Allergic diseases as risk factors for Long-COVID symptoms: Systematic review of prospective cohort studies
Doreen Wolff, Karl Philipp Drewitz and Angela Ulrich contributed equally to this study.
Abstract
Objective
The role of allergy as a risk factor for Long-COVID (LC) is unclear and has not been thoroughly examined yet. We aimed to systematically review and appraise the epidemiological evidence on allergic diseases as risk factors for LC.
Design
This is an initial systematic review. Two reviewers independently performed the study selection and data extraction using Covidence. Risk of bias (RoB) and certainty of evidence (GRADE) were assessed. Random effects meta-analyses were used to pool unadjusted ORs within homogeneous data subsets.
Data Sources
We retrieved articles published between January 1st, 2020 and January 19th, 2023 from MEDLINE via PubMed, Scopus, the WHO-COVID-19 database and the LOVE platform (Epistemonikos Foundation). In addition, citations and reference lists were searched.
Eligibility Criteria
We included prospective cohort studies recruiting individuals of all ages with confirmed SARS-CoV-2 infection that were followed up for at least 12 months for LC symptoms where information on pre-existing allergic diseases was available. We excluded all study designs that were not prospective cohort studies and all publication types that were not original articles.
Results
We identified 13 studies (9967 participants, range 39–1950 per study), all assessed as high RoB, due to population selection and methods used to ascertain the exposures and the outcome. Four studies did not provide sufficient data to calculate Odds Ratios. The evidence supported a possible relationship between LC and allergy, but was very uncertain. For example, pre-existing asthma measured in hospital-based populations (6 studies, 4019 participants) may be associated with increased risk of LC (Odds Ratio 1.94, 95% CI 1.08, 3.50) and findings were similar for pre-existing rhinitis (3 studies, 1141 participants; Odds Ratio 1.96, 95% CI 1.61, 2.39), both very low certainty evidence.
Conclusions
Pre-existing asthma or rhinitis may increase the risk of LC.
Graphical Abstract
We systematically reviewed and appraised the epidemiological evidence on allergic diseases as risk factors for Long-COVID. Meta-analysis revealed that pre-existing asthma measured in hospital-based populations and pre-existing rhinitis were significantly associated with increased Long-COVID incidences. Asthma and rhinitis may increase the risk of Long-COVID but the certainty of evidence is very low. CI, confidence interval; OR, odds ratio
Key Message
- In 13 included studies, the proportion of people with LC ranged from 11% to 90%.
- Pre-existing asthma measured in a hospital-based population and pre-existing rhinitis was associated with increased risk of LC.
- Evidence was of very low certainty, related to population selection and exposure and outcome measurements.
1 INTRODUCTION
Since the very first months of the pandemic, patients reported persistent symptoms following acute COVID-19 infection.1 In September 2020, in response to the request from Member States, the World Health Organisation (WHO) introduced additional emergency codes to the International Statistical Classification of Diseases and Related Health Problems (ICD) 10 and ICD-11 for post-COVID-19 condition (PCC).2
Many other terms are used to describe this condition, including ‘long COVID’, ‘post-COVID syndrome’, ‘chronic COVID syndrome’, ‘Post-acute sequelae of COVID-19 (PASC)’3 and ‘COVID-19 long-hauler’.1, 3, 4 According to the COVID-19 rapid guidelines (NG188) from the National Institute for Health Care Excellence (NICE), acute COVID-19 includes symptoms from illness onset up to 4 weeks, ongoing symptomatic COVID-19 is defined as symptoms of COVID-19 from 4 to 12 weeks and post-COVID-19 syndrome is associated with “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks and are not explained by an alternative diagnosis”.5 Long-COVID (LC) is a term developed by the patient community which does not have an agreed definition.5 It can be broadly defined as signs, symptoms, and sequelae that continue or develop after acute COVID-19 or SARS-CoV-2 infection for any period of time and are generally multisystemic. LC might present with a relapsing–remitting pattern and a progression or worsening over time, with the possibility of severe and life-threatening events even months or years after infection.6 LC can present with a variety of signs and symptoms.7, 8 Fatigue and dyspnoea are the most commonly reported symptoms in the adult population.9-16 These symptoms may persist for over a year17 and may appear after mild, moderate or severe SARS-CoV-2 infection in both hospitalised and non-hospitalised patients.18-20 Clinical treatment pathways remain unclear and there are currently no proven, evidence-based treatment modalities yet, for either subgroups or the entire LC population.21
Identifying the causes of LC symptoms is essential for targeted prevention and treatment, a crucial step towards avoiding long-term consequences and determining the rehabilitation needs of affected individuals.
In a 2022 review, Notarte et al.22 analysed data from 38 studies and identified age, female sex, and pre-existing medical comorbidities such as pulmonary disease, diabetes, obesity, and organ transplantation as primary risk factors associated with LC.22 The authors found three cohort studies that showed an association between asthma and longer symptom duration in patients recovering from COVID-19, but also one study that did not show this association. Nevertheless, they also noted that their results should be interpreted with caution, as the included studies exhibited moderate to high risk of bias. While the underlying mechanisms of LC are not yet fully understood, recent studies suggest that pre-existing comorbidities, asthma and allergic diseases are among the factors associated with LC. However, available evidence has never been systematically assessed.23
Therefore, the aim of this systematic review and meta-analysis was to identify, assess, and summarise existing evidence on associations between pre-existing allergic conditions and LC to inform aetiological research.
