The role of long non-coding RNAs in drug resistance of cancer
He-da Zhang, Lin-hong Jiang and Shan-liang Zhong contributed equally to this study.
Funding information: Jiangsu Provincial Key Research Development Program, Grant/Award Number: BE2019731; National Key Research and Development Program of China, Grant/Award Number: 2016YFC0905900
Abstract
Long non-coding RNAs (lncRNAs), a class of long RNAs, are longer than 200 nucleotides in length but lack protein-coding capacity. LncRNAs, as critical genomic regulators, are involved in genomic imprinting regulation, histone modification and gene expression regulation as well as tumor initiation and progression. However, it is also found that lncRNAs are associated with drug resistance in several types of cancer. Drug resistance is an important reason for clinical chemotherapy failure, and the molecular mechanism of tumor resistance is complex, which is a process of multi-cause, multi-gene and multi-signal transduction pathway interaction. Then comprehending the mechanisms of chemoresistance will help find ways to control the tumor progression effectively. Therefore, in this review, we will construct lncRNAs /drug resistance interaction network and shed light on the role of lncRNAs in drug resistance.
1 INTRODUCTION
Chemoresistance, roughly divided into intrinsic or acquired resistance, is one of the main reasons for the failure of tumor therapies, and chemoresistance is also correlated with individual differences and genetic changes in tumor.1 The main drug resistance mechanisms contain increasing intracellular drug efflux or decrease drug uptake,2 reducing apoptosis due to genetic mutation,3 and enhancing cell detoxification capacity.4 However, emerging evidence has shown that non-coding RNAs are also involved in drug resistance, such as up-regulation of miR-130a enhances BCL2 expression via mediating PTEN/PI3K/AKT signaling pathway, which reduces epithelial platinum-resistant ovarian cancer cell apoptosis5; miR-222 upregulation enhances breast cell sensitivity to ADR (adriamycin) via PTEN/Akt/FOXO1 or PTEN/Akt/CDKN1B pathway.6, 7 Moreover, a new set of non-coding RNAs, lncRNAs, comes into our sight. LncRNAs are mainly transcribed by RNA polymerase II and formed through a series of processes, including splice, 5′-capping and polyadenylation.3, 8 LncRNAs are mostly present in the nucleus and divided into five types: sense, antisense, bidirectional, intergenic, and intronic lncRNAs.9, 10 Recent studies have found that lncRNAs are dysregulated in many types of cancer,11 and could mediate gene expression at epigenetic levels by interacting with RNA, protein molecules, and forming RNA-RNA, RNA-protein complexes.12 In addition, lncRNAs also serve as important epigenetic regulators to participate in diverse cellular functions, including cell proliferation, apoptosis and tumorigenicity,13 as well as drug resistance, such as lower expression of lncRNA UCA1 suppresses the expression of CREB1, BCL2 and RAB22A via up-regulating miR-204-5p, thus improving colorectal cancer cell sensitivity to 5-fluorouracil (5-FU)14; lncRNA HOTAIR activates the PI3K/AKT pathway via targeting miR-126 upregulating the expression of VEGFA and PIK3R2, and promotes the expression of MRP, thus decreasing gastric cancer cells sensitivity to cisplatin and inhibiting cell apoptosis.15 However, the detailed mechanisms of the development of chemoresistance in other tumors are not fully illuminated. Therefore, we will focus on the molecular mechanism of lncRNAs on chemoresistance by reviewing and summarizing the latest research findings, and how lncRNAs participate in relevant signaling pathways of tumor cell resistance, which may offer new therapeutic regimens for clinical treatment of drug resistance.
