of persistent bacterial populations in urinary tract infections (Sharma et al., 2021).
These very recent works using organ-on-chips to study host-pathogen interactions reveal how powerful the technique is in particular to investigate human-restricted pathogens, co-infection studies, the impact of mechanical forces or multiple tissues reactions during infections.
4 CONCLUSION
Although recently developed, some studies employing organ-on-chip have demonstrated the high potential of this technology to understand infectious processes. By recapitulating the tissue physiology, with its particular physical cues and interface, organ-on-chip allow at addressing new questions or can revisit old dogma on host-pathogen interactions that will for sure unravel key findings in the future. Besides, this method proposes a way to reduce animal experimentation required by the international community. Finally, the emergence of body-on-chip will be key to investigate signal transfer, or immune cells recruitment, between organs to recapitulate the human disease in an integrated model. Furthermore, body-on-chip using human- derived organoids will be a breakthrough towards personalised medicine and drug testing.
ACKNOWLEDGEMENT
We are very grateful to Assu2000 and PTR232-2019 to fund our team and the fellowship of Thomas Feaugas. We would like to thank Emulate Company and in particular Brett Clair for providing us image. We thank the PBI (Imagopole) platform of Institut Pasteur for microscope maintenance and technical help.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.