2 METHODS
2.1 Protocol registration
The protocol for this review was registered a priori in the International Prospective Register of Systematic Reviews and Meta-analysis (PROSPERO): CRD42023391245.
2.2 Search strategy
We performed a systematic literature search and retrieved articles published between January 1, 2020 and January 19, 2023 from MEDLINE via PubMed, Scopus, the WHO-COVID-19 database and the LOVE platform (Epistemonikos Foundation). In addition, citations and reference lists were searched. Two reviewers (DW, AU) independently conducted the electronic search. For our search, we used and modified the recommended search query for studies on COVID-19 from the National Center for Biotechnology Information (NCBI) in PubMed.24, 25 The detailed search strategy for all databases is provided in the (Tables S1–S4).
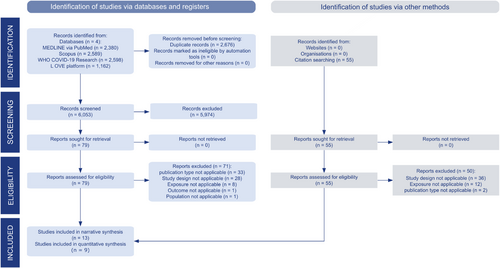
Results of each search were merged into one single file in CITAVI 27 (Swiss Academic Software), which was uploaded to Covidence systematic review software (Veritas Health Innovation) for duplicate removal as well as title abstract and full-text screening (Figure 1).
2.3 Inclusion and exclusion criteria
We included all original articles in English and German that reported on prospective cohort studies recruiting individuals of all ages with information on pre-existing allergic diseases and confirmed SARS-CoV-2 infection (positive RT-PCR test or clinical diagnosis) and following them for at least 12 months for LC symptoms. We adopted the definition of LC from the National Institute for Health Care Excellence (NICE) COVID-19 rapid guidelines (NG188).6 In reference to this guideline, we defined LC as any self-reported or physician-diagnosed symptoms continuing or developing after acute COVID-19 onset. We excluded all study designs that were not prospective cohort studies and all publication types that were not original articles.
2.4 Study selection
The initial phase of title and abstract screening in Covidence involved a pilot phase in which 10% of the items were independently reviewed and discussed in duplicate by four reviewers (DW, AU, KPD, DS) to ensure consistency. Then the same reviewers (DW, AU, KPD, DS) independently screened the remaining items in duplicate. Included items were uploaded as full texts. The same methodology has been implemented for full-text screening. Any disagreements were resolved through discussion involving an additional reviewer and other co-authors if needed, until consensus was reached.
Guidelines for title and abstract screening and full-text screening are available in Guideline S1 and S2.
2.5 Data extraction
Three reviewers (AU, DW, and KPD) independently extracted data of the included studies in duplicate using a modified extraction template in Covidence. Discrepancies were resolved by consensus.
All authors were contacted for additional data. The obtained data were subsequently converted into a Microsoft Excel spreadsheet for further processing of the data. Extracted information included authors, publication year, country, study setting, study population, diagnosis of COVID-19, exposures (allergic diseases), exposure assessment, outcomes (LC symptoms, duration, and assessment methods), and funding. The main exposures are summarized in Table S7.
2.6 Risk of bias assessment
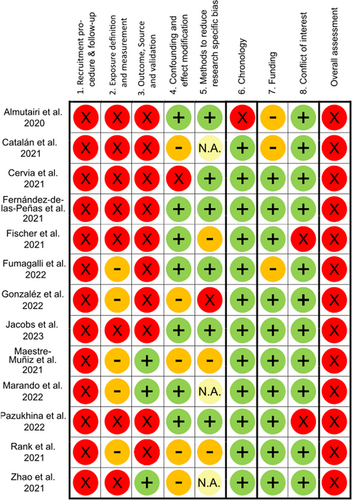
Risk of bias (RoB) was assessed using a standardised appraisal checklist developed by Romero Starke et al.26 In brief, RoB was evaluated across five major domains: (i) recruitment procedure and follow-up (in cohort studies), (ii) exposure definition and measurement, (iii) outcome source and validation, (iv) confounding, and (v) methods of analysis, and three minor domains: (vi) chronology, (vii) funding, and (viii) conflicts of interest. For each included study, two reviewers (DW, KPD) independently assessed the RoB for each domain as ‘high’, ‘low’, ‘unclear’, or ‘not applicable’. Any disagreements were resolved through discussion involving an additional reviewer and other co-authors if needed, until consensus was reached. A low overall RoB was attributed to a study only when all major domains were evaluated as posing a low risk. If any major domain was deemed to present a high or unclear risk, the overall RoB assigned to the study was consequently classified as high.
2.7 Grading the certainty of evidence
The certainty of evidence (CoE) for each outcome examined in the meta-analyses was rated independently by two separate reviewers (PK, AU) following the ‘Grading of Recommendations, Assessment, Development and Evaluations’ (GRADE) approach.27 Disagreements were resolved by discussions with a third reviewer (DW). GRADE was assessed for five domains (risk of bias, inconsistency, indirectness, imprecision, and other considerations). The GRADEpro tool28 was used to combine up- and downgrades of the individual domains, leading to an overall assessment with four possible grades: ‘high’, ‘moderate’, ‘low’, or ‘very low’. Reporting of the results builds on the ‘informative statements to communicate the findings of SRs on interventions’ by the GRADE working group.29
2.8 Data analysis, synthesis, and reporting
The characteristics and results of all studies were initially summarised descriptively. Studies that provided information on both the number of patients with and without allergic diseases and the number of patients with and without LC were eligible for quantitative synthesis.