2 LncRNAs REGULATE DRUG SUSCEPTIBILITY IN BREAST CANCER
2.1 LncRNAs and trastuzumab resistance
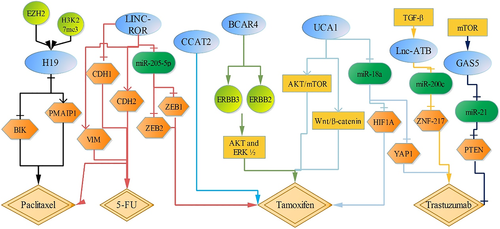
Breast cancer is the most common malignancy in women and the second leading cause of cancer death among women in the world.16, 17 However, about 15% to 20% of breast cancer patients detect HER2 overexpression or amplification, which is associated with poor prognosis and survival. Trastuzumab could actively and effectively control early stage and metastatic HER2-positive breast cancer.18 LncRNA GAS5 is downregulated in trastuzumab-resistant breast cancer tissues and cells. GAS5, upregulated through lapatinib by activating mTOR signaling pathway, functions as a sponge for miR-21, which upregulates miR-21-targeted PTEN expression, and then decreases breast cancer trastuzumab resistance and inhibits breast cancer cell proliferation.18 But lnc-ATB, activated by TGF-β, is upregulated in trastuzumab-resistant breast cancer tissues and cells. Downregulation of lnc-ATB inhibits ZNF-217 expression through setting free miR-200c, increasing the sensibility to trastuzumab and promoting cell apoptosis.19 Besides, upregulation of UCA1 inhibits miR-18a expression, which upregulates the target gene of miR-18a, Yes-associated protein 1 (YAP1), thus decreasing the sensitivity to trastuzumab in breast cancer cells.20
2.2 LncRNAs and tamoxifen resistance
However, UCA1 expression is also higher in tamoxifen-resistant breast cancer cells than in tamoxifen-sensitive cells. Estrogen receptor (ER) positive breast cancer accounted for 70%.21, 22 Tamoxifen, an antagonist of the ER, is widely used for the prevention and treatment of breast cancer with ER-positive, which remarkably increases survival of breast cancer patients.21 It shows that upregulation of UCA1 serves as a sponge for miR-18a that negatively regulates HIF1A expression, which significantly promotes tamoxifen resistance in ER-positive breast cancer cells.21 Moreover, downregulation of UCA1 also increases tamoxifen induced ER-positive breast cancer cell apoptosis by inhibiting AKT/mTOR and Wnt/β-catenin signaling pathways.23, 24 LncRNA CCAT2 is also upregulated in tamoxifen-resistant breast cancer cells, compared with tamoxifen-sensitive cells. Downregulation of CCAT2 increases the sensitivity to tamoxifen, promoting breast cancer cell apoptosis.25 Breast cancer anti-estrogen resistance 4 (BCAR4) is annotated as an lncRNA, and BCAR4 upregulation promotes tamoxifen resistance in breast cancer cells via activating ERBB2/ERBB3 signaling pathway and their downstream mediators, AKT and ERK ½.26-29 Also, LINC-ROR serves as a sponge for miR-205-5p, activating EMT signaling pathway by targeting ZEB1, and ZEB2, and then increases chemoresistance of breast cancer cells to tamoxifen.30
2.3 LncRNAs and other drugs resistance
In addition, LINC-ROR also decreases the sensibility of breast cancer cells to 5-FU and paclitaxel (PTX) by regulating EMT pathway, concomitant with decreased CDH1 expression and increased the expression of VIM and CDH2.31 H19 is upregulated in PTX-resistant ER-positive breast cancer cells. H19 suppresses the transcription of pro-apoptotic genes BIK and PMAIP1 through recruiting EZH2 and facilitating H3K27me3 modification, which enhances chemoresistance to PTX and reduces cell apoptosis.32 (Figure 1).
3 LncRNAs REGULATE DRUG SUSCEPTIBILITY IN LUNG CANCER
3.1 LncRNAs and docetaxel resistance
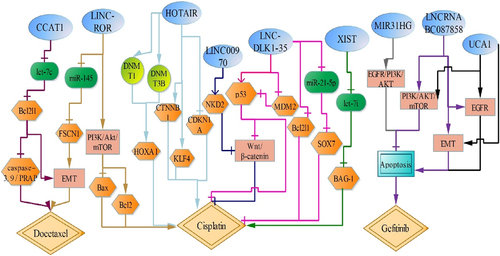
Docetaxel has been widely used in the treatment of lung adenocarcinoma (LAD). LncRNA CCAT1 is highly expressed in docetaxel-resistant lung cancer cells. CCAT1 could directly interact with its target let-7c and inhibit its expression. By contrast, let-7c significantly abolishes the effects of CCAT1 on docetaxel resistance via mediating let-7c-targeted Bcl2l1, and reverses EMT phenotype, including the expression of VIM, CDH2 decreased and the expression of CDH1 and CTNNB1 increased. Then it indicates that CCAT1 increases chemoresistance by regulating Bcl2l1 via competing with let-7c, activating EMT signaling pathway and inhibiting the activation of caspase-3, caspase-9 and PRAP, thus decreasing cell apoptosis and promoting cell proliferation.33 Besides, LINC-ROR is also upregulated in docetaxel-resistant LAD cells, compared with docetaxel-sensitive LAD cells, and LINC-ROR upregulates miR-145-targeted FSCN1 expression by sponging for miR-145, which contributes to the EMT process, concomitant with decreased CDH1 and CTNNB1 expression, and increased the expression of VIM and CDH2, thus increasing resistance to docetaxel.34 In addition, LINC-ROR knockdown also increases sensitivity of lung cancer cell to cisplatin by suppressing PI3K/Akt/mTOR signaling pathway, which increases Bax expression, but decreases Bcl2 expression, thus promoting cell apoptosis.35
