Identifying haplotypes in recessive inherited retinal dystrophies using whole-genome linked-read sequencing
Funding information: Suomen Kulttuurirahasto
Abstract
Conventional next-generation sequencing methods, used in most gene panels, cannot separate maternally and paternally derived sequence information of distant variants. In recessive diseases, two or more equally plausible causative variants with unsolved phase information prevent accurate molecular diagnosis. In reality, close relatives might be unavailable for segregation analysis. Here, we utilized whole genome linked-read sequencing to assign variants to haplotypes in two patients with inherited retinal dystrophies. Patient 1 with macular dystrophy had variants c.3442T>C, p.(Cys1148Arg), c.4209G>T, p.(Glu1403Asp), and c.1182C>T, p.(Cys394=) in CRB1, and Patient 2 with nonsyndromic retinitis pigmentosa had c.1328T>A, p.(Val443Asp) and c.3032C>G, p.(Ser1011*) in AHI1. The relatives were not available for genotyping. Using whole genome linked-read sequencing we phased the variants to haplotypes providing genetic background for the retinal dystrophies. In future, when the price of sequencing methods that provides long-read data decreases and their read-depth and accuracy increases, they are probably considered the primary or adjunctive sequencing methods in genetic testing, allowing the immediate collection of phase information and thus obviating the need for the carrier testing and segregation analysis.
1 INTRODUCTION
Genetic testing for monogenic diseases is usually done with gene panels and exome sequencing techniques. However, conventional next-generation sequencing (NGS) methods are not equipped to separate maternally and paternally derived sequence information.1 When a patient with recessive disorder carries multiple equally plausible causative variants or a variant of unknown significance (VUS), the variant phase information is important for accurate molecular diagnosis. Additionally, determining the phase of two heterozygous variants in a recessive disease gene will help to establish whether this gene is implicated in disease or not. Haplotyping is traditionally based on genotyping parents. When close relatives are not available for carrier testing, alternative methods are needed.
Microfluidics-based linked-read sequencing technology is a new haplotyping approach.2 High molecular weight genomic DNA is partitioned into droplets and transformed into short barcoded fragments for sequencing. After standard short-read sequencing of the libraries, a computational algorithm links the sequencing data into long-range haplotype information based on the barcodes.2
Inherited retinal diseases (IRDs) affect the function of the retina and retinal pigment epithelium, leading to reduced vision. They are caused by over 300 different genes and loci, making individual diseases rare (RetNet, https://sph.uth.edu/RetNet/). Genetic testing is crucial to resolve causative gene variants for accurate clinical and genetic counseling.3
Here we report the clinical characteristics and genetic findings of two patients with inherited retinal dystrophies. Both patients were initially identified with potential disease-causing variants using conventional NGS. The variants phase remained undecided and as close relatives were not available for genotyping, the haplotype ascertainment was resolved with whole-genome sequencing (WGS) with linked-reads enabling a genetic diagnosis.
2 METHODS
Eligible to this study were two patients with IRDs and incomplete molecular diagnosis based on initial NGS findings in the Department of Ophthalmology, Helsinki University Hospital, Finland. The study was conducted retrospectively as a registry study and was approved by the Institutional Review Board, and followed the tenets of the Declaration of Helsinki.
The patients ophthalmic examination included color fundus photographs (Topcon X, Topcon, Japan), spectral-domain optical coherence tomography (SD-OCT) scans and fundus autofluorescence (FAF) imaging (488-nm excitation) (Heidelberg Spectralis HRA + OCT device, Heidelberg Engineering, Heidelberg, Germany). The visual fields were taken using the Goldmann perimeter. Full-field electroretinogram (ERG) was recorded using the RETI-port/scan 21 unit (Roland Consult Stasche & Finger, Brandenburg an der Havel, Germany) according to the International Society for Clinical Electrophysiology of Vision standards.4
Genomic DNA was extracted from peripheral blood using standard methods. Genetic testing was performed at the Blueprint Genetics laboratory (Blueprint Genetics, Helsinki, Finland) using the retinal dystrophy gene panel of 266 genes. The identified variants were confirmed by Blueprint Genetics using Sanger sequencing. Details of the methodology are available at www.blueprintgenetics.com. The WGS with linked reads was performed in the Institute for Institute for Molecular Medicine Finland (FIMM, Helsinki, Finland). Further details are available in Supporting Information S1.
