T cell receptor gene-modified allogeneic T cells with siRNA for endogenous T cell receptor induce efficient tumor regression without graft-versus-host disease
Abstract
Adoptive immunotherapy using genetically engineered patient-derived lymphocytes to express tumor-reactive receptors is a promising treatment for malignancy. However, utilization of autologous T cells in this therapy limits the quality of gene-engineered T cells, thereby inhibiting the timely infusion of the cells into patients. In this study, we evaluated the anti-tumor efficacy and the potential to induce graft-versus-host disease (GVHD) in T cell receptor (TCR) gene-engineered allogeneic T cells that downregulate the endogenous TCR and HLA class I molecules with the aim of developing an “off-the-shelf” cell product with expanded application of genetically engineered T cells. We transduced human lymphocytes with a high-affinity TCR specific to the cancer/testis antigen NY-ESO-1 using a novel retrovirus vector with siRNAs specific to the endogenous TCR (siTCR vector). These T cells showed reduced expression of endogenous TCR and minimized reactivity to allogeneic cells in vitro. In non-obese diabetic/SCID/γcnull mice, TCR gene-transduced T cells induced tumor regression without development of GVHD. A lentivirus-based CRISPR/Cas9 system targeting β-2 microglobulin in TCR gene-modified T cells silenced the HLA class I expression and prevented allogeneic CD8+ T cell stimulation without disrupting their anti-tumor capacity. This report is the first demonstration that siTCR technology is effective in preventing GVHD. Adoptive cell therapy with allogeneic T cells engineered with siTCR vector may be useful in developing an “off-the-shelf” therapy for patients with malignancy.
Abbreviations
-
- ACT
-
- adoptive cell therapy
-
- CAR
-
- chimeric antigen receptor
-
- CFSE
-
- carboxyfluorescein succinimidyl ester
-
- GVHD
-
- graft-versus-host disease
-
- HLA
-
- human leukocyte antigen
-
- NK
-
- natural killer
-
- NOG
-
- non-obese diabetic/SCID/γcnull
-
- PD-1
-
- programmed death-1
-
- TCR
-
- T cell receptor
1 INTRODUCTION
Immunotherapy has become an important cancer treatment modality. Immunotherapy with immune checkpoint blockade has greatly improved the survival of patients with various types of malignancies, including melanoma, non-small cell lung carcinoma, ovarian cancer, and renal cell carcinoma.1-9 However, a large proportion of patients still experience disease progression with these therapies.
Adoptive cell therapy (ACT) may provide an additional treatment option for these patients. ACT uses patient-derived lymphocytes that are genetically engineered to express tumor-reactive T-cell receptor (TCR), which is a promising treatment for malignancy.10-17 Most current TCR clinical trials utilize autologous T cells; however, using patients’ own lymphocytes limits the application of adoptive immunotherapy in several ways. First, patients’ lymphocytes often have impaired quality and quantity owing to the influence of the tumor on the host immune status as well as the effects of treatments such as chemotherapy. Second, manufacturing autologous T cell products is a lengthy process; therefore, patients may miss the optimal window to benefit from the therapy. Third, patient-specific production of gene-engineered T cells is associated with high costs. To circumvent these limitations, we aimed to develop “off-the-shelf” cell products utilizing allogenic T cells.
We established a retroviral vector system (siTCR vector) that express tumor antigen-specific TCR along with siRNAs specific for endogenous TCR genes. T cells transduced with tumor-specific TCR with siTCR vector showed enhanced expression of transduced tumor-specific TCR while minimizing potential TCR-mispairing.18-20, 22 In the present study, we evaluated whether allogeneic T cells transduced with the siTCR vector system would show anti-tumor activity with reduced potential to induce graft-versus-host disease (GVHD). We also evaluated the effect of human leukocyte antigen (HLA) class I knockdown on the T cells transduced with siTCR vector to avoid recognition by allogeneic CD8+ T cells.
2 MATERIALS AND METHODS
2.1 Sample collection
PBMCs were isolated from healthy donors who provided informed consent. The study protocol was approved by the clinical research ethics committee of Nagasaki University Hospital. PBMCs were cultured in GT-T551 culture medium (Takara Bio) supplemented with 0.6% autologous plasma, 0.2% human serum albumin, and 600 IU/mL interleukin-2.