3.2 LncRNAs and gefitinib resistance
Gefitinib, a specific epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), could block the signal transduction pathway of tyrosine protein kinase, promoting tumor cell apoptosis.36 The expressions of lncRNA MIR31HG, LNCRNA BC087858 and UCA1 are significantly increased in gefitinib-resistant non-small cell LAD cells. Downregulation of MIR31HG inhibits the expression of pEGFR, pAKT, pMDM2, Bcl2, but increases the expression of p53, caspase-3, caspase-9 and Bax. These results suggest that downregulation of MIR31HG decreases gefitinib resistance through the EGFR/PI3K/AKT pathway, which induces cell mitochondrial apoptosis pathway, promoting lung cancer cell apoptosis.37 Loss of LNCRNA BC087858 decreases VIM, SNAI1, Bcl2, ZEB1, pEGFR, pAKT, pERK expression, but increases the expression of caspase-3, caspase-8, caspase-9, BCL2L11, Bax, CDH1. Meanwhile, silencing UCA1 could decrease the expression of pEGFR, pAKT, pERK, pmTOR, VIM, SNAI1, CDH2, and increase CDH1 expression. Therefore, both LNCRNA BC087858 and UCA1 may contribute to gefitinib-resistance by activating the PI3K/AKT/mTOR, EGFR and EMT signaling pathways.38, 39 (Figure 2).
3.3 LncRNAs and cisplatin resistance
LncRNA AK126698 (LINC00970) (lncRNAs official name comes from LNCipedia database,40 https://lncipedia.org/) not only suppresses cell proliferation and migration by regulating Wnt/β-catenin,41 but also participates in cisplatin resistance in non-small cell lung cancer.42 LINC00970 expression is decreased in cisplatin-resistant non-small cell lung cancer cells, and LINC00970 knockdown decreases NKD2 expression that prevented the β-catenin from being flagged for degradation by combining with Dvl protein, which activates canonical Wnt/β-catenin signaling pathway and leads to reduced cisplatin-induced apoptosis in lung cancer cells.42 LncRNA MEG3 (LNC-DLK1-35), acted as a tumor suppressor gene, is downregulated in lung cancer cisplatin-resistant cells. Upregulation of LNC-DLK1-35 not only activates p53 signaling pathway through inhibiting MDM2, but also reduces Bcl2l1 expression, thus enhancing the sensibility of lung cancer cells to cisplatin.43 Furthermore, LNC-DLK1-35 also may decrease cisplatin resistance through suppressing Wnt/β-catenin signaling pathway by p53 activation, concomitant with decreased CTNNB1 and survivin.44 Additionally, LNC-DLK1-35 functions as a sponge for miR-21-5p, which positively upregulates miR-21-5p-targeted SOX7, inducing the sensitivity of non-small cell lung cancer cells to cisplatin.45
However, lncRNA XIST is highly expressed in cisplatin-resistant A549/DDP cells. Indeed, upregulation of lncRNA XIST competitively binds let-7i and inhibits its activity, which abolishes the inhibitory effect of let-7i on its target BAG-1, increasing the chemosensitivity to cisplatin and decreasing LAD cell apoptosis.46 LncRNA HOTAIR is significantly highly expressed in cisplatin-resistant LAD cells, while the expression of CDKN1A is downregulated, consistent with the results of the clinical response of patients to cisplatin-based chemotherapy.47 CDKN1A (p21), a cyclin-dependent kinase inhibitor, induces cell cycle arrest at the G1.48 And it is found that downregulation of HOTAIR decreases the cisplatin resistance of lung cancer cells by increasing CDKN1A expression.47 Besides, HOTAIR also promotes resistance to cisplatin by upregulating KLF4 and tumor stem cell related biomarkers CTNNB1 and its downstream (POU5F1, Sox2, MYC).49 Moreover, loss of HOTAIR decreases HOXA1 methylation by inhibiting the expression of DNMT1 and DNMT3B, thus increasing the sensitivity to cisplatin.50 So we speculate that HOTAIR regulates cisplatin resistance of lung cancer by combining multiple targets. (Figure 2).
