Three novel patients with epileptic encephalopathy due to biallelic mutations in the PLCB1 gene
Funding information: the French Foundation for Rare Diseases
Abstract
Biallelic mutations in the PLCB1 gene, encoding for a phospholipase C beta isoform strongly expressed in the brain, have been reported to cause infantile epileptic encephalopathy in only four children to date. We report here three additional patients to delineate the phenotypic and genotypic characteristics of the disease. Our three patients were one sporadic case with an intragenic homozygous deletion and two cousins with the homozygous p.(Arg222*) nonsense variant in PLCB1. These patients had severe to profound intellectual disability, epileptic spasms at age 3-5 months concomitant with developmental arrest or regression, other seizure types and drug-resistant epilepsy. With this report, we expand the clinical, radiologic and electroencephalographic knowledge about the extremely rare PLCB1-related encephalopathy. Since the first report in 2010, the overall number of reported patients with our additional patients is currently limited to seven. All seven patients had epileptic encephalopathy, mainly infantile spasms and 6/7 had profound intellectual disability, with one only being able to walk. Truncal hypotonia was the most frequent neurological sign, sometimes associated with pyramidal and/or extrapyramidal hypertonia of limbs. Microcephaly was inconstant. In conclusion, the phenotypical spectrum of PLCB1-related encephalopathy is relatively narrow, comprises infantile spasms and severe to profound intellectual disability, and does not seem to define a recognizable clinical entity.
1 INTRODUCTION
The PLCB1 gene is located on chromosome 20p12 and encodes for a phospholipase C beta isoform strongly expressed in the brain of mammals. Its coding region is organized into 32 exons and spans more than 250 kb.1 It produces two isoforms (PLCB1a and PLCB1b) co-expressed in the human fetal and adult brain, mainly in the amygdala, caudate nuclei and hippocampi.1 The PLCB1 enzyme cleaves membrane phosphatidylinositol 4,5-biphosphate into cytosol-soluble inositol 1,4,5-triphosphate and membrane-bound diacylglycerol,2 two key intracellular transducers of extracellular signals. This metabolism involved in neurotransmission, hormonal signals and other processes related to G-proteins signaling, is essential for the central nervous system.3 This is particularly illustrated by Plcb1 knockout mice that die after early-onset recurrent seizures or status epilepticus.4
In humans, biallelic PLCB1 mutations have been reported to cause infantile epileptic encephalopathy (EE, MIM 613722) in only four patients to date5-8 (Table 1). We report here three additional patients to delineate the phenotypic and genotypic characteristics of the PLCB1-related EE (PREE).
| Present report | Previous reports | ||||||
|---|---|---|---|---|---|---|---|
| Patient ID/reference | Patient #1 | Patient #2 | Patient #3 | Kurian et al. | Poduri et al. | Ngoh et al. | Schoojans et al. |
| Gender | Male | Female | Male | Male | Male | Female | Male |
| Current age/age at description | 5 y 2 mo | 1 y 2 mo | Deceased at 8 y 7 mo | Deceased at 2.9 y | 9 mo | 3 y | 4 y |
| Molecular characteristics | |||||||
| PLCB1 gene anomaly | hmz nonsense mutation | hmz 373-kb intragenic deletion | hmz 546-kb intragenic deletion | hmz 486-kb intragenic deletion | Intragenic 476-kb deletion + splice site mutation | hmz 32-kb intragenic deletion | |
| Variants (genomic)/coordinates deletions (hg19) | chr20:g.8637900C>T | chr20:8,314,301-8,688,028 | chr20:8,034,441-8,520,723 | chr20:8,094,049-8,580,284 | c.99+1G>A/del chr20:8,094,442-8,575,333 | del chr20:8,645,677-8,683,411 | |
| cDNA level (NM_015192.3) | c.664C>T | ? | ? | ? | ? | ? | |
