Does proactively asking about allergens before ordering improve customer outcomes? An in-business randomised controlled trial
Key messages
- We report a cluster-randomised trial of proactively asking customers about food hypersensitivity in food outlets.
- A self-selected sample of 936 customers at 18 outlets responded to an experience survey.
- Customers at intervention group outlets who were asked about food hypersensitivity reported slightly increased confidence.
- Staff proactively asking about allergens increased customer satisfaction, on three different customer satisfaction metrics.
Roughly 5% of the UK population report having a food hypersensitivity, which includes both intolerances and allergic reactions,1 and 60% of those (3% of the UK population) have immunoglobulin E (IgE)-mediated food allergy.2 Hospital admissions for food anaphylaxis are increasing3; most deaths due to food allergens between 1992 and 2012 occurred as a result of food being bought from food businesses.4 However, qualitative research suggests that customers with allergies may be reluctant to actively seek information about allergens.5, 6
Following a successful feasibility study run by the Behavioural Practice from 3 to 20 March 2020, the Food Standards Agency (FSA) commissioned the Behavioural Practice to run a full field trial, to test whether Food Business Operator (FBO) staff proactively asking customers about allergies would increase customer confidence that they could identify any ingredients, comfort asking about ingredients and perceptions of food safety regarding food and drink sold at the chain. The study was pre-registered on Open Science Framework before trial launch, and details about data collection and materials are available there. The study was funded by the Food Standards Agency, who have published a report (https://www.food.gov.uk/research/proactively-asking-about-allergens-full-report).
We ran a matched-pairs clustered randomised trial, where the clusters were branches of a national FBO. Participants were customers who entered the FBO between 28 March 2022 and 30 June 2022, who placed a food order at the till and who completed a voluntary survey about their experience. Half of the branches implemented the intervention, with staff being told to ask, ‘Do you have any food allergies or intolerances?’ before customers placed their order. If a customer replied in the affirmative, the FBO's standard protocol in handling these cases was followed, which involved directing customers to scan a QR code to access allergen information. The other half of the branches were a control, the staff were not instructed to ask; they continued with business as usual. In both intervention and control branches, leaflets were handed out and there were table toppers directing customers to scan a QR code to take them to a survey. The materials advertised that participants completing the survey could be entered into a prize draw for 25 Love2Shop vouchers each worth £50.
The primary outcome measures were three questions on the survey, which were shown in a random order using 5-point Likert scales: confidence that, if needed, they could identify any ingredients in the food or drink products (1 = ‘Not at all confident’ to 5 = ‘Very confident’), comfort asking about ingredients (1 = ‘Not at all comfortable’ to 5 = ‘Very comfortable’) and perception of food safety in the FBO (1 = ‘Very unconcerned’ to 5 = ‘Very concerned’). Secondary outcomes were three questions on common customer satisfaction metrics (also shown in a random order and using 5-point Likert scales): customer satisfaction, customer trust in the business and whether the customer would recommend the business to a friend or family member. We also asked whether customers declared an allergy and/or food intolerance on their visit. Fidelity had been poor in the feasibility study, so we pre-registered a per-protocol analysis in order to investigate the effect of the intervention, when it was delivered as intended. We measured fidelity via a question on the survey, asking customers to report whether the employee asked whether they had a food allergy or intolerance before they made their purchase.
Staff in the treatment arm knew that they were delivering an intervention, staff in the control arm knew that they were a business-as-usual arm that was being used to compare the effectiveness of changes that were being implemented in other branches. Customers did not know about the trial arms. Randomisation was done using pair matching on footfall, location (London/non-London) and whether or not the branch had seating, treating each pair as a strata for stratified randomisation.
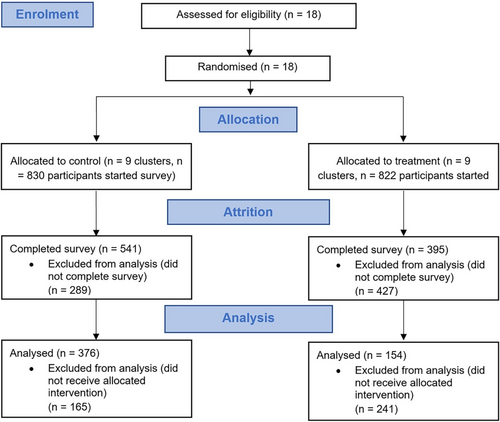
We randomised 18 branches, 9 in each arm. We received n = 936 survey completions across the two trial conditions (n = 395 in the treatment arm and n = 541 in the control arm). In addition, there were a number of incomplete surveys, which we did not analyse, (n = 427 in the treatment arm, n = 289 in the control arm), the majority of which (635 of 716) dropped out on the initial information and consent pages. Of the completed surveys, n = 530 reported receiving the correct treatment and were analysed (n = 376 in the treatment arm and n = 154 in the control arm). See Figure 1. There were n = 198 males who completed the survey and received the allocated intervention and the modal age group of participants was 16–25 (n = 239 or 45.1%). There were n = 89 (16.8%) who reported having an allergy or food intolerance (n = 30 in the treatment arm, n = 59 in the control arm); we were not powered for sub-group analysis. We analysed the data using a series of pre-registered two-level hierarchical linear models (https://osf.io/973d8/), controlling for the impacts of individual-level covariates—food hypersensitivity, age and gender, See Table 1. Results were corroborated using an ordinal probit model as a sensitivity analysis.