4 LncRNA REGULATE DRUG SUSCEPTIBILITY IN ENDOMETRIAL CANCER
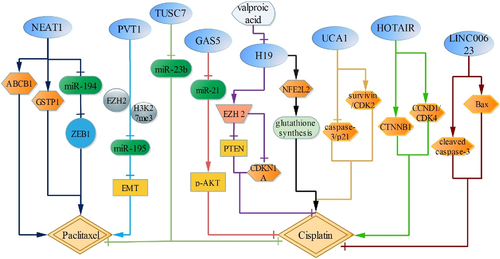
Endometrial cancer is one of the most common malignancies in gynecology. LncRNA TUSC7 is downregulated in cisplatin and PTX-resistant endometrial carcinoma tissues and cells (HEC1A/CR cells). Indeed, TUSC7 upregulation attenuates HEC1A/CR cell proliferation and increases cell apoptosis. However, miR-23b upregulation partly reverses TUSC7 inhibitory effect on HEC1A/CR cells, and it is further proved TUSC7 could bind to miR-23b directly by RNA pull-down assay. Therefore, TUSC7 enhances HEC1A/CR cells sensitivity by targeting miR-23b51 (Figure 3).
4.1 LncRNAs regulate drug susceptibility in cervical cancer
LncRNA PVT1 is correlated with PTX resistance in cervical cancer. It shows that lncRNA PVT1 directly inhibits miR-195 via promoting EZH2 binding and H3K27me3 expression in the miR-195 promoter region in cervical cancer, which reduces the inhibition of PTX-induced cell viability through modulating EMT pathway, concomitant with CDH1 decreased, FN1 and VIM increased.52
LncRNA UCA1 is highly expressed in cisplatin-resistant cervical cancer cells, and downregulation of UCA1 increases the expression of caspase-3 and CDKN1A, but reduces the expression of survivin and CDK2, which attenuates cisplatin-resistant cervical cancer cell growth and intensified cell apoptosis.53 LncRNA GAS5 upregulation decreases pAKT expression via targeting miR-21, then inhibits cisplatin-resistant cervical cancer cell proliferation after cisplatin intervention.54 (Figure 3).
5 LncRNAs REGULATE DRUG SUSCEPTIBILITY IN OVARIAN CANCER
ENST00000457645 (LINC00623) expression is lower in cisplatin-resistant ovarian cancer cells than in cisplatin-sensitive cells, up-regulation of LINC00623 intensifies the expression of Bax and cleaved caspase-3, thereby contributing to the apoptosis of cisplatin resistant cells.55 Besides, valproic acid is also connected with cisplatin resistance in ovarian cancer. It shows that valproic acid attenuates the resistance to cisplatin by inhibiting lncRNA H19, and then reduces EZH2 expression, concomitant with increased EZH2 target genes, CDKN1A and PTEN, thus increasing cell apoptosis.56 Furthermore, H19 silence also reduces the expression of NQO1, GSR, G6PD, GCLC, GCLM and GSTP1 by inhibiting NFE2L2, which decreases glutathione synthesis, thus enhancing ovarian cancer cell sensitivity to cisplatin.57 LncRNA HOTAIR is upregulated in cisplatin resistant ovarian carcinoma cells,58 and HOTAIR upregulation also increases of CTNNB1, CCND1 and CDK4 expression, which reduces the sensitivity to cisplatin. Therefore, HOTAIR may regulate cisplatin resistance by mediating Wnt/β-catenin and cell cycle arrest.59
LncRNA NEAT1 expression is higher in PTX-resistant ovarian cancer tissues and cells, and NEAT1 silence not only reduces the expression of ZEB1, the target gene of miR-194, by sponging for miR-194, but also reduces the protein levels of ABCB1 and GSTP1, that reinforces the sensitivity to PTX, thus enhancing cell apoptosis60 (Figure 3).