2.2 Retroviral and lentiviral transduction
We created a retroviral vector encoding NY-ESO-1-specific TCRα and TCRβ genes, including siRNA constructs that specifically downregulated endogenous TCR (siTCR retroviral vector; MS3-NY-ESO-1-siTCR). The vector includes DNA encoding TCR-α and TCR-β chains specific for NY-ESO-1157-165 and HLA-A*02:01; the Gly50 and Ala51 residues in the CDR2 region of the TCR-β chain were replaced with Ala and Glu, respectively, resulting in a reduction in the Kd from 21.4 mM for the wild type to 1.9 mM.21 The coding sequences of the TCR-α and -β transgenes are codon-optimized and therefore resistant to siRNA against endogenous TCR.18 Human lymphocytes transduced with MS3-NY-ESO-1-siTCR showed NY-ESO-1-specific cytotoxicity in human tumor cells.22
To target β-2 microglobulin, which is required for the expression of HLA class I, we created a guide RNA (GAGTAGCGCGAGCACAGCTAAGG) that was ligated into the pLVSIN-EF1α (Takara Bio) lentiviral vector.
PBMCs were stimulated with 30 ng/mL OKT-3(eBioscience) and 600 IU/mL interleukin-2 prior to transduction. Plates were coated with 20 μg/mL RetroNectin (Takara Bio) and incubated overnight at 4°C. Retroviral or lentiviral solutions were preloaded onto RetroNectin-coated plates, centrifuged at 2000 g for 2 h, and rinsed with PBS, according to the RetroNectin-bound virus infection method. The cells were then applied onto preloaded plates. We transduced viral vector twice on two consecutive days with MOI between three and five; PBMCs transduced with the retroviral or lentiviral vector were designated as gene-modified cells. Control PBMCs were stimulated with OKT3 in a similar manner and cultured in the same medium; these specimens were designated as unmodified cells.
2.3 Enrichment of Human leukocyte antigen-negative, CD8-positive, or CD4-positive T cells
Cells were washed with buffer (PBS containing 2% FBS) and incubated for 15 min with phycoerythrin-conjugated anti-HLA, -CD8, or -CD4 mAbs at 4°C. After two washes, the cells were incubated for 15 min with phycoerythrin microbeads at 4°C. After two more washes, the cells were passed through an LS Column (Miltenyi Biotec), and the flow-through fraction (HLA-negative cells) or non-flow through fractions (CD8-positive or CD4-positive cells) were collected for further use. All enrichment was over 98%.
2.4 Intracellular cytokine staining assay
The cells were plated at an effector-to-target ratio of 2:1 (2 × 105 effectors: 1 × 105 targets) in 200 μL of RPMI medium containing 10% FBS in a 96-well plate. The effector cells were unmodified PBMCs or gene-modified PBMCs, and the target cells were peptide-pulsed T2 cells. Next, allophycocyanin-labeled anti-CD107a antibody was added, and the plate was incubated at 37°C for 1 h before adding Golgi Stop, followed by incubation for another 4 h. Therefore, the effector cells and the target cells were co-cultured for a total of 5 h. The surface marker was stained using the following antibodies: anti-CD3 (Becton Dickinson Biosciences, Franklin Lakes, NJ, USA), anti-CD4 (BD Biosciences), and anti-CD8 (BD Biosciences). After washing with PBS, the cells were incubated in Cytofix/Cytoperm (BD Biosciences) for 20 min. After washing twice with Perm wash buffer (BD Biosciences), the cells were incubated with intracellular staining-antibodies (anti-IFNγ [BioLegend] and anti-TNFα [BioLegend]) for 30 min. After washing with fluorescence-activated cell sorting buffer, the samples were analyzed using a BD LSRFortessa flow cytometer (BD Bioscience). Data analysis was performed using FlowJo (Tree Star).
