RNAi Silencing of IL-1β and TNF-α in the Treatment of Post-traumatic Arthritis in Rabbits
Abstract
Post-traumatic arthritis is a secondary complication to severe joint trauma. With the disease progression, it may eventually lead to osteoarthritis in patients whose age is considerably younger than patients with traditional bone arthritis. The main objective of this study was to explore the feasibility of using lentiviral-mediated RNA interference silencing of IL-1β and TNF-α to treat post-traumatic arthritis in rabbits. About 48 New Zealand rabbits underwent bilateral knee joint surgery to stimulate traumatic arthritis. They were then randomly divided into four groups of 12 rabbits each. The histopathology of the cartilage was observed, and the changes were assessed by Mankin scoring. ELISA was used to detect the expression of IL-1β and TNF-α in the synovial fluid. (i) Compared with the control group, the transfection and co-transfected groups displayed reduced cartilage damage and speed of degeneration. The co-transfected group showed the greatest alleviation of symptoms. The Mankin score was statistically different (p < 0.01). (ii) Compared with the control group, the expression of IL-1β or TNF-α was reduced in the respective transfection groups (p < 0.01 in both groups) and IL-1β and TNF-α were reduced in the co-transfected group (p < 0.01). The co-transfected group showed the lowest expression of the three experimental groups of both IL-1β and TNF-α (p < 0.01). Lentivirus-mediated RNA interference can knock down the expression of IL-1β and TNF-α in joint fluids and, in a synergistic effect when two siRNAs are co-transfected, ease cartilage degeneration.
Abbreviations
-
- dsRNA
-
- double-stranded RNA
-
- ELISA
-
- the enzyme-linked immunosorbent assay
-
- IL-1β
-
- interleukin-1 beta
-
- RNAi
-
- RNA interference
-
- siRNA
-
- small interfering RNAs
-
- TNF-α
-
- tumor necrosis factor-alpha
Post-traumatic arthritis is a secondary complication to severe joint trauma 1. With the disease progression, it may eventually lead to osteoarthritis 2 in patients whose age is considerably younger than patients with traditional bone arthritis 3, 4. Even with the intervention of modern advanced surgical techniques, 50% of patients who experience severe joint trauma will eventually suffer from osteoarthritis 5. Gene therapy is currently a topic of considerable interest in the treatment of these conditions.
There is much research that shows that interleukin-1β (interleukin 1β, IL-1β) and tumor necrosis factor-α (tumor necrosis factor α, TNF-α) are the two most important inflammatory cytokines resulting in post-traumatic osteoarthritis 6. In this study, RNA interference (RNAi) using synthetic, packaged recombinant lentiviruses targeting TNF-α and IL-1β was utilized to study the inhibition effects of post-traumatic arthritis in the synovial fluid of rabbits and to observe the effects on articular cartilage injury.
Methods and Materials
Main reagents and instruments
The GV118 lentiviral three-plasmid vector systems was acquired from the Shanghai Jukai Gene Company. Trizol and Lipofectamine 2000 reagents were purchased from Invitrogen (Carlsbad, CA, USA). 293T cell lines and rabbit knee joint synovial cells were purchased from Real-time PCR kit (QPS201; TOYOBO Japanese Company, Osaka, Japan), Reverse transcription kit (Promega, Madison, WI, USA), and PRISM ® 7500 Sequence Detection System (ABI, Foster City, CA, USA). Medium: DMEM-F12 and fetal bovine serum (Gibco, Gaithersburg, MD, USA): 20%, Penicillin (Bi Yuntian): 1% protein-free cryopreservation of stem cells with a liquid (Cyagen Biotechnology, Santa Clara, CA, USA), and Supernatant TNF-α ELISA and IL-1β detection kit (Usnc Life Science, Inc, Wuhan, China).
Establishment of post-traumatic arthritis rabbit model
Forty-eight (48) New Zealand rabbits, aged 12 months and weighing 2.0–2.5 kg, were provided by the Branch of the Department of Medicine, Nanchang University. The experimental procedures were approved by the Animal Ethics Committee of the Second Affiliated Hospital of Nanchang University. Animal care was in compliance with the IASP and European Community (E.C. L358/1 18/12/86) guidelines on the use and protection of animals in experimental research. All efforts were made to minimize animal sufferance and to reduce the number of animals used. Both male and female rabbits were included in this study. Each rabbit underwent bilateral knee surgery to develop the trauma model. The rabbits were anesthetized with 4% chloral hydrate, and a 2-cm vertical incision was made on the inner side of the knee, under sterile surgical conditions. Once the knee was exposed, the anterior cruciate and medial collateral ligaments were cut and the medial meniscus was removed. The limbs were not repaired after surgery, but the rabbits received penicillin 400 000 U intramuscularly for 3 days to prevent infection.