3 RESULTS
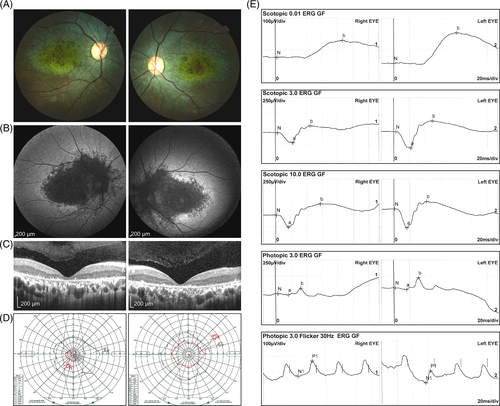
Patient 1 was a 36-year-old woman of Southeast Asian ancestry with reduced visual acuity and bilateral macular dystrophy. She reported no family history of poor vision, nyctalopia or consanguinity. BCVA was 0.03 (Snellen equivalents) in the right and 0.05 in the left eye. Fundus examination showed nummular pigmentation, beaten bronze appearance and outer retinal atrophy in the macula. Otherwise, a biomicroscopic examination was unremarkable, including the peripheral retina (Figure 1A). FAF images showed hypoautofluorescence in the central macula, extending to the peripapillary retina in the both eyes (Figure 1B). SD-OCT revealed loss of physiologic lamination of the outer retina, leading to macular atrophy and degeneration (Figure 1C), and the foveal ellipsoid zone (EZ) was absent. Small hyperreflective dots were found in the inner and outer retinal layers.5 Visual fields showed a relative scotoma in central field and concentrically constricted peripherals fields (Figure 1D). Full-field ERG at the age of 30 years showed reduced cone function with preserved rod function (Figure 1E).
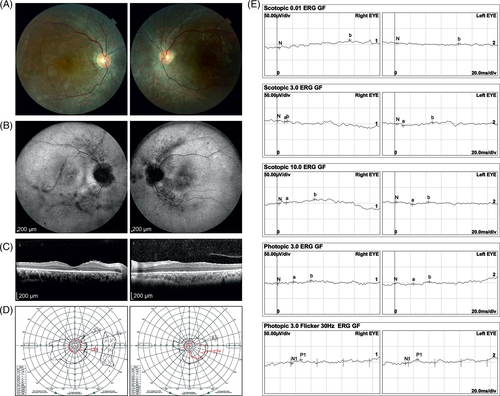
Patient 2 was a 26-year-old man of Northern African ancestry with the referral diagnosis of nonsyndromic retinitis pigmentosa. The family history revealed no retinal disorders. BCVA was 0.6 in the right and 0.7 in the left eye. The anterior segments of the eyes were unremarkable. Fundus examination revealed waxy pallor of the optic discs, narrowed vessels, and atrophic retina with moderate bone-spicules pigmentation. Macular region showed mild pigmentary changes and shiny reflex (Figure 2A). FAF images showed hypofluorescent changes in the peripapillary area and along the arcades, and reduced fluorescence in the macular areas. Hyperautofluorescent changes were noted in the papillomacular region, especially in the right eye (Figure 2B), however AF images in the right eye were obscured by some central vitreous opacities. SD-OCT of the macular demonstrated loss of EZ outside the foveal area (Figure 2C). Goldmann perimetry revealed a concentric constriction visual fields (Figure 2D). ERG showed severe generalized retinal dysfunction involving both rods and cones (Figure 2E).
The initial genetic testing was done with a retinal dystrophy gene panel. Patient 1 was heterozygous for three different rare variants in Crumbs Cell Polarity Complex Component 1 (CRB1) gene: c.3442T>C, p.(Cys1148Arg), c.4209G>T, p.(Glu1403Asp), and c.1182C>T, p.(Cys394=) (Figure 3A, Table S1). Pathogenic CRB1 variants are typically recessive and lead to varying range of inherited retinal dystrophies.6 The variant c.3442T>C has been described in patients with retinitis pigmentosa but was not present in gnomAD.7 The c.1182C>T (rs115352681, ClinVar 771 066) does not alter the amino acid sequence but potentially might alter splicing (Table S1). It is found in gnomAD with a frequency of 0.000096. The variant c.4209G>T has not been previously described. According to ACMG guidelines the variant c.3442T>C was classified as pathogenic, and the variants c.4209G>T and c.1182C>T as VUS (Table S1).