2.5 ELISA
Target cells were washed and suspended at a density of 1 × 105 cells/mL in RPMI, and then 100 μL of each target cell type was added in duplicate to a 96-well round-bottom plate. Effector T cells were washed and resuspended at 1 × 106 cells/mL in RPMI medium, and then 100 μL of T cells was combined with the target cells in the indicated wells. The plates were incubated at 37°C for 18–24 h and the supernatant was harvested. INF-γ ELISA was performed as previously described.23
2.6 Mouse studies
The animal experiments were conducted using 8–9-week-old immunodeficient non-obese diabetic/SCID/γcnull (NOG) mice. The mice were irradiated (2.5 Gy). The next day, they were injected with 2.5 × 106 NY-ESO-1-expressing human tumor cell line NW-MEL-38, followed by administration of 3.0 × 106 TCR gene-modified or unmodified cells through the tail vain; 25%–35% tetramer-positive cells were detected in the non-selected gene-modified cells. We monitored the mice for tumor growth and the development of GVHD two to three times per week. The development of GVHD was evaluated as weight loss. The study protocol was approved by the ethics committee of animal experiments at Nagasaki University.
2.7 Mixed lymphocyte reaction
We labeled allogeneic effector lymphocytes with 5 μM carboxyfluorescein succinimidyl ester (CFSE) and cultured with mitomycin C-treated target cells at an effector-to-target ratio of 1:1 (2 × 105 cells) in 200 μL of RPMI medium containing 10% FBS in a 96-well plate. After 5 days, cells were harvested and stained using the following antibodies: anti-CD3 (Becton Dickinson Biosciences), anti-CD4 (BD Biosciences), and anti-CD8 (BD Biosciences). Proliferation of effector lymphocytes were evaluated by CFSE intensity using a BD LSRFortessa flow cytometer (BD Bioscience).
2.8 Immunohistochemistry
Bone marrow tissues were harvested from NOG mice and were processed into paraffin-embedded and frozen tissue sections for hematoxylin and eosin staining. Frozen tissues were stained by anti-human CD4 (BioLegend) and anti-human CD8 (BioLegend) mAbs.
2.9 Statistical analysis
Categorical values were compared using the χ2 test. Nonparametric continuous values were compared using the Mann–Whitney U-test. Statistical significance was defined as a p-value of <0.05.
3 RESULTS
3.1 Gene-modified cells with siTCR vector showed an anti-tumor effect and reduced reactivity to allogeneic lymphocytes
We previously reported that human lymphocytes transduced with a retroviral vector encoding siRNA constructs specific to endogenous TCR reduced the expression of endogenous TCR and exhibited high surface expression of the introduced tumor-specific TCR.18-20 Human lymphocytes transduced with a high-affinity TCR specific to a cancer/testis antigen, NY-ESO-1, by a retrovirus vector with siRNA specific to endogenous TCR (siTCR vector) were reported to induce significant tumor response in patients with NY-ESO-1 expressing synovial sarcoma.22
Figure 1A shows a representative flow cytometry analysis result of NY-ESO-1-specific TCR expression in the human lymphocytes transduced with siTCR retroviral vector: MS3-NY-ESO-1-siTCR. By staining with an NY-ESO-1157-165/HLA-A*02:01 tetramer, the expression of NY-ESO-1-specific TCR was found to be 29.2% in CD4+ cells and 33.8% in CD8+ lymphocytes (Figure 1A). As this TCR showed high affinity for and bound to the tetramer in a CD8-independent manner, we concluded that the tetramer could detect TCR on CD4+ T cells.
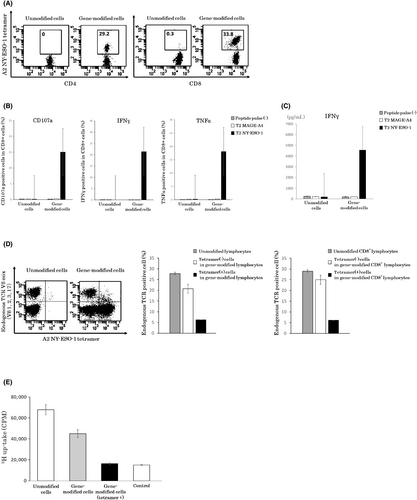
To test the tumor antigen-specific activity of these gene-modified cells, we evaluated the release of the degranulation marker CD107a and cytokine secretion in an intracellular staining assay and ELISAs. Release of CD107a and secretion of IFNγ and TNFα were observed only when siTCR gene-engineered T cells were stimulated with the NY-ESO-1 peptide, indicating specific anti-tumor activity (Figure 1B,C).