Based on these studies, aggregate-data meta-analyses were performed within four homogeneous study participant subsets that differed in the underlying condition and population: (a) pre-existing asthma in a hospital-based population, (b) asthma measured in the general population, (c) allergic rhinitis, and (d) allergies (Figure 3). Unadjusted odds ratios were used as outcome measure. As considerable between-study heterogeneity was still anticipated in each subset, random-effect models were used to pool odds ratios. The Mantel–Haenszel method30, 31 was applied for study weight calculation and the Paule–Mandel estimator32 to calculate the heterogeneity variance . To calculate confidence intervals around the pooled odds ratios, Knapp-Hartung adjustments33 were used. Statistical significance was set at 2-sided p < .05. As measures of heterogeneity, the heterogeneity variance and the I2 statistic were reported along with their respective confidence intervals, using a rough interpretation of the I2 statistics (low: 0%–25%, moderate: 25%–75%, high: 75%–100%).34 In each subset, subgroup analyses were performed to explore heterogeneity using study-level variables if the analysis included more than five studies and yielded moderate to high heterogeneity. Sensitivity analyses were conducted to test the robustness of the results regarding the model choice and RoB using fixed-effect models and stratification by RoB, respectively. Publication bias was evaluated by assessing funnel plot asymmetry and applying the Egger's test if ten or more studies were included in the analysis.35 The results of each meta-analysis were presented in forest plots (Figure 3). Statistical analyses were performed using R free software for statistical computing version 4.2.3 and the R package ‘meta’.36, 37
3 RESULTS
3.1 Study selection and characteristics
The systematic search yielded a total of 8729 references.
After removing duplicates, a total of 6053 references remained for title and abstract screening. 79 publications were eligible for full-text screening, of which eight were considered. In addition, citations (n = 55) and reference lists (n = 79 with 3181 entries) were scanned, which yielded five further studies for inclusion. A total of 13 articles were finally selected for narrative synthesis and nine for quantitative synthesis.
Reasons for exclusion for all studies excluded during full-text screening are listed in Table S5. The most common reason for exclusion was “type of publication not applicable” (n = 33). The complete references of all included studies are shown in Table S6. Figure 1 shows the PRISMA flowchart for the overall study selection.
The studies were conducted in Spain (n = 4),17, 38-40 Switzerland (n = 2),41, 42 China (n = 1),43 Germany (n = 1),44 Italy (n = 1),45 Luxembourg (n = 1),46 Russia (n = 1),47 Saudi-Arabia (n = 1),48 and the United States (n = 1).49
The majority of the studies were set in a hospital.17, 38-45, 47, 48 One study was performed among plasma donors44 and two were population-based.46, 49 The 13 included studies differed in terms of their primary objectives, sample size, proportion of LC affected, as well as exposure and outcome measurements. Five studies' primary goal was to identify risk factors for LC,38, 41, 47-49 while the remaining assessed the long-term health consequences of COVID-19 descriptively or otherwise.17, 39, 40, 42-46
Sample sizes ranged from 39 to 2826 persons, shares of females from 18.4% to 66.1%, and incidence/prevalence of LC varied from 11.1% to 89.5% (median: 53.3%). Except for one study, all studies examined adults with a mean age ranging from 37.5 to 65.1 years. Table 1 provides detailed characteristics of the included studies.
| Reference, year, country | Time conducted | Follow-up duration | Participantsa | Setting/setting description |
|---|---|---|---|---|
| Almutairi et al., 2022, Saudi Arabia | May 2020 – August 2021 2020 | 1 year |
Patients ≥18 years Total: n = 372 Response rate: not reported Female: 46.2% Mean age: 37.45 ± 13.44 years |
Hospital, district general hospital in Riyadh, bed capacity of 690 beds |
| Catalán et al., 2021, Spain | March 2020 – April 2021 | 1 year |
Patients ≥18 years Total: n = 124 Response rate: not reported Female: 25.0% Median age: 61.5 years (no steroids group), 68.5 years (steroid group) |
Hospital, Tertiary hospital |
| Cervia et al., 2022, Switzerland | April 2020 – August 2021 | 1 year |
Patients ≥18 years Total: n = 175 Response rate: 77% Female: 47.1% Median age: 33 (controls), 43 (mild COVID-group), 63 (severe COVID-group) |
Hospital, four hospitals |
| Fernández-de-Las-Peñas et al., 2021, Spain | March 2020 – May 2021 | 1 year |
Patients ≥18 years Total: n = 1950 Response rate: 61.2% Female: 50.2% Mean age: 61 ± 16 years |
Hospital, Multicentre, 3 public hospitals of Madrid |
| Fischer et al., 2022, Luxembourg | April 2020 – November 2021 | 1 year |
Patients ≥18 years Total: 330 Response rate: not reported Female: 66.7% Mean age: 40.2 ± 12.5 years |
Test centre, Certified laboratories in Luxembourg |
