Neutrophil extracellular traps can activate alternative complement pathways
H. Wang
Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China
Institute of Nephrology, Peking University, Beijing, China
Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China
Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China
Peking-Tsinghua Center for Life Sciences, Beijing, China
Search for more papers by this authorC. Wang
Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China
Institute of Nephrology, Peking University, Beijing, China
Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China
Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China
Peking-Tsinghua Center for Life Sciences, Beijing, China
Search for more papers by this authorM.-H. Zhao
Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China
Institute of Nephrology, Peking University, Beijing, China
Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China
Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China
Peking-Tsinghua Center for Life Sciences, Beijing, China
Search for more papers by this authorCorresponding Author
M. Chen
Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China
Institute of Nephrology, Peking University, Beijing, China
Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China
Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China
Peking-Tsinghua Center for Life Sciences, Beijing, China
Correspondence: M. Chen, Renal Division, Department of Medicine, Peking University First Hospital, Beijing 100034, China. E-mail: [email protected]Search for more papers by this authorH. Wang
Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China
Institute of Nephrology, Peking University, Beijing, China
Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China
Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China
Peking-Tsinghua Center for Life Sciences, Beijing, China
Search for more papers by this authorC. Wang
Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China
Institute of Nephrology, Peking University, Beijing, China
Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China
Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China
Peking-Tsinghua Center for Life Sciences, Beijing, China
Search for more papers by this authorM.-H. Zhao
Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China
Institute of Nephrology, Peking University, Beijing, China
Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China
Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China
Peking-Tsinghua Center for Life Sciences, Beijing, China
Search for more papers by this authorCorresponding Author
M. Chen
Renal Division, Department of Medicine, Peking University First Hospital, Beijing, China
Institute of Nephrology, Peking University, Beijing, China
Key Laboratory of Renal Disease, Ministry of Health of China, Beijing, China
Key Laboratory of Chronic Kidney Disease Prevention and Treatment (Peking University), Ministry of Education, Beijing, China
Peking-Tsinghua Center for Life Sciences, Beijing, China
Correspondence: M. Chen, Renal Division, Department of Medicine, Peking University First Hospital, Beijing 100034, China. E-mail: [email protected]Search for more papers by this authorSummary
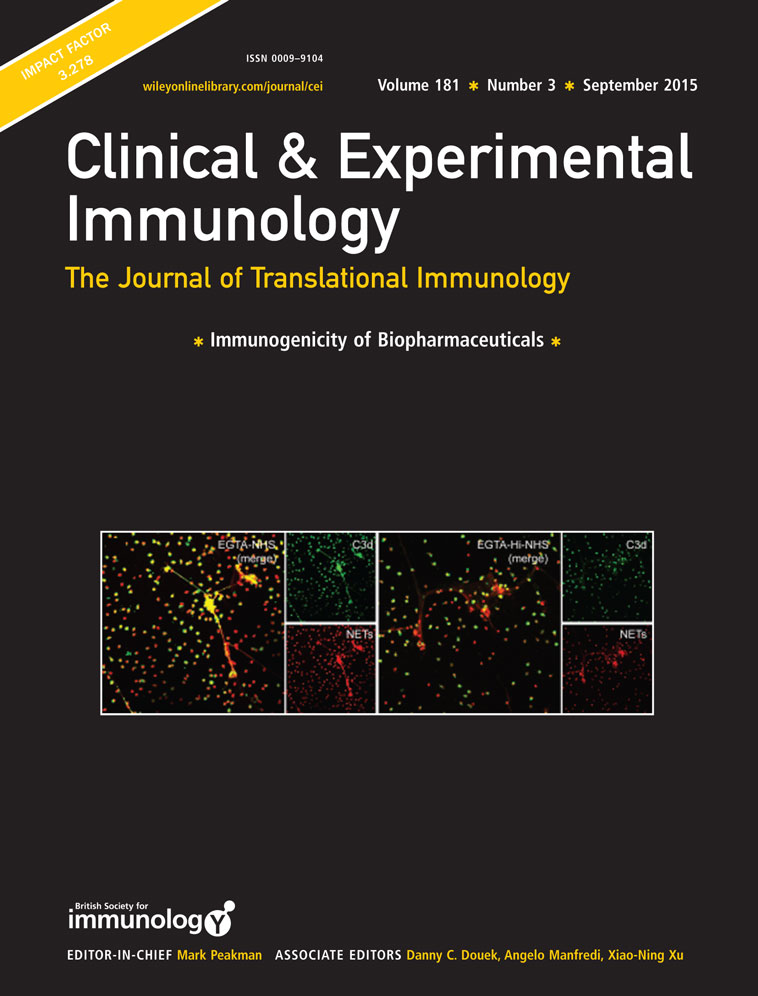