Next, we evaluated the expression of endogenous TCR in gene-modified cells. Lymphocytes were stained with an A2 NY-ESO-1-specific tetramer and endogenous TCR Vβ mixture (Vβ1, 2, 3, 17) antibody. The percentage of endogenous TCR-positive cells was approximately 30% in unmodified lymphocytes, whereas this value in gene-modified lymphocytes was reduced to approximately 20%. Furthermore, in NY-ESO-1 tetramer-positive gene-modified lymphocytes, the percentage significantly decreased to 6% (Figure 1D). This result was similar to that observed for CD8+ lymphocytes. These results indicated that the expression of endogenous TCR was downregulated in TCR gene-engineered T cells with siTCR vector.
The reactivity of siTCR gene-modified cells to allogeneic lymphocytes was examined in a thymidine uptake proliferation assay. Along with decreased expression of endogenous TCR, NY-ESO-1 tetramer-positive gene-modified cells exhibited dramatically reduced reactivity to allogeneic lymphocytes (Figure 1E).
3.2 Adoptive transfer of T cell receptor gene-modified T cells into immunodeficient non-obese diabetic/SCID/γcnull mice induced complete tumor regression without graft-versus-host disease development
To investigate whether human lymphocytes genetically engineered to express NY-ESO-1-specific TCR could inhibit the growth of NY-ESO-1-expressing human tumors, we adoptively transferred TCR gene-modified T cells into immunodeficient NOG mice. Eight- to nine-week-old irradiated NOG mice were inoculated with the NY-ESO-1-expressing HLA-A*02:01-positive human tumor cell line NW-MEL-38 and then administered TCR gene-transduced T cells. We monitored the mice for tumor growth and the development of GVHD two to three times per week. The development of GVHD was evaluated by weight loss (Figure 2A).
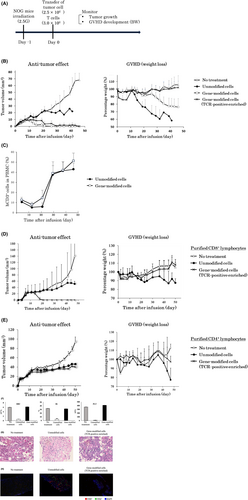
Transfer of unmodified cells failed to control the growth of human tumor cells, and lethal GVHD developed. Tumor growth was slightly suppressed in mice receiving unmodified cells compared to the untreated mice because of the unhealthy states caused by lethal GVHD. In the mouse model into which gene-modified whole cells were transferred, tumor growth and weight loss were slower than those in the untreated model. Furthermore, transfer of TCR-positive enriched cells induced complete tumor regression without GVHD development (Figure 2B). We evaluated peripheral bloods harvested from the mice after administration of human T cells. Human CD3+ lymphocytes were detectable in both the groups receiving unmodified cells and gene-modified cells, indicating that the transferred T cells resided in mice during the monitor period (Figure 2C).
We transferred purified CD8+ (Figure 2D) or CD4+ (Figure 2E) lymphocytes into a similar mouse model and evaluated the tumor size and body weight. Transfer of CD8+ TCR gene-modified cells suppressed tumor growth, whereas CD4+ TCR gene-modified cells did not. In addition, both CD8+ and CD4+ TCR gene-modified cells suppressed GVHD development. Namely, CD8+ T cells contributed to tumor rejection, whereas both CD8+ and CD4+ T cells contributed to GVHD development. The NOG mice transduced with unmodified human PBMCs developed severe anemia and thrombocytopenia on day 47. In contrast, the mice receiving TCR-positive enriched cells maintained normal red blood cell and platelets counts (Figure 2F). The bone marrow showed severe fibrosis and low cellularity in mice transferred with unmodified human PBMCs, whereas mice transferred with TCR-positive enriched cells maintained a normal bone marrow tissue structure (Figure 2G), suggesting that destruction of the bone marrow caused the anemia and thrombocytopenia. Increased infiltration of human CD4+ and CD8+ T cells were found in mice transferred with unmodified human PBMCs compared with that of mice that received TCR-positive enriched cells (Figure 2H).