| Fumagalli et al., 2022, Italy | April 2020 – September 2021 | 1 year |
Patients ≥18 years Total: n = 1825 Response rate: 13.9% Female: 40.2% Mean age: 62 ± 15 years |
Hospital, Tertiary Care Hospital |
| González et al., 2022, Spain | March 2020 – August 2021 | 1 year |
Patients ≥18 years Total: n = 144 Response rate: 34.7% Female: 33.1% Median age: 61 years (IQR 52.0–67.0) |
Hospital, ICU and Hospital, post-COVID unit |
| Jacobs et al., 2023, USA | May 2020 – May 2022 | Up to one year and above |
Persons ≥18 years Total: n = 1224 Response rate: not reported Female: 69.7% Median age: 48 years (IQR 33–60) |
State of Arizona (population based) |
| Maestre-Muñiz et al., 2021, Spain | March 2020 – May 2021 | 1 year |
Patients ≥18 years Total: n = 766 Response rate: 87% Female: 49.3% Mean age: 65.1 ± 17.5 years |
Hospital, 160-bed community medical centre located in a rural area |
| Marando et al., 2022, Switzerland | March 2020 – April 2021 | 1 year |
Patients ≥18 years Total: n = 39 Response rate: 97.43% Female: 21.1% Median age: 64.5 years (IQR 52.7–72.2) |
Hospital |
| Pazukhina et al., 2022, Russia | April 2020 – August 2021 | Adults: median 383 days, (IQR: 376–390), Children: median 367 days (IQR: 351–379) |
Patients <18 years and patients ≥18 years Total: n = 2826 (1994 adults / 832 children) Response rate: 79.47% adults / 97.99% children Female: 50.6% adults / 51.6% children Median age: 56.8 years (IQR 47.0–65.8) adults, 9.5 years (IQR 2.4–14.8) children |
Hospital, four large tertiary adult hospitals / the primary paediatric COVID-19 hospital in Moscow throughout the time of pandemic |
| Rank et al., 2021, Germany | Not reported | 1 year |
Plasma donors ≥18 years Total: n = 98 Response rate: 84.69% Female: 24.1% Median age: 42 years (range 19–62) |
Hospital, Institute for Transfusion Medicine, University Hospital Augsburg |
| Zhao et al., 2021, China | January 2020 – February 2021 | Median 366 days (IQR: 355–376) |
Patients ≥18 years Total: n = 94 Response rate: not reported Female: 42.55% Mean age: 48.11 years |
Hospital, local hospitals in Henan Province |
- Abbreviation: IQR, Interquartile range.
- a Target population, total number of participants at baseline, response rate, proportion of females, average age.
3.2 Acute COVID-19 and pre-existing allergic disease definition
Nine studies defined COVID-19 infection as a laboratory-confirmed positive PCR test.17, 38, 40-43, 46-48 Three studies did not provide detailed definition, but included patients admitted to hospital with COVID-19.39, 44, 45 One study relied on self-reported test results including the date of testing.49
Nine studies derived information on pre-existing allergic diseases by screening electronic medical records.17, 38, 45, 47, 48 For three studies, the assessment method of allergic diseases was not reported.41, 42, 46 In one study, self-report was used.49 Diagnostic and assessment methods applied within the studies are depicted in Table 2.
| Reference, year, country | COVID-19 diagnosis | (Pre-existing) comorbidities assessment/data source | Long-COVID assessment |
|---|---|---|---|
| Almutairi et al., 2022, Saudi Arabia | SARS-CoV-2 detection by real-time polymerase chain reaction (RT-PCR), extracted from Electronic Medical Records | Electronic medical records |
|
| Catalán et al., 2021, Spain | SARS-CoV-2 detection by RT-PCR | Electronic medical records |
|
| Cervia et al., 2022, Switzerland | SARS-CoV-2 detection by RT-PCR | Not reported |
|
| Fernández-de-Las-Peñas et al., 2021, Spain | SARS-CoV-2 detection by RT-PCR and radiological examination | Not reported |
|
| Fischer et al., 2022, Luxembourg | SARS-CoV-2 detection by RT-PCR | Electronic medical records |
|
| Fumagalli et al., 2022, Italy | Not reported (post-discharge COVID-19 patients) | Electronic medical records |
|
| González et al., 2022, Spain | SARS-CoV-2 detection at critical care admission (test not specified) | Not reported – Recorded at hospital or ICU admission. |
|
| Jacobs et al., 2023, USA | Self-reported SARS-CoV-2 test results with specified date of the test | Self-report, questionnaire |
|
| Maestre-Muñiz et al., 2021, Spain | SARS-CoV2 detection by laboratory confirmed test | Electronic medical records |
|
| Marando et al., 2022, Switzerland | SARS-CoV-2 detection by RT-PCR | Electronic medical records |
|
| Pazukhina et al., 2022, Russia | SARS-CoV-2 detection by RT-PCR | Electronic medical records |
|
| Rank et al., 2021, Germany | Not reported (Patients were included after recovery from COVID-19/analysis of humoral immunity against SARS-CoV-2 by IgA/IgG ELISA was carried out as part of 6-week follow-up visit) | Electronic donor records |
|
| Zhao et al., 2021, China | SARS-CoV-2 detection by laboratory-confirmed test | Electronic medical records |
|
- Abbreviations: ISARIC, International Severe Acute Respiratory and emerging Infection Consortium; PCR, polymerase chain reaction; PSQI, Pittsburgh Sleep Quality Index; RT-PCR, reverse transcription-polymerase chain reaction.