| Protein level | p.(Arg222*) | ? | ? | ? | ? | ? | |
| Epilepsy | |||||||
| Age of first seizure (months) | 5 | 3 | 3 | 2.5 | 6 | 10 | 4 |
| Type of the first seizures | Spasms | Spasms | Spasms | Focal sz followed by tonic stiffening and flexion of arms and legs | Perioral cyanosis, limpness, mouth automatism, eyelid fluttering | Bilateral upper limb jerking, eye deviation, staring | Febrile status epilepticus |
| Others seizure types | Focal sz, myoclonic jerks | Focal tonic and clonic sz | Nocturnal tonic clonic sz | Spasms, tonic sz, partial sz | Tonic sz | Tonic and clonic sz | Spasms, focal sz, generalized tonic–clonic sz |
| Maximum number of seizures | Multiple/day | Multiple/day | 3-4/day | Multiple/day | Up to 27/day | Multiple/day | NA |
| Status epilepti-cus? | None | None | Several | None | None | Some prolonged seizures | Yes |
| Epilepsy syndrome | Infantile spasms | Infantile spasms | Infantile spasms | Infantile spasms | MMPSI | NSEE | Infantile spasms |
| Initial development | |||||||
| Initial psycho-motor develop-menta | Normal | Normal except absence of vocalization | Delayed – unable to hold his head at 3 months | Normal but mild axial hypotonia | Delayed but progressing | Delayed | Normal |
| Regression or stagnation of skills? (age) | Yes (5 mo) |
Yes (5 mo) |
Yes (3 mo) |
Yes (8 mo) |
Yes (6 mo) |
Yes (NA) |
Yes (4 mo) |
| Clinical examination | |||||||
| Motor develop-ment (age) | No head support, unable to sit (5 y) | Head support acquired, unable to sit (20 mo) | No head support, unable to sit, unable to walk (7 y 6 mo) | No head control, unable to sit (2.5 y) | Unable to roll (6 mo) | No head support, unable to sit (3 y) | Able to walk at 3 (4 y) |
| Eye contact | Transient | Transient | NA | Transient | NA | Able to track object | Limited |
| Use of hands | None (some uncontrolled movements) | None with dystonic posturing | None | NA | Limited voluntary movements | Able to hold small objects | Palmary grasping |
| Language | Babbling | Babbling | Absent | None | None | None | None |
| Other | Swallowing difficulties | NA | Feeding difficulties since the neonatal period, squint | NA | NA | Swallowing difficulties | Autistic features, self-mutilating behavior |
| Neurological examination | Severe truncal hypotonia, dystonia of hands | Mild axial hypotonia, poor gesticulation, dystonia of left upper limb | Generalized weakness, hypertonia of limbs, hyperreflexia | Axial hypotonia, spastic quadriparesis | No eye contact, severe hypotonia, poor gesticulation | Right esotropia, rotatory nystagmus, axial and peripheral hypotonia | Mild axial hypotonia |
| Height/weight/HC in kg/cm/cm (SD) | At 6 y: 23.5 kg (+1.5 SD)/117 cm (+1 SD)/52 cm (0 SD) | At 5 m: 5.950 kg (−1 SD)/62.5 cm (0 SD)/42.5 cm (+0.3 SD) | At 7 y 6 m: 9 kg (−5 SD)/94 cm (−5 SD)/47 cm (−3.7 SD) | Second centile/NA/0.4 centile | NA | HC 75e p | −1.9 DS/−1.5 DS/−3 DS |
| Other | Thrombocytosis | Post-axial polydactyly | NA | NA | NA | NA | NA |
Brain MRI (age) |
Normal (10 and 26 mo) | Normal (5 mo) | Brain CT scan: mild atrophy (19 mo) | Normal (6 m) | Midly prominent cerebrospinal fluid spaces (6, 7, 8, 9 mo) | Supratentorial atrophy and a mildly hypoplastic corpus callosum (11 mo) | Mild generalized atrophy (3 y) |
- Abbreviations: y, year(s); m, month(s); hmz, homozygous; sz, seizure(s); MMPSI, malignant migrating partial seizures in infancy; NSEE, non-syndromic encephalopathic epilepsy; HC, head circumference.
- a According to the patient's parents.
2 PATIENTS AND METHODS
All patients were assessed clinically by at least one of the authors. Patient #3 was recruited through the EuroEPINOMICS RES consortium.
Details about the used molecular methods are available in Supporting information.