3.3 β-2 microglobulin gene-edited T cells lost the capacity to stimulate allogeneic lymphocytes with retained anti-tumor activity
To suppress the expression of HLA class I molecules, we designed guide RNA constructs targeting β-2 microglobulin, which is required for the expression of HLA class I. Using a lentiviral vector containing these guide RNA constructs, we generated HLA class I-deficient T cells. We analyzed the expression of HLA in gene-edited T cells using flow cytometry. HLA-deficient cells were detected in approximately 15% of lymphocytes by staining with HLA-A, B, and C antibodies (Figure 3A). To enrich HLA class I-deficient cells, we performed bead selection and sorted HLA-deficient cells with high purity (99%; Figure 3B).
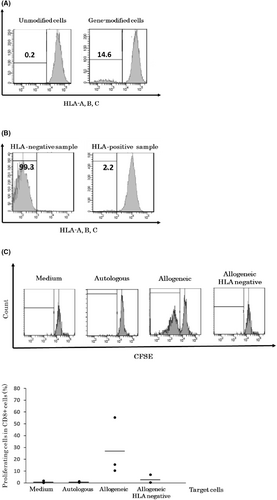
To evaluate whether HLA-deficient T cells were recognized by allogeneic lymphocytes, we labeled allogeneic effector lymphocytes with carboxyfluorescein succinimidyl ester (CFSE) and investigated the proliferation of effector lymphocytes in a mixed lymphocyte reaction (MLR). CD8+ cells were sorted as effector cells and labeled with CFSE. Autologous lymphocytes, allogeneic lymphocytes, and HLA class I-deficient allogeneic lymphocytes were evaluated. Autologous lymphocytes did not stimulate effector cells, whereas allogeneic lymphocytes stimulated 50% of the effector cell fraction. However, when stimulated with HLA-negative allogeneic lymphocytes, this reactivity decreased to the same level as that observed for autologous cells (Figure 3C). Therefore, HLA class I-deficient T cells were considered to have lost the capacity to stimulate allogeneic lymphocytes. The efficiency of β-2 microglobulin knockout was not high, which was expected due to the low titer of the lentivirus. In the MLR analysis, HLA class I-deficient cells showed reduced antigenicity, indicating that the design of the gRNA sequence was appropriate and that effective HLA class I knockout was achieved.
Next, we generated T cells deficient in endogenous TCR and HLA class I molecules by serial transduction of both viral vectors: the siTCR retrovirus vector and the lentivirus vector targeting β-2 microglobulin. We conducted flow cytometry analysis of NY-ESO-1-specific TCR expression and HLA expression. After staining with a specific tetramer, the expression of NY-ESO-1-specific TCR was approximately 40%, and the percentage of HLA-A-, B-, and C-negative cells was approximately 10% in lymphocytes (Figure 4A). NY-ESO-1-specific TCR-positive T cells deficient in endogenous TCR and HLA class I accounted for approximately 3% of cells. To enrich these T cells, we performed bead selection and sorted HLA-deficient cells.
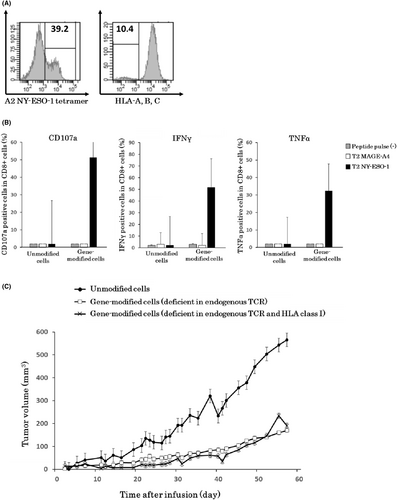
To test the anti-tumor activity of these T cells, we evaluated the release of the degranulation marker CD107a and the secretion of cytokines using an intracellular staining assay. CD107a release and IFNγ and TNFα secretion were confirmed following stimulation with NY-ESO-1 peptide, indicating that the specific anti-tumor activity had been retained (Figure 4B).
