Clinical aspects of hereditary spastic paraplegia 76 and novel CAPN1 mutations
Mutations in CAPN1 may lead to pure or complicated autosomal recessive (AR) hereditary spastic paraplegia (HSP), classified as spastic paraplegia 76 (SPG76, OMIM # 616907). In the past 2 years, several groups have identified SPG76 patients.1-6 Herein, we report eight additional cases from four Brazilian unrelated families and reviewed all clinical features of published reports on this condition.
1 FAMILY A
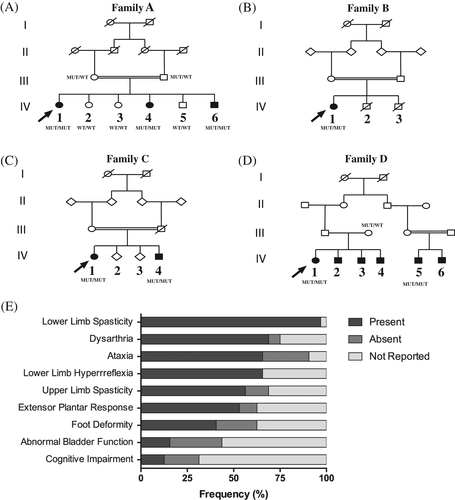
Three affected individuals born from a first-degree consanguineous marriage (Figure 1A) were diagnosed with pure HSP. Clinical and neurological evaluation showed that all three patients presented progressive lower limb spasticity and dysarthria, and two of them exhibited mild-ataxia as well. Peripheral blood (5 mL) from patients and family unaffected relatives was collected for DNA extraction. All individuals that had blood samples collected for DNA extraction in this work signed informed consent approved by the Human Ethics Committee from the Biosciences Institute, University of São Paulo (CAAE: 60861316.0.0000.5464). Whole exome sequencing (WES) in one affected individual revealed a stop gain mutation in homozygosis in CAPN1 (NM_001198868, c.1176G>A; p.Trp392*). All affected siblings carry the causative mutation in homozygosis, whereas none of the unaffected relatives has this same genotype. This variant that have been previously reported in Turkish patients5 was not present in population databases (ExAC, GnomAD and ABraOM).
2 FAMILY B
A 37-year-old affected woman (Figure 1B) reported onset at age 22 with frequent falls and spastic gait. Neurological evaluation disclosed lower limb spasticity and hyperreflexia, without ataxia. WES detected the same c.1176G>A (p.Trp392*) homozygote mutation seen in Family A.
3 FAMILY C
Two siblings, a 46-year-old woman with disease onset at age of 20, and her 51-year-old brother (Figure 1C), with symptoms starting at age of 35, had spastic gait and mild ataxia. WES in the proband detected a novel LoF mutation in CAPN1 (NM_001198868, c.675C>A; p.Tyr225*) in homozygous state. This mutation was also present in both alleles of the affected brother and was not present in the population databases.
4 FAMILY D
A 42-year-old female (Figure 1D) was ascertained presenting lower limb spasticity and mild-ataxia symptoms, starting at age of 30. WES identified compound heterozygous LoF mutations: a previously described c.1176G>A (p.Trp392*) and a novel pathogenic variant (NM_001198868, c.618_619delAG; p.Gly208Glnfs*7). The c.618_619delAG variant was present in heterozygosity in the ExAC database with low frequency (0.005%). Her mother was tested for both mutations, and she is heterozygote for the c.1176G>A (p.Trp392*) mutation. Also, her affected cousin (IV-5) was ascertained presenting lower limb spasticity and ataxia at age 38, and he was tested for both mutations. We detected the c.1176G>A (p.Trp392*) mutation in homozygosis in this patient.
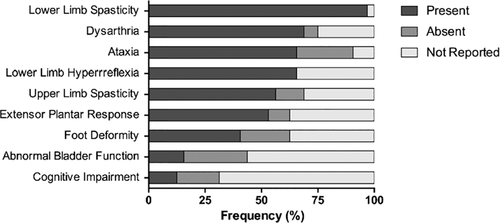
Here we describe eight additional SPG76 patients and compare them with 24 recently reported cases in the literature.1-6 Disease onset occurred on average at 25 years of age (±9.1) and ~72% of affected individuals were females. The most frequently observed clinical symptoms were lower limb spasticity (96.9%), dysarthria (68.8%) and ataxia (65.6%) (Figure 1E). Half of the patients had upper limb spasticity and extensor plantar response. Cognitive decline was observed in 12.5% of the reported cases; in 18.8%, this feature was absent and in 68.7%, this information was not reported. Our data support that SPG76 is characterized mainly by lower-limb spasticity, ataxia and dysarthria. Upper-limb spasticity was observed in half of patients and should be examined in order to better characterize clinically candidate patients for SPG76. In short, we reinforce the importance of screening the CAPN1 gene using next-generation sequencing in individuals with AR-HSP.
ACKNOWLEDGEMENTS
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo/Centro de Pesquisa, Inovação e Difusão and National Council of Technological and Scientific Development.
Editorial Process File
The Editorial Process File is available in the online version of this article.