| Predictors | Perceptions of food safety | Confidence in identifying ingredientsa | Comfort asking staff about ingredients | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimates (β) | CI | p | Estimates (β) | CI | p | Estimates (β) | CI | p | |
| Intercept | 2.03 | 1.74 to 2.31 | <.001 | 3.97 | 3.78 to 4.17 | <.001 | 4.21 | 4.02 to 4.40 | <.001 |
| Treatment (Treatment) | −0.02 | −0.31 to 0.28 | .920 | 0.26 | 0.07 to 0.45 | .008 | 0.36 | 0.17 to 0.56 | <.001 |
| Hypersensitivity (Yes) | 0.13 | −0.20 to 0.46 | .434 | −0.10 | −0.34 to 0.13 | .383 | −0.21 | −0.42 to 0.01 | .064 |
| Age (26–35) | 0.10 | −0.19 to 0.39 | .505 | 0.01 | −0.19 to 0.22 | .904 | −0.05 | −0.25 to 0.14 | .583 |
| Age (36–49) | 0.09 | −0.25 to 0.43 | .609 | 0.05 | −0.20 to 0.29 | .701 | −0.10 | −0.32 to 0.13 | .400 |
| Age (50+) | −0.06 | −0.55 to 0.42 | .800 | −0.13 | −0.48 to 0.22 | .465 | 0.26 | −0.07 to 0.58 | .118 |
| Gender (female) | 0.12 | −0.13 to 0.38 | .339 | 0.02 | −0.16 to 0.20 | .822 | 0.09 | −0.08 to 0.25 | .320 |
| Random effects | |||||||||
| σ 2 | 1.92 | – | 0.86 | ||||||
| τ 00 | 0.02Location | – | 0.00Location | ||||||
| ICC | 0.01 | – | 0.01 | ||||||
| N | 18Location | – | 18Location | ||||||
| Observations | 508 | 508 | 508 | ||||||
| Marginal R2/conditional R2 | .004/.012 | .017/.006 | .043/.049 | ||||||
- Note: Bold indicates the p values that are significant.
- a This model was conducted using a model without a random intercept, which had significantly better fit, χ2(1) = 19.476, p < .001.
Customers in the treatment arm who received the intervention were more confident in their ability to identify ingredients if need be than those in the control who did not receive the intervention. Among those in the treatment group, almost 83.2% were either ‘very’ or ‘somewhat’ confident they could identify ingredients, compared with 77.2% in the control group. Controlling for the influence of demographic covariates, those in the treatment group were, on average, 0.26 points more confident than those in the control group (β = 0.26, p < .01).
Those in the intervention arm expressed a greater level of comfort in asking a member of staff for information about product ingredients. For those in the treatment group, a majority (69.5%) were ‘very’ comfortable, while in the control group, only half (50.8%) were ‘very’ comfortable. Controlling for the influence of demographic covariates, those in the treatment group were, on average, 0.36 points more comfortable than those in the control group (β = 0.36, p < .001).
However, the intervention did not have an effect on customers' level of concern regarding the safety of the food that is sold in the FBO. Overall, a majority (65.3%) were ‘very’ or ‘fairly’ unconcerned about the quality of food that is sold in the FBO, compared with 68.1% in the control. The intervention did not have a significant impact on level of concern in the primary model (β = −0.02, p = .920).
Fidelity to the intervention was poor. In the treatment arm, 39.0% of participants (154 of 395) said they were asked whether they had a food allergy or intolerance (which was higher than the 30.0% who were asked as a part of business as usual in the control arm). Fidelity in the treatment arm varied between branches, ranging from 20.8% to 75.9%. None of the primary outcomes were effective in an intention-to-treat analysis.
There were improvements on all the secondary outcomes: customer satisfaction, customer trust in the business and whether the customer would recommend the business to a friend or family member. Customers were also more likely to report having declared an allergy or intolerance in the treatment arm (OR = 8.58, p < .001).
The key limitation in the design of the trial is possible bias due to low coverage, self-selection of customers into the survey and any differential (biased or non-random) attrition of customers during the survey. We ran the trial in a national FBO, in branches both inside and outside London. Nevertheless, all branches were in cities and, of course, the type of people who go to a particular FBO is not random. In particular, the sample tended to be quite young, the modal age group being 16–25. We do not know the footfall in the branches during the trial, so we do not know the percentage of customers who responded to the survey (and therefore the ultimate extent of selection bias). We also note that attrition was higher in the treatment branch than in the control; we have no explanation for this.
Another limitation of this trial is that it was only conducted in one FBO: As such, we cannot be sure that the results generalise to other types of food business. In this trial, we partnered with a national FBO; in the feasibility study, we partnered with an international FBO.
Future research should seek to investigate whether this trial's intervention is effective in other types of business and for other types of consumers, and might consider study designs which capture more complete outcome data (including adverse effects).
AUTHOR CONTRIBUTION
Robert McPhedran contributed to the methodology (experimental design), formal analysis, data curation and writing—original draft (results section). Pieter Cornel contributed to the resources (study materials), investigation (overseeing evidence collection) and original draft (conclusion). Yuchen Yang and Sanjeev Devarajan contributed to the resources (study materials), writing—review and editing. Ben Toombs contributed to the conceptualisation and writing—review and editing. Anya Mohideen, Alice Rayner and Phil Jones contributed to the conceptualisation, methodology, writing review and editing. Natalie Gold contributed to the project administration, supervision, conceptualisation, methodology (experimental design) and writing—original draft (introduction and methods).
ACKNOWLEDGEMENTS
We thank Bethan Mead for helpful comments.
FUNDING INFORMATION
This work was supported by the Food Standards Agency. Employees of the FSA were involved in commissioning and conceptualising the project and critically reviewed the paper.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Due to a non-disclosure agreement with the data owner, we are unable to share the data used for this study. However, we will make our analysis code available in a public repository upon publication. The study protocol was pre-registered and publicly available on the Open Science Framework (https://osf.io/3zwhs).