3.3 LC (outcome) definition across studies
Except for one study, all studies used the term “Long-COVID” or its equivalents. Almutairi et al.48 however, did not explicitly use the term or one alternative expressions, but assessed the recovery of patients' chemosensory dysfunctions. In line with NICE guideline5 and our inclusion criteria, persons showing chemosensory dysfunction up to 1 year, are considered as LC patients.
Two studies46, 47 used the WHO definition for post-COVID (Post-COVID-19 as the presence of any symptom which started no later than 3 months after hospital discharge and lasted for at least 2 months). Catalan et al.40 defined LC as the persistence of symptoms from the acute phase of illness, the onset of new symptoms in the post-infection period, exacerbation of symptoms that were present before the viral infection, and a wide range of post-COVID-19 sequelae. Cervia et al.41 referred to LC as persistence of one or more COVID-19-related symptoms for more than 4 weeks (i.e. 29 days and more) after the onset of the first COVID-19-related symptom. Fernández-de-Las-Peñas et al.38 used the term ‘Persistent post-COVID-19’ which included symptoms lasting longer than 24 weeks after infection. Gonzales et al.39 described “Post-acute COVID-19” as the presence of symptoms such as fatigue, shortness of breath, and cognitive impairment that affect daily quality of life after 3 months of probable or confirmed SARS-CoV-2 infection and cannot be explained by other alternative diagnoses. Jacobs et al.49 used the term ‘post-acute sequelae of COVID-19’ (PASC) which referred to self-reported persistent or new symptoms 28 days or more after the test date for acute infection. Maestre-Muñiz et al.,17 in contrast, used the term “persistent symptoms in the context of COVID-19 infection” (PCS), i.e. the persistence of at least one clinically relevant symptom, spirometry disturbance, or significant radiological change in patients after COVID-19 infection. Marando et al.42 defined LC as the persistence of changes in lung function tests and chest imaging up to 6 months after the onset of symptoms and the reduction in health-related quality of life. In the context of LC, Rank et al.44 used the term ‘prolonged symptoms’. Zhao et al.43 referred to LC as the long-term health consequences of COVID-19 survivors 1 year after discharge and Fumagalli et al.45 referred to LC as the persistence of symptoms consistent with COVID-19.
Corresponding to the different definitions, the outcome was measured differently: Six studies exclusively relied on self-report through questionnaires to assess outcomes.38, 40, 45, 46, 48, 49 Seven studies combined questionnaires with other outcome measures.17, 39, 41-44, 47 Three studies also included a chest CT scan, pulmonary function tests and a six-minute walk test.39, 42, 43 Three studies combined questionnaires with medical history data or electronic medical records.17, 41, 47 Two studies investigated blood or plasma samples.41, 44 Six studies investigated patient-reported outcomes such as health-related quality of life, anxiety, depression, or well-being in addition to somatic LC symptoms.40, 42, 43, 45-47
3.4 Pre-existing allergic diseases in individual studies
Asthma was the most common pre-existing allergic disease measured separately in twelve studies, with a single study looking at allergic diseases in general.45 Four of the twelve studies reporting asthma additionally reported allergic diseases.44, 47-49 Almutairi et al.,48 Jacobs et al.49 and Rank et al.44 additionally surveyed allergic rhinitis, Pazukhina et al.47 allergic respiratory diseases without asthma and allergic rhinitis/hay fever (in children only). The relevant exposures are listed in Table 3.
| Reference, year, country | Baseline Population | Follow-up population | Long-COVID cases (total number and percent of follow-up population) | Exposures of the study population (total number and percent at follow-up) | Meta-analysis conducteda |
|---|---|---|---|---|---|
| Almutairi et al., 2022, Saudi Arabia | 372 | 372 | 46 12.37% | Asthma: 26 (6.98%) | a |
| Rhinitis: 51 (13.70%) | c | ||||
| Catalán et al., 2021, Spain | 124 | 76 | 68 89.47% | Asthma: 2 (2.63%) | |
| Cervia et al., 2022, Switzerland | 175 | 134 | 85 63.43% | Asthma: 17 (12.68%) | a |
| Fernández-de-Las-Peñas et al., 2021, Spain | 1950 | 1950 | 1583 81.18% | Asthma: 126 (6.46%) | a |
| Fischer et al., 2022, Luxembourg | 330 | 289 | 172 59.52% | Asthma: 6 (2.07%) | b |
| Fumagalli et al., 2022, Italy | 1825 | 254 | 103 40.55% | Allergies: 10 (3.93%) | d |
| González et al., 2022, Spain | 144 | 93 | 57 61.29% | Asthma: 10 (10.75%) | |
| Jacobs et al., 2023, USA | 1224 | 413 | 205 49.64% | Asthma: 67 (16.22%) | b |
| Allergic Rhinitis: 175 (42.37%) | c | ||||
| Maestre-Muñiz et al., 2021, Spain | 766 | 543 | 309 56.91% | Asthma: 51 (9.39%) | a |
| Marando et al., 2022, Switzerland | 39 | 38 | 6 15.79% | Asthma: 5 (13.15%) | a |
| Pazukhina et al., 2022, Russia | 1994 | 1013 | 345 34.06% | Asthma: 48 (4.73%) | a |
| Adults | |||||
| Children | 832 | 360 | 40 11.11% | Asthma: 5 (1.38%) | |
| Allergic respiratory diseases: 5 (1.38%) | d | ||||
| allergic rhinitis: 26 (7.2%) | c | ||||
| Rank et al., 2021, Germany | 98 | 83 | 23 27.71% | Asthma: 2 (2.40%) | |
| Rhinitis: 4 (4.81%) | |||||
| Zhao et al., 2021, China | 94 | 94 | 58 61.70% | Asthma: 2 (2.12%) |
- a a-d reflect the different subsets used for meta-analyses: (a) pre-existing asthma measured in a hospital-based population; (b) pre-existing asthma measured in the general population; (c) pre-existing rhinitis; (d) pre-existing allergies.