3 DESCRIPTION OF PATIENTS
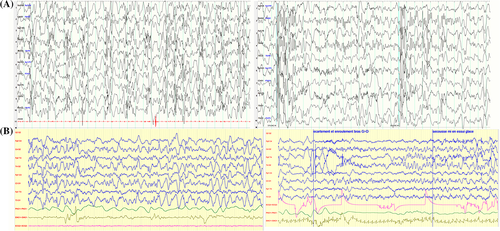
Patient #1 is a 5-year-old boy born full term after normal pregnancy and delivery with normal parameters (birth weight 3495 g, length 49 cm, head circumference [HC] 37 cm, Apgar score 10/10). His parents were healthy, consanguineous individuals from Mauritania. They had a daughter who died of dehydration in Africa at 2 years old. This girl had severe epilepsy and psychomotor delay like Patient #1 (Figure S1). Patient #1 had a normal development during his first months of life according to his parents. At 5 months, they noticed a loss of acquisitions and paroxysmal events proven to be epileptic spasms. When examined, the child had poor eye contact, did not smile and could not hold his head. His EEG displayed hypsarrhythmia (Figure 1A), which confirmed the diagnosis of West syndrome. Spasms presented in cluster of 30 symmetric flexor spasms involving axial muscles and limbs. They were resistant to several antiepileptic drugs (Table S1). During the first year, other types of seizures appeared, including focal tonic seizures and myoclonic jerks. At 3 years old, Patient #1 had severe hypotonia, was unable to hold his head or sit, had no eye contact, very limited vocalization and poorly responded to stimuli. He underwent gastrostomy because of choking episodes. At 4 years old, the epilepsy was stable under valproate acid, clonazepam and topiramate (2-3 short seizures per week). At 5 years old, clinical examination was unchanged, except for mild dystonia of hands, HC was 51.5 cm (+0.2 SD), weight 24.5 kg (+3 SD). He had occasional seizures only triggered by fever.
Brain MRIs at 6, 10 and 26 months were normal (Figure S2). Blood cell count revealed a chronic thrombocytosis of unknown origin. Laboratory investigations for inborn metabolic diseases and lymphocytic karyotype were normal. SNP microarray analysis (Illumina) showed no copy number variant.
Patient #2 is the female cousin of Patient #1 (Figure S1) aged 14 months. She was born full term after normal pregnancy and delivery with normal birth weight. Her parents were healthy and consanguineous. Patient #2 had a normal development during her first months of life. She could smile, hold her head and tried to grab objects at 3 months but did not vocalize. She had unilateral post-axial polydactyly but no other congenital anomaly. At 3 months, she started to lose acquired skills and had spasms and other seizures characterized by activity arrest, apnea and movements of four limbs. At 5 months epilepsy was diagnosed. At examination, she had no eye contact and marked axial hypotonia. EEG showed a slow background activity with multifocal spikes, without physiological features, but no hypsarrhythmia (Figure 1B). Her epilepsy was resistant to multiple treatments (Table S1). Corticosteroids and ketogenic diet showed partial efficiency. Epileptic spasms and partial seizure gradually disappeared and EEG progressively improved with time (Table S1). Clinical examination at 20 months showed a girl with transient eye contact, severe truncal hypotonia, unable to sit or grab objects, with intermittent dystonia of left upper limb. Her weight, height and HC were normal. Brain MRI performed at 5.5 months was normal (Figure S2).
Patient #3 was an Egyptian boy of 7.5 years old. He was the third child from healthy consanguineous parents and born full term after a normal pregnancy with normal birth weight (Figure S3). At the age of 3 months, he presented clusters of epileptic spasms with extension of the limbs and axial contraction which persisted few minutes. At that time, he was not able to hold his head. He first attended the neurology clinic in Cairo at the age of 19 months. At examination, he showed hypertonia of limbs, hyperreflexia more prominent on the left side and a squint of his left eye. His height was −2.7 SD and HC −2.3 SD below the mean. EEG performed at 21 months showed left temporal spike-waves. Metabolic screening was normal and brain CT showed global atrophy. During the following years, Patient #3 had nocturnal tonic clonic seizures with loss of consciousness for few minutes occurring 3-4 times per night. He also had several status epilepticus usually triggered by fever and controlled with intravenous phenytoin. EEG performed at 4 years old showed a background of 4-5 Hz theta activity, sleep spindles and a focus of right occipital slow spikes-waves. At 7.5 years old, Patient #3 displayed profound intellectual disability (ID), absent head support and absent speech. HC was 47 cm (−3.7 SD), height 94 cm (−5 SD), weight 9 kg (−5 SD). He still had daily seizures, consisting of flexion spasms despite trials of several antiepileptic treatments. EEG showed focal right parietal and temporal epileptogenic activity. Patient #3 died of pneumonia at 8 years 7 months.
4 MOLECULAR RESULTS
We identified the homozygous nonsense variant Chr20(GRCh37):g.8637900C>T, NM_015192.3:c.664C>T, p.(Arg222*) in PLCB1 in Patients #1 and #2. This variant was absent in gnomAD database, confirmed by Sanger sequencing, as was the heterozygous status of the patients' parents.