Preparation of the recombinant lentivirus
The siRNAs were designed and synthesized by RiboBio Co. (Guangzhou, China) according to the sequences shown in Table 1. According to the designed sequence, the synthesis for the TNF-α siRNA was carried out in a reaction solution containing 2 μL of 10× buffer, 0.4 μL of upstream and downstream primers, 0.8 μL of dNTP (2.5 mmol/L), 0.2 μL Taq polymerase, and 1 μL Template. Ultrapure water was added to make the total reaction volume to about 20 μL, and the oligos were synthesized. According to the GV118 lentiviral plasmid vector kit instructions, the vector was linearized, purified, and absorbed. The synthetic oligos were linked and transformed into competent E. coli cells. The positive clones were picked, and their DNA was extracted and amplified. The DNA fragments were linearized and transfected, using Lipofectamine 2000, into 293T cells for lentivirus packaging. The lentivirus was tittered and prepared for use. The IL-1β siRNA was synthesized and packaged into lentivirus using the same technique.
| Sequence | TNF-α-siRNA | IL-1β-siRNA |
|---|---|---|
| Target sequence | CACGTTTTCCGTGAAAACAGAGC | GAGAACTTTCTTTTCCTTAATCT |
| Positive-sense strand (5′-3′) | CACGUUUUCCGUGAAAACAGAGCdTdT | GAGAACUUUCUUUUCCUAAUCUdTdT |
| Antisense strand (3′-5′) | dTdTGUGCAAAAGGCACUUUUGUCUCG | dTdTCUCUUGAAAGAAAAGGAUUAGA |
Cell transfection
Primary synovial cells were obtained from normal rabbit knee joints. The first-generation synovial cells were seeded into 250-mL flasks at a density of 5 × 105 cells per flask. When the cells were approximately 70% confluent, they were treated with recombinant lentivirus suspension (MOI = 20) while adding polybrene to a final transfection concentration of 8 μg/mL.
Experimental grouping
About 48 New Zealand rabbits were randomly divided into four experimental groups after being prepared according to the post-traumatic arthritis model. Each group consisted of 12 rabbits. Group A was the control group and would receive intra-articular injections of saline solution. Groups B and C were experimental groups and would receive injections of either TNF-α or IL-1β siRNA recombinant lentivirus-infected cell suspension, respectively. Group D was the final experimental group and would receive injections of both TNF-α and IL-1β siRNA recombinant lentivirus-infected synovial cells. At weeks 1, 2, and 4, three animals from each group were killed for analysis.
Injection method
Seven (7) days after modeling, the rabbits were given 4% chloral hydrate intraperitoneal anesthesia. An injection of 1 mL of TNF-α siRNA, IL-1β siRNA or TNF-α, and IL-1β siRNA recombinant lentivirus-infected synovial cells (5 × 106 cells/mL) was given to each rabbit in the knee joint cavity near the patellar ligament. The knee joint was moved manually for 5 min to distribute the transfected cells.
Experimental observations
Cartilage staining
Cartilage samples were taken from the region around the damaged medial femoral condyle. The samples were stained with hematoxylin-eosin (HE) and toluidine blue for observation. The HE-stained cartilage samples were observed under a microscope to assess chondrocyte morphology and determine whether the physical structure was regular and lacking surface cracks and whether the tidal line was complete. The degree of cartilage loss was determined by observing the toluidine blue-stained samples, and the results were graded according to the Mankin scoring criteria.
ELISA
The expression of TNF-α and IL-1β in the synovial fluid was determined by ELISA (double antibody sandwich method).
Statistics
All of the experimental data are expressed as means ± standard deviation. The results were analyzed by spss 17.0 [IBM (SPSS), Chicago, IL, USA] software for anova and Fisher's least significant different (LSD) test.
Results
Construction of recombinant lentiviral packaging
Recombinant lentiviruses were successfully synthesized and packaged for TNF-α and IL-1β siRNAs (Figure 1). The sequences were tested and found to be in line with the expected DNA sequences. This means that the silencing synthetic sequences were inserted correctly. The viral titre of the TNF-α siRNA was 4.0 × 108 TU/mL, and the titre of the IL-1β siRNA was 2 × 109 TU/mL. These were sufficient for use in the subsequent experiments.
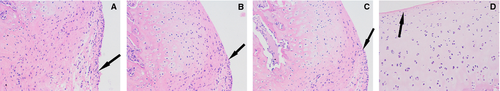
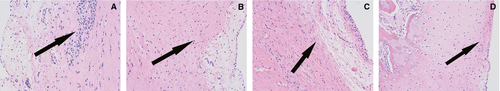
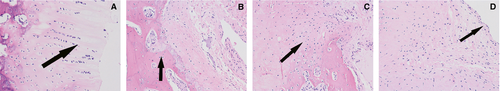
Cartilage assessment
All four groups were seen to have articular cartilage damage that increased with time. The control group (A) had visible moderate joint articular cartilage damage 4 weeks after injection (Figures 2-4). Compared to the control group, the experimental groups (B, C, D) show the cartilage injury is reduced during the same time-period. Experimental group D, which received injections of both TNF-α and IL-1β siRNA recombinant lentivirus-infected synovial cells, is the most obviously relieved.