3.5 Risk of bias assessment
All included studies were subject to high RoB regarding recruitment (high selection bias). Eleven studies17, 38-43, 45, 47, 48 used data on highly selective populations (previously hospitalised individuals).
Rank et al.44 recruited convalescent plasma donors after mild COVID-19 infection, while Fischer et al.46 looked at Sars-CoV-2-positive people. The authors explained this by “virtually all positive patients could be included in the (…) cohort”.46 However, loss to follow-up reached 46.40% and was therefore above the predefined threshold of 20%, resulting in a high RoB. Jacobs et al.49 also recruited participants from the general population of the U.S. state of Arizona (every resident was eligible) through test centres, contact tracing, postcard mailings throughout the state, or vaccination sites. Loss to follow-up was 66.26% and also above the predefined threshold, resulting in a high RoB rating.
The studies' exposure measurements were rated as either high38, 40, 41, 43, 46-49 or unclear17, 39, 42, 44, 45 RoB. Pre-existing allergic diseases were not tested or clinically diagnosed beforehand. Outcome assessment for LC was rated to be at low RoB in three studies: On top of a questionnaire on persistent symptoms and other instruments, Maestre-Muñiz et al.17 reviewed electronic medical records and performed a physical examination. Marando et al.42 performed lung function tests, chest CT scans, and the six-minute walk test and used health-related quality of life questionnaires. Zhao et al.43 used several questionnaires (e.g. SF-36), performed face-to-face interviews, lung function tests, chest CT scans, and the six-minute walk test. All other studies assessed LC by self-reports from the study participants.
Funding (n = 10) and conflict of interest (n = 11) were mostly rated at low RoB. An overview of all RoB ratings is presented in Figure 2. The overall RoB rating was high for all studies.
3.6 Certainty of evidence assessments
Table 4 shows the CoE assessments for the outcomes examined in the meta-analyses based on the four data subsets, which were rated as ‘very low’ for all outcomes. Reasons for downgrades were the very high RoB of the included primary studies, imprecision and indirectness. Due to the study design of the primary studies, confounding was plausible for all outcomes.
| Certainty assessment | Certainty | ||||||
|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | |
| Risk of developing long-COVID (Population: hospital-based, exposure: asthma) | |||||||
| 6 | Observational studies | Very seriousa | Not seriousb | Not serious | Seriousc | All plausible residual confounding would reduce the demonstrated effect |
⨁◯◯◯ Very low |
| Risk of developing long-COVID (Population: general, exposure: asthma) | |||||||
| 2 | Observational studies | Very seriousa | Not seriousd | Not serious | Seriouse | All plausible residual confounding would reduce the demonstrated effect |
⨁◯◯◯ Very low |
| Risk of developing long-COVID (Exposure: allergic rhinitis) | |||||||
| 3 | Observational studies | Very seriousa | Not seriousd | Seriousf | Not seriousg | All plausible residual confounding would reduce the demonstrated effect |
⨁◯◯◯ Very low |
| Risk of developing long-COVID (Exposure: allergies) | |||||||
| 2 | Observational studies | Very seriousa | Not seriousd | Seriousf | Not seriousg | All plausible residual confounding would reduce the demonstrated effect |
⨁◯◯◯ Very low |
- Note: Explanations: a. The primary studies show serious limitations in outcome measurement and no randomisation was conducted. b. I2 statistics indicates low heterogeneity (24%, p > .05); Confidence intervals of the included primary studies are overlapping. c. The 95% confidence interval includes no clinical meaningful difference between both groups. d. I2 statistics indicates low heterogeneity (0%, p > .05), Confidence intervals of the included primary studies are overlapping. e. The 95% confidence interval is very wide, including substantial benefit and harm from the exposure. f. Important differences across trials regarding populations (children and adults), settings (population-based or hospital), and exposure and outcome measurements. g. The 95% confidence interval is narrow, and the sample size in each study is high.