The interaction between neutrophils and activation of alternative complement pathway plays a pivotal role in the pathogenesis of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV). ANCAs activate primed neutrophils to release neutrophil extracellular traps (NETs), which have recently gathered increasing attention in the development of AAV. The relationship between NETs and alternative complement pathway has not been elucidated. The current study aimed to investigate the relationship between NETs and alternative complement pathway. Detection of components of alternative complement pathway on NETs in vitro was assessed by immunostain and confocal microscopy. Complement deposition on NETs were detected after incubation with magnesium salt ethyleneglycol tetraacetic acid (Mg-EGTA)-treated human serum. After incubation of serum with supernatants enriched in ANCA-induced NETs, levels of complement components in supernatants were measured by enzyme-linked immunosorbent assay (ELISA). Complement factor B (Bb) and properdin deposited on NETs in vitro. The deposition of C3b and C5b-9 on NETs incubated with heat-inactivated normal human serum (Hi-NHS) or EGTA-treated Hi-NHS (Mg-EGTA-Hi-NHS) were significantly less than that on NETs incubated with NHS or EGTA-treated NHS (Mg-EGTA-NHS). NETs induced by ANCA could activate the alternative complement cascade in the serum. In the presence of EGTA, C3a, C5a and SC5b-9 concentration decreased from 800·42 ± 244·81 ng/ml, 7·68 ± 1·50 ng/ml, 382·15 ± 159·75 ng/ml in the supernatants enriched in ANCA induced NETs to 479·07 ± 156·2 ng/ml, 4·86 ± 1·26 ng/ml, 212·65 ± 44·40 ng/ml in the supernatants of DNase I-degraded NETs (P < 0·001, P = 0·008, P < 0·001, respectively). NETs could activate the alternative complement pathway, and might thus participate in the pathogenesis of AAV.
Supporting Information
| Filename | Description |
|---|---|
| cei12654-sup-0001-suppinfo01.docx21.4 KB |
Supporting information |
| cei12654-sup-0002-suppinfo02.tif864.7 KB |
Figure S1. Neutrophil extracellular trap (NET) induced by anti-neutrophil cytoplasmic antibody (ANCA). Neutrophils were primed with tumour necrosis factor (TNF)-α and incubated with 250 µg/ml ANCA-positive immunoglobulin (Ig)G or normal human IgG. We also stimulated neutrophils with ANCA-positive IgG alone without TNF-α priming. Phorbol myristate acetate (PMA)-activated neutrophils were used as the positive control. (a) After 180 min of incubation, the DNA release was visualized by fluorescence microscopy of 4',6-diamidino-2-phenylindole (DAPI)-stained specimens. A representative example of three independent experiments is shown. Magnification ×200. (b) Quantitative assessment of the percentage of NETs-forming cells revealed robust NET formation after ANCA activation. After ANCA activation, 24·26 ± 5·62% of neutrophils produced NETs, compared to 8·90 ± 2·78% of normal IgG-treated neutrophils, 13·51 ± 3·50% neutrophils treated with ANCA-positive IgG alone without TNF-α priming. Incubation with PMA, known as a strong inducer of NETs, triggered NETs production in 40·91 ± 6·50% of all neutrophils. |
| cei12654-sup-0003-suppinfo03.tif1.1 MB |
Figure S2. Isolated anti-neutrophil cytoplasmic antibody (ANCA)-positive immunoglobulin (Ig)G could bind to neutrophil extracellular trap (NETs). Neutrophils were stimulated with 50 nM PMA for 3 h at 37°C. After fixation with 4% paraformaldehyde (PFA), the samples were washed with phosphate-buffered saline (PBS) and then incubated with 250 µg/ml isolated ANCA-positive immunoglobulin (Ig)G (a) or normal human control IgG (b) for 1 h at 37°C. After washing with phosphate-buffered saline (PBS), AF488-conjugated anti-human IgG antibodies (1 : 500 dilution; Jackson Immuno Research Laboratories) were applied for 1 h at 37°C. Isolated ANCA-positive IgG bound to the NETs was seen. Representative figures are shown. Magnification ×200. |
| cei12654-sup-0004-suppinfo04.tif7.7 MB |
Figure S3. Immunofluorescence identifying neutrophil extracellular traps (NETs) induced by anti-neutrophil cytoplasmic antibodies (ANCA). Neutrophils were primed with tumour necrosis factor (TNF)-α and incubated with 250 µg/ml ANCA-positive-IgG. NETs induced by ANCA were identified by co-localization of DNA (blue), histone (red) and MPO (green). A representative example of three independent experiments is shown. Magnification ×400. |