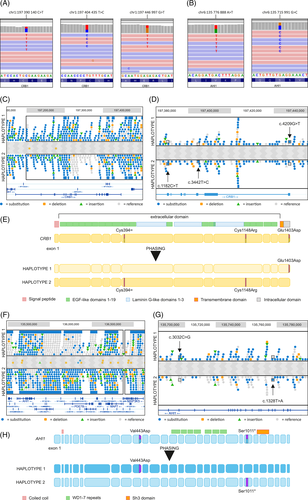
Patient 2 had a missense variant c.1328T>A, p.(Val443Asp) and a truncating variant c.3032C>G, p.(Ser1011*) in the Abelson Helper Integration Site 1 (AHI1) gene (Figure 3B, Table S1). The truncating variant c.3032C>G is found heterozygous in the gnomAD database with a frequency of 0.000064. The variant c.1328T>A is known to affect AHI1 function8 and both variants have been reported in patients with Joubert syndrome.8 The truncating variant is additionally reported in nonsyndromic retinitis pigmentosa in ClinVar. Apart from the retinal dystrophy our patient showed no findings suggesting Joubert syndrome.9 Although both variants are considered as pathogenic according to ACMG classification (Table S1), the phase remained unknown. Additional findings for both patients are available in Supporting Information S1.
Initial NGS could not determine the variants' phase. As no close relatives were available from either patient for carrier testing, patients were subjected to WGS with linked reads for phasing. All three CRB1 variants are located in a single phase block enabling variant separation into haplotypes (Figure 3C). The synonymous change c.1182C>T was traced to the same chromosome as the pathogenic variant c.3442T>C and is likely benign though it remains a VUS according to ACMG (Figure 3D,E, Table S1). The variant c.4209G>T is inherited in the other allele (Figure 3D,E, Table S1), and is now considered likely pathogenic. AHI1 region phasing positioned the c.1328T>A and c.3032C>G on different alleles (Figure 3G,H) and both are classified as pathogenic (Figure 3F).
4 DISCUSSION
We show that sequencing with linked-reads2 can be used to phase variants without parental reference. Conventional NGS had failed to complete the molecular diagnosis of two patients with a recessively inherited retinal disorder. Linked-read sequencing was able to separate identified variants into alleles perfecting the patients' molecular diagnosis. Furthermore, we report new likely pathogenic variants in CRB1 and a novel combination of AHI1 variants causing autosomal recessive retinitis pigmentosa.
The clinical presentation and genetic findings of patient 1 indicate macular or cone-rod dystrophy, a diagnosis suggestive of a macular dystrophy caused by biallelic variants in CRB1.10 Although the described variant c.4209G>T p.(Glu1403Asp) is novel, a variant c.4207G>C, p.(Glu1403Gln) in the same codon has previously been reported in patients with retinitis pigmentosa.11
Biallelic variants in AHI1, encoding the Jouberin protein, were first found to cause Joubert syndrome-3 (JBTS3, MIM# 608629),12 a ciliopathy characterized by cerebellar vermis dysplasia, hypotonia, developmental delay, and retinal dystrophy.13 Recessive variants in AHI1 are reported to cause also nonsyndromic retinitis pigmentosa.14 The retinitis pigmentosa of patient 2 phenotypically recapitulates the nonsyndromic retinitis pigmentosa caused by AHI1 variants.14 The patient has a missense variant upstream and a truncating variant downstream of the WD40 domain. It is thought that in JBTS3 variants in AHI1 abolish Jouberin function whereas variants that retain some protein function leads to nonsyndromic retinitis pigmentosa. As the missense variant p.(Val443Asp) causes Joubert syndrome when homozygous, it seems likely that the N-terminal truncating variant p.(Ser1011*) preserves enough function for other than retinal photoreceptors to survive. This is a novel combination of AHI1 variants causing retinitis pigmentosa.
It has been shown previously, and we concur here, that linked-read sequencing technologies is reliable in phasing performance.15 Additionally, no carrier testing of close relatives is needed. When the price of sequencing methods providing long-read data such as WES with linked reads, or genuine long-read methods such as PacBio16 and Oxford Nanopore Technologies17 decreases while both read-depth and accuracy increase, these methods will most likely be considered first-line for genetic testing. Accurate genetic diagnosis will become increasingly important as new targeted therapies are developed.
ACKNOWLEDGEMENTS
We thank research nurse Ms. Ilona Mikkonen for assistance. This work was supported by The Finnish Cultural Foundation.
CONFLICT OF INTEREST
E.-M.S. has served as a consultant for Blueprint Genetics, Finland, and has served in the advisory board of Novartis Finland and Bayer Finland. T.T.K. received lecture fees from Santen Finland. J.A.T received lecture fees from Thea Finland and Blueprint Genetics, and has served in the advisory board of Novartis Finland. The other authors declare no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
Data Availability The data supporting the findings of this study are available on request from the corresponding author.