3.7 Reported association of allergic diseases and LC symptoms in individual studies
Four studies41, 47-49 provided estimates on the association between pre-existing allergic conditions such as asthma or hay fever and LC. Pazukhina et al.47 reported allergic respiratory diseases as a risk factor for LC in children (OR: 2.66, 95% CI: 1.04–6.47). One study did not identify any risk factor associated with long-term post-COVID-19 cough38 and one study excluded allergies as a variable from their model.45
Although we contacted all authors of the primary studies to obtain missing data, the availability of data from four studies proved insufficient to calculate ORs and 95% confidence intervals (CIs).39, 40, 43, 44 We were able to calculate/replicate the ORs and 95% CIs for nine studies.17, 38, 41, 42, 45-49
3.8 Meta-analyses on the association between allergic diseases and LC symptoms
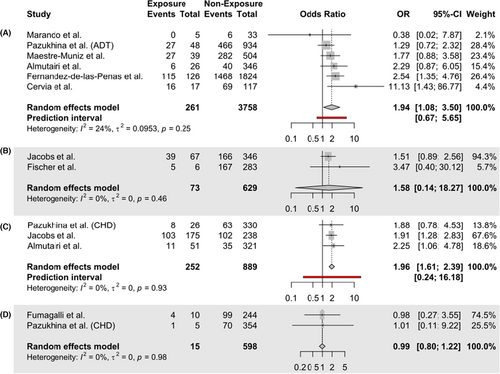
Analysis “a” included pooled data from six studies, based on 261 hospital-based individuals with pre-existing asthma and 3758 hospital-based individuals without asthma. Pre-existing asthma may be associated with an increased risk of LC, but the evidence was very uncertain (OR = 1.94; 95% CI [1.08, 3.50]). The GRADE certainty of evidence was very low due to high RoB and serious imprecision. Between-study heterogeneity was low ( = 0.09, 95% CI [0.00, 5.5890]; = 24.1%, 95% CI [0.0%, 67.8%]).
Analysis “b” examined associations between pre-existing asthma and LC risk in the general population based on n = 73 individuals with pre-existing asthma and n = 629 individuals without asthma. We found no statistically significant associations between pre-existing asthma and risk of LC (OR = 1.58; 95% CI [0.14, 18.27]). The GRADE CoE was very low due to high RoB and imprecision. Explorative analyses revealed that without Knapp-Hartung adjustments, the confidence interval was reduced to a range similar to the ones found in the other subsets (OR = 1.58; 95% CI [0.95, 2.65]).
In subset “c”, n = 252 participants with pre-existing allergic rhinitis were compared to n = 889 individuals without pre-existing allergic rhinitis based on three studies. Pre-existing rhinitis was significantly associated with an increased risk of LC (OR = 1.96; 95% CI [1.61, 2.39]). The GRADE CoE was very low due to high RoB and indirectness. In subset “d”, n = 15 participants with pre-existing allergies were compared to n = 598 individuals without pre-existing allergies based on two studies. There was no statistically significant association between pre-existing allergies and LC (OR = 0.99; 95% CI [0.80, 1.22]). The GRADE CoE was very low due to high RoB and indirectness. For subsets “b”, “c”, and “d”, between-study heterogeneity was estimated to be very low ( = 0.00; = 0.00%). Sensitivity analyses using fixed-effects models resulted in similar effect estimates (Figure S1). Due to low heterogeneity, no subgroup analyses were conducted. Due to the small number of studies in each meta-analysis, publication bias was not evaluated. Forest plots of the four meta-analyses are presented in Figure 3.
4 DISCUSSION
4.1 Statement of principal findings
The purpose of this systematic review was to explore whether pre-existing allergic diseases are risk factors for the development of LC. We identified 13 studies that met our eligibility criteria. Four studies proved to be insufficient for the calculation of effect estimates. Because of the differences in the studies found in terms of allergic disease, measurements and settings, we conducted meta-analyses in four homogenous subgroups. Pre-existing asthma measured in a hospital-based population (6 studies) and pre-existing rhinitis (3 studies) may be associated with an increased risk of LC (OR = 1.94; 95% CI [1.08, 3.50]; OR = 1.96; 95% CI [1.61, 2.39]), based on very uncertain evidence. The evidence is very uncertain about the associations between pre-existing asthma measured in the general population and incidences of LC (K = 2, OR = 1.58 95% CI [0.14, 18.27]), as well as pre-existing allergies and incidences of LC (K = 2, OR = 0.99 95% CI [0.80, 1.22]).
The very low certainty of the evidence for all results synthesised in different subgroups was due to the high risk of bias in primary studies, plausible confounding, indirectness and imprecision. The overall high risk of bias in primary studies arose mostly due to highly selective study populations (hospital patients) or high loss to follow-up. Only three studies assessed LC with physical examinations and comprehensive questionnaires.17, 42, 43 Compared to the other studies only asking about persistent symptoms, there seems to be a general insecurity or imbalance in research on how to specifically assess LC.
4.2 Strengths and limitations of the current review
To gather relevant evidence, we accepted exposure and outcome detection by means of self-reports that are prone to recall bias. We decided to include studies with reported asthma even if it was not clearly defined as allergic asthma. If asthma was summed up with other chronic respiratory diseases (e.g. COPD), we excluded these studies. Differentiating between COPD and asthma seems to be important in the disease course of acute COVID-19: A systematic review about chronic diseases as predictors for severity and mortality of COVID-19 with 217 included studies indicated that COPD was the strongest predictor for a severe COVID-19 course, for admission to an intensive care unit (ICU) and mortality. Asthma showed an association with a reduced risk of a fatal COVID-19 course.50 The broad eligibility criteria regarding outcome and exposure measures and populations were also reflected in clinical and statistical heterogeneity across the identified studies. Although grouping the studies in subsets resulted in more homogeneous subgroups, the studies still differed in key aspects. This, and the underlying study design of the primary studies, made it difficult to draw firm conclusions about the role of allergic diseases as a risk factor for developing LC because the associations may also be confounded or not generalisable to the target population.