| cei12654-sup-0005-suppinfo05.tif2.1 MB |
Figure S4. Detection of deposition of Bb and properdin on neutrophil extracellular trap (NETs) induced by phorbol myristate acetate (PMA) or lipopolysaccharide (LPS) in vitro. Neutrophils isolated from healthy donors were stimulated with 50 nM PMA or 100ng/ml LPS. NETs were identified by co-localization of DNA (blue) and elastase (green). Deposition of Bb and properdin (red) on NETs was assessed by confocal microscopy. Magnification ×400. (a) Deposition of Bb (red) on NETs induced by PMA stained by DNA (blue) and elastase (green). (b) Deposition of Bb (red) on NETs induced by LPS stained by DNA (blue) and elastase (green). (c) Isotype control for staining antibody was perfomed. Primary antibodies were replaced by normal rabbit immunoglobulin (Ig)G and mouse IgG2a. (d) Deposition of properdin (red) on NETs induced by PMA stained by DNA (blue) and elastase (green). (e) Deposition of properdin (red) on NETs induced by LPS stained by DNA (blue) and elastase (green). (f) Isotype control for staining antibody was performed. Primary antibodies were replaced by normal rabbit IgG and mouse IgG2a. |
| cei12654-sup-0006-suppinfo06.tif246.6 KB |
Figure S5. Immunoblotting of complements on neutrophil extracellular trap (NETs). Human neutrophils were left untreated, treated with anti-neutrophil cytoplasmic antibody (ANCA)-positive immunoglobulin (Ig)G after tumour necrosis factor (TNF)-α priming. Supernatants were collected, and Bb, properdin were examined by Western blotting. Supernatants enriched in NETs and degraded NETs (treated with commercial DNase I) were incubated by normal human serum in the presence of ethyleneglycol teraacetic acid (EGTA), C5b and C3d were examined by Western blotting. Primary antibodies were used as below: mouse anti-human Bb antibody (Quidel), rabbit anti-human properdin antibody (Abcam), rabbit anti-human C5b antibody (Abcam) and rabbit anti-human C3d anibody (Abcam). (a) Detection of Bb on NETs by immunoblotting. (b) Detection of properdin on NETs by immunoblotting. (c) Detection of C5b on NETs incubated with serum in the presence of EGTA by immunoblotting. (d) Detection of C3d on NETs incubated with serum in the presence of EGTA by immunoblotting. |
| cei12654-sup-0007-suppinfo07.tif1.2 MB |
Figure S6. Immunofluorescence detection for C4d on neutrophil extracellular trap (NETs). Neutrophils were primed with TNF-α and incubated with 250µg/ml anti-neutrophil cytoplasmic antibody (ANCA)-positive immunoglobulin (Ig)G. The induced NETs were then incubated with normal human serum (NHS) at the presence of ethyleneglycol teraacetic acid (EGTA) or not for 40 min at 37°C. After incubation with serum, coverslips with NETs were washed, and C4d was detected using rabbit anti-human C4d polyclonal antibodies (1 : 100 dilution; Abcam) as primary antibodies with secondary antibodies AF488-labelled donkey anti-rabbit IgG (1 : 500 dilution, Jackson ImmunoResearch Laboratories). NETs were visualized using 4',6-diamidino-2-phenylindole (DAPI) (blue). (a) C4d deposited on NETs during incubation with normal serum. (b) Little or no deposition of C4d on NETs during incubation with normal serum at the presence of EGTA could be observed. Representative images of three independent experiments are shown. Magnification ×200. |
| cei12654-sup-0008-suppinfo08.tif516.9 KB |