6 LncRNAs REGULATE DRUG SUSCEPTIBILITY IN BLADDER CANCER
6.1 LncRNAs and gemcitabine/cisplatin resistance
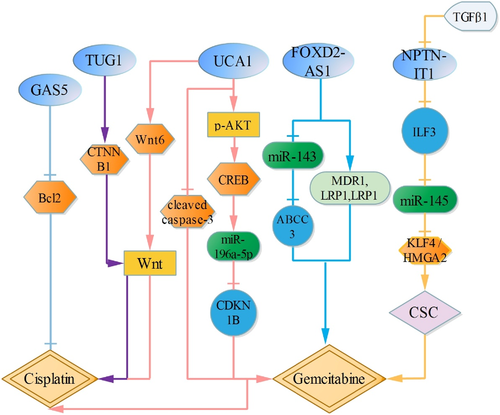
LncRNA FOXD2-AS1 is upregulated in gemcitabine resistant cells. Downregulation of FOXD2-AS1 attenuates the IC50 activity, and inhibits the expression of MDR1, MRP2 and LRP1. Furthermore, FOXD2-AS1 competes with miR-143, which reinforces miR-143-targeted gene ABCC3, thus enhancing gemcitabine resistance.61 TGFβ1 is highly expressed in bladder cancer cells treated with gemcitabine, and TGFβ1 upregulation reduces lncRNA-LET (NPTN-IT1) expression. Besides, NPTN-IT1 also attenuates the expression of the target of miR-145, ILF3, which upregulates miR-145 expression, and then inhibits cancer stem-like cells markers, ALDH1A1, CD44, KLF4 and HMGA2. Meanwhile, KLF4 and HMGA2 are also shown as target genes for miR-145. Therefore, TGFβ1 induced by gemcitabine reinforces cancer cell stemness through NPTN-IT1/ILF3/miR-145/KLF4/HMGA2, which increases gemcitabine resistance.62
Besides, bladder cancer cells treated with si-lncRNA UCA1 attenuate the IC50 activity of cells induced by gemcitabine/cisplatin, and UCA1 upregulation reduces cleaved caspase-3 expression and cell apoptosis. Furthermore, UCA1 silence inhibits the expression of p-AKT and p-CREB, and CREB has been shown to be a downstream effector of AKT signaling pathway.63 Meanwhile, miR-196a-5p is also the transcriptional target of CREB, and regulation of miR-196a-5p by UCA1 depends on the activation of CREB. Besides, miR-196a-5p upregulation increases gemcitabine/cisplatin resistance by directly targeting CDKN1B and reducing cleaved caspase-3, which reinforces cell activity and reduces cell apoptosis. Therefore, UCA1 reduces the sensitivity to gemcitabine/cisplatin by p-AKT/CREB/miR-196a-5p/CDKN1B.64 Additionally, UCA1 also increases cell resistance to cisplatin via activating Wnt signaling pathway in a Wnt6-dependent manner.65
6.2 LncRNAs and doxorubicin resistance
LncRNA TUG1 is highly expressed in doxorubicin resistant cells, and TUG1 silence reinforces the sensitivity to doxorubicin via Wnt/β-catenin pathway through downregulating CTNNB1.66 Besides, lncRNA-GAS5 upregulation enhances the sensitivity to doxorubicin by inhibiting Bcl2, which attenuates doxorubicin-induced cell IC50 activity and increases cell apoptosis.67 (Figure 4).
7 CONCLUSION AND PERSPECTIVES
LncRNAs have been recognized as oncogenes or tumor suppressors to regulate cell biological behavior, including cell proliferation, apoptosis, invasion and migration. However, drug resistance still affects the therapeutic effect, and brings resistance to clinical treatment. Although the cell drug resistance has been continuously studied, the mechanisms and pathways of drug resistance mediated by lncRNAs are still insufficient. In this article, we find that lncRNAs are frequently dysregulated in different drug resistant cells or tissues, and increase or decrease the sensitivity via targeting genes or mediating related signaling pathways (Table 1). Most important of all, comprehending drug resistant mechanisms of lncRNAs will lay a solid foundation for clinical treatment. Although our current research on the mechanism of lncRNAs on drug resistance is not complete, and may only be the tip of the iceberg, then in future studies, we need to improve the schematic diagram of lncRNAs in drug-resistance, including looking for new lncRNAs, signaling pathways or genes, and provide new therapeutic concept for tumor chemotherapy.