We undertook a robust systematic electronic literature search in four major databases including the WHO COVID-19 Global Research Database established by the WHO evidence retrieval sub-group.51 Nonetheless, we cannot guarantee that we identified all relevant articles, even though citations and reference lists were searched. We contacted all authors for raw data, but did not receive a response from all and thus were unable to obtain all aspired data. We strictly adhered to the respective reporting guideline.52 Every relevant task was carried out independently by at least two reviewers.
4.3 Mechanistic considerations
Research indicates that a type-2 T-helper lymphocyte (Th-2) allergic immune response may potentially offer protection against severe COVID-19 infection53 while paradoxically may be associated with an increased risk of LC. Some theories were proposed originating from observations that elevated Th-2 activity and eosinophil counts are associated with recovery from acute infection, and that pre-existing allergic asthma is associated with less severe COVID infection.54 Nonetheless, recent studies have also linked asthma and allergies to a higher prevalence of LC. Understanding this dual role could guide the development of novel therapeutic strategies for acute COVID-19 infections and the prevention of LC.
In the context of viral respiratory infections, innate immune responses activate innate lymphocytic cells of a type-2 phenotype (ILC-2) which release a range of cytokines associated with allergic diseases.55 This process has been observed in COVID-19 patients and linked with more favourable outcomes. However, it could also exacerbate the severity of pre-existing allergic conditions. In line with this, evidence suggests that pre-existing asthma might reduce acute COVID-19 infection severity. Other immunological (dys) regulation processes are also suspected to influence the development of LC, e.g., Th-17 or cytokine activity, in particular interleukin (IL) 6.53, 54 In a recent systematic review and meta-analysis of 16 studies, Yin et al. even postulate that increased IL-6 levels might predict LC.55
4.4 Studies in progress and integration in previous literature
We are aware of at least 5 studies in progress56-60 which are investigating long-term consequences of COVID-19 and will provide future results. We intend to update the current systematic review once results from these studies become available, approximately every 6 months. We encourage investigators of ongoing studies to publish results together with their datasets.
4.5 Differences between study protocol and actual review
In the course of the Systematic Review, the inclusion criterion “persistent symptoms >28 days or >4 weeks” was removed because hardly any study reported on specific symptom duration periods. Nevertheless, screening was performed to ensure that all study populations considered met the predefined LC definition.
4.6 Implications for future research and practice
More high-quality research is needed to clarify the role of pre-existing allergic diseases as risk factors for LC. This could be achieved by improving the validity of exposure and outcome assessment and minimising attrition in the respective cohorts. It must be acknowledged that no agreement existed until recently which health outcomes should be measured in people living with LC. An international Delphi exercise involving more than 1500 people has meanwhile agreed on a Core Outcome Set (COS) for post-COVID-19 condition.61 The COS includes fatigue, pain, post-exertion symptoms, work or occupational and study changes, survival and functioning, symptoms, and conditions for each of cardiovascular, respiratory, nervous system, cognitive, mental health, and physical outcomes in addition to recovery that was included a priori. We do hope that this COS will impact the way in which LC is measured in epidemiological cohort studies. In terms of exposure assessments, a clearer distinction of the different allergic diseases would be desirable. Meanwhile, the allergy community should be aware that individuals with asthma or rhinitis might be at increased risk of LC after SARS-CoV-2 infection.
5 CONCLUSION
The allergy community should be aware that individuals with asthma or rhinitis might be at increased risk of LC after SARS-CoV-2 infection. The evidence for these associations is very uncertain, therefore more robust epidemiological research is needed to clarify the role of allergy in the aetiology of LC.
AUTHOR CONTRIBUTIONS
CA had the idea for this systematic review and conceived it with DW, KPD and AU. AU, KPD, DW and CA developed the search strings with support of DM, SD and DS. DW and AU conducted the literature search, supported by KPD. DW, KPD, AU and DS screened literature. AU, DW and KPD extracted the data independently and consented extraction. Meta-Analysis was performed by AS, PK and interpreted by all authors. Risk of bias was assessed by DW and KPD, certainty of evidence by DW, AU, PK and AS. DW, KPD, AU and CA wrote the manuscript with support of PK, AS, DM, JS, SD, DS. All authors commented and reviewed the manuscript for important intellectual content and approved the final version.
ACKNOWLEDGEMENTS
We thank Elena Brushinski and Taurai Hahne for their helpful preceding work. Furthermore, we highly appreciate the cooperation of Elizabeth T. Jacobs, César Fernández-de-Las-Peñas, Aurélie Fischer, Alfredo J. Lucendo, Marco Marando, and Ekaterina Pazukhina by providing additional data on their studies. Additionally, we thank Katharina Heldt for her helpful advice in developing our search string. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
This review was partly supported by egePan Unimed (Grant number 01KX2021), funded by the Federal Ministry of Education and Research in Germany (BMBF) as part of a Network of University Medicine (NUM).
CONFLICT OF INTEREST STATEMENT
DM is principal investigator of the StopCOVID observational cohort study and co-leads the PC-COS project, developing Core Outcome Set for post-COVID-19 condition. The authors DW, KPD, AU, DS, SD, AS, PK, JS and CA declared to have no conflicts of interest with respect to the research, authorship, and publication of this article.
SYSTEMATIC REVIEW REGISTRATION
PROSPERO CRD42023391245. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=391245.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.