Figure S7. Quantification of mean fluorescence intensity of C3b staining on neutrophil extracellular trap (NETs) after incubation with serum. The mean fluorescence intensity (MFI) of C3b staining on NETs incubated with normal human serum (NHS), heat inactivated (Hi)-NHS (lack of active complements), magnesium salt-ethyleneglycol teraacetic acid (Mg-EGTA)-treated NHS, Mg-EGTA-treated Hi-NHS (lack of active complements). Data are presented as mean of three independent experiments ± standard deviation (s.d.). The MFI of C3b on NETs incubated with NHS was 49·74 ± 8·36, while the MFI of C3b on NETs incubated with Hi-NHS was 11·95 ± 2·85 (P<0·001). The MFI of C3b on NETs incubated with Mg-EGTA-treated NHS was 30·96 ± 5·37, while the MFI of C3b on NETs incubated with Mg-EGTA-treated Hi-NHS was 7·14 ± 1·40 (P<0·001). |
| cei12654-sup-0009-suppinfo09.tif1.2 MB |
Figure S8. Neutrophil extracellular trap (NETs) induced by phorbol myristate acetate (PMA) or lipopolysaccharide (LPS) could activate the alternative complement cascade in the serum. Neutrophils were stimulated by 50 nM PMA or 100 ng/ml LPS. Bars represent mean ± standard deviation (s.d.). Each measured on neutrophils of five independent experiments and donors. Supernatants from the cell suspensions were incubated for 40 min with normal serum or magnesium salt-ethyleneglycol teraacetic acid (Mg-EGTA)-treated normal serum, and C3a, C5a, SC5b-9 generation was measured by specific enzyme-linked immunosorbent assay (ELISA). *P < 0·05, **P < 0·01, ***P < 0·001. |
| cei12654-sup-0010-suppinfo10.tif434 KB |
Figure S9. Quantification of the generation of C5a in serum after specific inhibition of tissue factor or thrombin. Neutrophils were incubated with anti-TF antibody (10 μg/ml) for 30 min in 37°C or 1 μM D-Phe-Pro-Arg-choro-methylketone dihydrochloride (PPACK) for 40 min at room temperature, and were then stimulated by ANCA-positive immunoglobulin (Ig)G after tumour necrosis factor (TNF)-α-priming or 50 nM phorbol myristate acetate (PMA). Bars represent mean ± standard deviation (s.d.). Each measured on neutrophils of four independent experiments and donors. Supernatants from the cell suspensions were incubated for 40 min with normal serum or magnesium salt-ethyleneglycol teraacetic acid (Mg-EGTA)-treated normal serum, and C5a generation was measured by specific. D-Phe-Pro-Arg-choro-methylketone dihydrochloride (PPACK) was used to inactivate thrombin. Anti-TF antibody (murine monoclonal antibody against human tissue factor) was used to inhibit the pro-coagulant activity of tissue factor; n.s. no significant difference. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Jennette JC, Falk RJ, Bacon PA et al. 2012 revised International Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum 2013; 65: 1–11.
- 2 Chen M, Yu F, Zhang Y, Zhao MH. Antineutrophil cytoplasmic autoantibody-associated vasculitis in older patients. Medicine (Baltimore) 2008; 87: 203–9.
- 3 Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA 1990; 87: 4115−19.
- 4 Kessenbrock K, Krumbholz M, Schonermarck U et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 2009; 15: 623–5.
- 5 Lande R, Gregorio J, Facchinetti V et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007; 449: 564–9.
- 6 Sangaletti S, Tripodo C, Chiodoni C et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood 2012; 120: 3007–18.
- 7 Xing GQ, Chen M, Liu G, Zheng X, E J, Zhao MH. Differential deposition of C4d and MBL in glomeruli of patients with ANCA-negative pauci-immune crescentic glomerulonephritis. J Clin Immunol 2010; 30: 144–56.
- 8 Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol 2007; 170: 52–64.
- 9 Gou SJ, Yuan J, Chen M, Yu F, Zhao MH. Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int 2013; 83: 129–37.
- 10 Xing GQ, Chen M, Liu G et al. Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. J Clin Immunol 2009; 29: 282–91.
- 11 Huugen D, van Esch A, Xiao H et al. Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int 2007; 71: 646–54.