Mankin score
Compared to the control group (A), experimental groups B, C, and D showed significantly different (p < 0.01) Mankin scores at each time-point. At 1, 2, and 4 weeks after injection (Table 2, Figures 2-4), there was a significant difference in the Mankin scores among the experimental groups.
| Group | 1 week | 2 weeks | 4 weeks |
|---|---|---|---|
| A | 3.33 ± 0.52 | 4.33 ± 0.52 | 6.33 ± 0.52 |
| B | 2.50 ± 0.55* | 3.33 ± 0.52*,▵ | 4.33 ± 0.52*,▵ |
| C | 2.33 ± 0.52* | 3.33 ± 0.51*,▵ | 4.32 ± 0.52*,▵ |
| D | 2.00 ± 0.63* | 2.50 ± 0.84* | 3.67 ± 0.52* |
- Compared with the control group over the same period, *p < 0.01. Compared with the same period in group D, ▵p < 0.01.
Synovial fluid TNF-α and IL-1β expression
Compared with the control group (A), the expression of TNF-α in the group B rabbits decreased at each time-point (p < 0.01) (Table 3). At each time-point, the expression of IL-1β was reduced for the group C animals, compared to the control group. Compared to the control group, experimental group D showed a decrease in TNF-α and IL-1β levels at each time-point (p < 0.01). At each time-point, the expression of these cytokines was statistically significant (p < 0.01) for each experimental group.
 , pg/mL)
, pg/mL)| Group | Time | TNF-α | IL-β |
|---|---|---|---|
| A | 1 week | 186.30 ± 1.42 | 87.16 ± 0.32 |
| 2 weeks | 174.00 ± 1.81 | 60.22 ± 0.83 | |
| 4 weeks | 166.43 ± 0.87 | 51.25 ± 0.84 | |
| B | 1 week | 172.94 ± 2.59*,▵ | 86.55 ± 0.27 |
| 2 weeks | 152.14 ± 1.76*,▵ | 58.55 ± 1.25 | |
| 4 weeks | 142.52 ± 3.41*,▵ | 46.42 ± 1.47 | |
| C | 1 week | 182.21 ± 3.53 | 77.44 ± 0.74*,▵ |
| 2 weeks | 169.87 ± 3.10 | 50.84 ± 0.81*,▵ | |
| 4 weeks | 162.58 ± 1.82 | 40.91 ± 0.61*,▵ | |
| D | 1 week | 158.98 ± 1.29* | 66.40 ± 1.14* |
| 2 weeks | 142.20 ± 0.95* | 41.09 ± 1.33* | |
| 4 weeks | 135.65 ± 1.84* | 37.05 ± 0.80* |
- Compared with the control group over the same period, *p < 0.01. Compared with the same period in group D, ▵p < 0.01.
Discussion
RNA interference (RNAi) is a gene-silencing phenomenon in which double-stranded RNAs (dsRNAs) are introduced into cells, are cut into small interfering RNAs (siRNA), and cause the sequence-specific degradation of mRNA, thereby inhibiting the expression of the corresponding gene7. A small amount of dsRNA can inhibit the expression of the target gene 8, making RNAi the simplest and most effective method of blocking gene expression in mammalian cells 9.
Lentiviral vectors are a new development in recent years, with high transfection efficiency, stable expression in passaged cells, and higher transfection efficiency in quiescent cells 10. Direct transfection of synthetic siRNA can inhibit gene expression, but the siRNA is easily degraded in vivo 11. Using lentiviral-mediated RNAi can produce a more stable, long-lasting effect because of the longer time needed to generate siRNA. Thus, the RNA interference becomes more stable and sustained 12, 13.
TNF-α and IL-1β are popular targets for gene therapy in the treatment of post-traumatic arthritis. This study found that the introduction of the gene for the IL-1 receptor antagonist into the cells of the rabbit knee joint will inhibit the progression of osteoarthritis. Combining the IL-1 receptor antagonist gene with the soluble TNF-α receptor gene had a synergistic effect, lessening the damage and progression of OA even further 14. In this study, RNAi principle was used to inhibit the expression of cytokines. The siRNAs for TNF-α and IL-1β were synthesized and packaged into the corresponding recombinant lentiviruses. After infection of synovial cells harvested from normal knee joints, the stably transfected cells were injected into the joints of post-traumatic arthritis model animals and the expression levels of TNF-α and IL-1β and the dynamics of articular cartilage damage were observed in vivo. The results showed that, compared to the untreated control group, the expression of the inflammatory cytokines in the joint fluid was decreased after injection with recombinant lentiviral-packaged siRNA. The degree of joint cartilage damage and the rate of tissue degeneration were observed and found to be reduced.
In conclusion, this study provides evidence that lentiviral-mediated RNAi may be an effective strategy to target post-traumatic arthritis patients, lessening the degree of damage to the joints and alleviating joint degradation commonly seen in these cases.
Acknowledgements
We thank Xiao-Long Shen for providing technical support and Ning-Ning Huang for providing technical support in RNAi Silencing examination.
Authors’ contributions
QT, LH, YZ, KS, FC, CL, and YP carried out all experiments and performed the statistical analysis. QT and QL conceived of the study, carried out the design and drafted the manuscript, and participated in the RT-PCR study and Western blot analysis. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.