| LncRNAs | Regulation | Pathway/genes/proteins | Affected chemotherapy drugs | Cancer type | References |
|---|---|---|---|---|---|
| BCAR4 | Up | ERBB2/ERBB3, AKT and ERK ½ | Tamoxifen | Breast cancer | 27-29 |
| CCAT1 | Up | let-7c/Bcl2l1/EMT caspase-3/ 9, PRAP | Docetaxel | Lung cancer | 33 |
| CCAT2 | Up | Tamoxifen | Breast cancer | 25 | |
| FOXD2-AS1 | Up | MDR1, MRP2, LRP1, miR-143/ABCC3 | Gemcitabine | Bladder cancer | 61 |
| GAS5 | Down | mTOR /miR-21/PTEN | Trastuzumab | Breast cancer | 18 |
| GAS5 | Down | miR-21/pAKT | Cisplatin | Cervical cancer | 54 |
| GAS5 | Down | Bcl2 | Doxorubicin | Bladder cancer | 67 |
| H19 | Up | BIK/PMAIP1 | PTX | Breast cancer | 32 |
| H19 | Up | CDKN1A, PTEN, EZH2, glutathione synthesis | Cisplatin | Ovarian cancer | 56, 57 |
| HOTAIR | Up | CDKN1A, KLF4 DNMT1/DNMT3B | Cisplatin | Lung cancer | 47, 49, 50 |
| HOTAIR | Up | CTNNB1, CCND1, CDK4 | Cisplatin | Ovarian cancer | 58, 59 |
| LINC-ROR | Up | miR-205-5p, ZEB1, ZEB2 | Tamoxifen | Breast cancer | 30 |
| LINC-ROR | Up | EMT | 5-FU/PTX | Breast cancer | 31 |
| LINC-ROR | Up | miR-145/FSCN1, EMT | Docetaxel | Lung cancer | 34 |
| LINC-ROR | Up | PI3K/Akt/mTOR Bcl2, Bax | Cisplatin | Lung cancer | 35 |
| LINC00970 | Down | Wnt/β-catenin | Cisplatin | Lung cancer | 42 |
| Lnc-ATB | Up | miR-200c/ZNF-217 | Trastuzumab | Breast cancer | 19 |
| LNC-DLK1-35 | Down | p53/Wnt/β-catenin miR-21-5p /SOX7 | Cisplatin | Lung cancer | 43-45 |
| LncRNA BC087858 | Up | PI3K/AKT/mTOR, EGFR, EMT | Gefitinib | Lung cancer | 38 |
| LINC00623 | Down | Bax, cleaved caspase-3 | Cisplatin | Ovarian cancer | 61 |
| MIR31HG | Up | EGFR/PI3K/AKT | Gefitinib | Lung cancer | 37 |
| NEAT1 | Up | miR-194/ZEB1,P-gp, GSTP1 | PTX | Ovarian cancer | 60 |
| NPTN-IT1 | ILF3/miR-145/KLF4/ HMGA2 | Gemcitabine | Bladder cancer | 62 | |
| PVT1 | Up | miR-195/EMT | PTX | Cervical cancer | 52 |
| TUG1 | Up | Wnt/β-catenin | Doxorubicin | Bladder cancer | 66 |
| TUSC7 | Down | miR-23b | CDDP/taxol | Endometrial carcinoma | 51 |
| UCA1 | Up | miR-18a/HIF1A | Tamoxifen | Breast cancer | 21 |
| UCA1 | Up | miR-18a/YAP1 | Trastuzumab | Breast cancer | 20 |
| UCA1 | Up | PI3K/AKT/mTOR, EGFR, EMT | Gefitinib | Lung cancer | 39 |
| UCA1 | Up | caspase-3, CDKN1A, survivin, CDK2 | Cisplatin | Cervical cancer | 53 |
| UCA1 | Up | caspase-3, p-AKT/CREB/miR-196a-5p/CDKN1B, | Cisplatin/gemcitabine | Bladder cancer | 64 |
| UCA1 | Up | Wnt6 | Cisplatin | Bladder cancer | 65 |
| XIST | Up | let-7i/BAG-1 | Cisplatin | Lung cancer | 46 |
- Abbreviations: 5-FU, 5-fluorouracil; LncRNAs, long non-coding RNAs; PTX, paclitaxel.
ACKNOWLEDGEMENTS
This research was supported by the National Key Research and Development Program of China (No. 2016YFC0905900), and grants from the Jiangsu Provincial Key Research Development Program (No. BE2019731).
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data are available on request.