Array-CGH performed in Patient #3 revealed a homozygous 373-kb deletion encompassing exons 3 to 11 of the PLCB1 gene (hg19 coordinates chr20:8,314,301-8,688,028).
5 DISCUSSION
In all seven patients with PREE reported to date, seizures began during the first year of life (mean 4.8 months, range 2.5-10 months) and were resistant to multiple antiepileptic drugs (Table 1). All four previously reported patients with PREE had severe epilepsy of infancy: one had malignant migrating partial seizures in infancy,7 two had epileptic spasms or West syndrome5, 8 and one had an non-syndromic EE6 (Table 1 and Table S1). Our three patients had epileptic spasms or West syndrome as the main and first epileptic presentation. Overall, spasms seem to be the most frequent seizure type observed in PREE. Other seizure types were noticed in 6/7 patients in the disease course, such as clonic and tonic focal seizures, generalized tonic-clonic seizures, myoclonic jerks. Seizures occurred several times per day in all patients (when known). EEGs showed severe alterations of the background activity associated with multifocal spikes in all seven patients, which is a hallmark of hypsarrhythmia. Three patients had seizures arising from temporal lobes.
Fever was a trigger of seizures in two patients only. Prolonged seizures and status epilepticus were also reported (Patients #1 and #3).
Developmental regression was noticed in 5/7 patients after epilepsy onset. Patients #1 and #2 were said to have normal development before the start of seizures, like those reported by Kurian et al5 and Schoonjans et al8 All patients had severe to profound ID and one patient only was able to walk, but he had severe autism. Our three patients were 20 months to 7.5 years old at last examination. Patient #2 had acquired head control, began to vocalize but was unable to sit; Patients #1 and #3 were also unable to sit, to use their hands and had no language and poor or absent eye contact. All had axial hypotonia. Hand dystonia was noticed in two of them. This description is in line with that of 3/4 patients of the literature, except that limb hypertonia was mostly attributed to spastic paresis rather than extrapyramidal involvement.5-7 Two previously reported patients had microcephaly,5, 8 like Patient #3, but not Patients #1 and #2 reported here. Thus, microcephaly is a possible and inconstant feature of PREE (3/6 patients). It may be associated with brain atrophy (2/3 microcephalic patients). Overall, the phenotype of PREE is not conspicuously distinctive when compared with other early-onset EE.
Mutations responsible for EE are mostly de novo heterozygous variants involving about one hundred genes to date.9 Recessive mutations, although less frequent, have also been reported in specific genes, including ALDH7A1, AP3B2, BRAT1, CAD, FRRS1L, PIGG, PNPO, SLC12A5, SLC25A22, ST3GAL3, ST3GAL5, SZT2, UBA5, WWOX. WOREE, the phenotype due to biallelic mutations in WWOX10 is the most frequent of these recessive disorders in our diagnostic laboratory (Groupe Hospitalier Pitié-Salpêtrière). Mutations in PLCB1 seems to be extremely rare, since four patients have been described to date,5-8 which makes only seven patients including ours over a period of eight years. Pathogenic PLCB1 variants reported to date are five intragenic deletions (homozygous in four cases), one splice-altering variant and one homozygous nonsense variant (Patients #1 and #2), thus the residual enzyme activity related to this combination of alleles is probably null. Notably, there is currently no phenotype ascribed to biallelic hypomorphic variants in PLCB1. Heterozygous individuals with one null PLCB1 allele (the patient's parents) are apparently healthy and fertile. This suggests that the small number of patients with PREE is not related to a detrimental effect of the heterozygous status. The small number of patients with PREE in comparison to WWOX is rather due to intrinsic characteristics of these genes because the o/e (observed/expected) ratios of missense and loss-of-function variants reported in gnomAD is 0.57 (374/658, Z = 4.02) and 0.19 (13/68.5, pLI = 0.97), respectively, for PLCB1 and 1.8 (434/241, Z = -4.5) and 1.11 (22/19.8, pLI = 0), respectively, for WWOX. These data suggest that the PLCB1 gene sequence is less prone to base substitution than WWOX, which probably explains the scarcity of patients with PREE.
ACKNOWLEDGEMENTS
This work was supported by the French Foundation for Rare Diseases (C.N., C.M.).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, upon reasonable request.