- 12 Leffler J, Martin M, Gullstrand B et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol 2012; 188: 3522–31.
- 13 Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun 2010; 34: J276–86.
- 14 Van Timmeren MM, Chen M, Heeringa P. Review article: pathogenic role of complement activation in anti-neutrophil cytoplasmic auto-antibody-associated vasculitis. Nephrology (Carlton) 2009; 14: 16–25.
- 15 Schreiber A, Rolle S, Peripelittchenko L et al. Phosphoinositol 3-kinase-gamma mediates antineutrophil cytoplasmic autoantibody-induced glomerulonephritis. Kidney Int 2010; 77: 118–28.
- 16 Choi M, Rolle S, Rane M, Haller H, Luft FC, Kettritz R. Extracellular signal-regulated kinase inhibition by statins inhibits neutrophil activation by ANCA. Kidney Int 2003; 63: 96–106.
- 17 Aga E, Katschinski DM, van Zandbergen G et al. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J Immunol 2002; 169: 898–905.
- 18 Jiang H, Wang Z, Serra D, Frank MM, Amalfitano A. Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-ad antibodies. Mol Ther 2004; 10: 1140–2.
- 19 Nakao M, Miura C, Itoh S et al. A complement C3 fragment equivalent to mammalian C3d from the common carp (Cyprinus carpio): generation in serum after activation of the alternative pathway and detection of its receptor on the lymphocyte surface. Fish Shellfish Immunol 2004; 16: 139–49.
- 20 Nakazawa D, Tomaru U, Suzuki A et al. Abnormal conformation and impaired degradation of propylthiouracil-induced neutrophil extracellular traps: implications of disordered neutrophil extracellular traps in a rat model of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2012; 64: 3779–87.
- 21 Saffarzadeh M, Juenemann C, Queisser MA et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLOS ONE 2012; 7: e32366.
- 22 Mershon-Shier KL, Vasuthasawat A, Takahashi K, Morrison SL, Beenhouwer DO. In vitro C3 deposition on Cryptococcus capsule occurs via multiple complement activation pathways. Mol Immunol 2011; 48: 2009–18.
- 23 Sahu A, Lambris JD. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol Rev 2001; 180: 35–48.
- 24 Nakazawa D, Shida H, Tomaru U et al. Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis. J Am Soc Nephrol 2014; 25: 990–7.
- 25 Nakazawa D, Tomaru U, Suzuki A et al. Abnormal conformation and impaired degradation of propylthiouracil-induced neutrophil extracellular traps: implications of disordered neutrophil extracellular traps in a rat model of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2012; 64: 3779–87.
- 26 Camous L, Roumenina L, Bigot S et al. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood 2011; 117: 1340–9.
- 27 Wirthmueller U, Dewald B, Thelen M et al. Properdin, a positive regulator of complement activation, is released from secondary granules of stimulated peripheral blood neutrophils. J Immunol 1997; 158: 4444–51.
- 28 Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol 2009; 20: 289–98.
- 29 Huber-Lang M, Younkin EM, Sarma JV et al. Generation of C5a by phagocytic cells. Am J Pathol 2002; 161: 1849–59.
- 30 Chen M, Xing GQ, Yu F, Liu G, Zhao MH. Complement deposition in renal histopathology of patients with ANCA-associated pauci-immune glomerulonephritis. Nephrol Dial Transplant 2009; 24: 1247–52.
- 31 Hao J, Meng LQ, Xu PC, Chen M, Zhao MH. p38MAPK, ERK and PI3K signaling pathways are involved in C5a-primed neutrophils for ANCA-mediated activation. PLOS ONE 2012; 7: e38317.
- 32 Kambas K, Chrysanthopoulou A, Vassilopoulos D et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis 2014; 73: 1854–63.
- 33 Huber-Lang M, Sarma JV, Zetoune FS et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med 2006; 12: 682–7.
- 34 Krisinger MJ, Goebeler V, Lu Z et al. Thrombin generates previously unidentified C5 products that support the terminal complement activation pathway. Blood 2012; 120: 1717–25.
- 35 O'Flynn J, Dixon KO, Faber Krol MC, Daha MR, van Kooten C. Myeloperoxidase directs properdin-mediated complement activation. J Innate Immun 2014; 6: 417–25.