By using T cells deficient in endogenous TCR and HLA class I molecules, we evaluated the in vivo anti-tumor effect in NOG mice. T cells deficient in endogenous TCR and HLA class I showed similar in vivo anti-tumor effects as HLA class I unmodified cells in mice based on tumor growth (Figure 4C).
4 DISCUSSION
This report is the first demonstration that siTCR technology is effective to avoid GVHD. We have previously shown that siTCR technology reduced the expression of endogenous TCR and enhanced the expression of transduced tumor-specific TCR resulting in effective tumor eradication.18-20 This report showed for the first time that siTCR technology exhibits another advantage and the allogeneic T cells engineered with siTCR vector will be useful for the treatment of tumor-bearing hosts, with effective anti-tumor effects without GVHD.
T cell receptor-T cell therapy has attracted attention in treating malignancies, including solid tumors that have been challenging targets for chimeric antigen receptor (CAR)-T cells because of the limited number of ideal cell surface antigens. We targeted the NY-ESO-1 antigen, a well-known cancer/testis antigen expressed in a wide range of tumor types, including neuroblastoma, myeloma, metastatic melanoma, synovial sarcoma, bladder cancer, esophageal cancer, hepatocellular cancer, head and neck cancer, non-small cell lung cancer, ovarian cancer, prostate cancer, and breast cancer.24 The frequency of NY-ESO-1 expression varies among these tumor types, with the highest expression observed in myxoid and round cell liposarcoma (89%–100%), neuroblastoma (82%), synovial sarcoma (80%), melanoma (46%), and ovarian cancer (43%).25-30 Other tumor types express NY-ESO-1 in the range of 20% to 40%. Therefore, NY-ESO-1 is an attractive target for TCR-T cell therapy, the efficacy and safety of which have been reported in clinical trials.17
Potential drawbacks of TCR-T cell therapy include the limited efficacy associated with the inefficient surface expression of transduced TCR caused by competition for CD3 molecules between endogenous and transduced TCRs,31-34 and potential self-reactivity derived from mispairing between the introduced TCRα/β chains and the endogenous TCR chain.35 To address these issues, technologies to suppress the expression of endogenous TCR have been investigated.18, 33, 34, 36, 37 We developed retroviral vectors (siTCR vectors) encoding siRNA constructs that specifically silence endogenous TCR, resulting in improved expression of introduced TCR and minimized potential TCR-mispairing.18-20 In this study, we demonstrated that siTCR vector can also efficiently produce gene-modified cells with reduced reactivity to allogeneic lymphocytes in vitro and with lowered potential to induce GVHD in an animal model in vivo.
Allogeneic T cells with gene modification are promising candidates to create “off-the-shelf” T cell products that can be widely applied to provide an effective T cell therapy for the right patient at the right time. CRISPR gene-edited CAR-T cells that were deficient for endogenous TCR, β-2 microglobulin, and programmed death-1 (PD-1) were reported with reduced alloreactivity and GVHD potential.38 CAR-T cells targeting CD19 with TALEN-mediated gene editing of TCR α chain and CD52 were generated from non-HLA-matched donor cells and infused into two infants with relapsed refractory B cell acute lymphoblastic leukemia.39 In this trial, molecular remission was achieved within 28 days and persistence of CAR-T cells was detected in both infants, suggesting the therapeutic potential of gene-editing technology; however, the effect of gene editing of endogenous TCR in clinical use was not clear and needs further investigation. Patients’ autologous T cells with CRISPR-engineering of TCRα, TCRβ, and PD-1, along with the NY-ESO-1 TCR transgene were administered to cancer patients to test the safety and feasibility of the engineered cells in cancer immunotherapy.37 When applied to allogeneic T cells, this approach may also help to increase the number of patients who benefit from TCR-T cell therapy.
We co-transduced a retroviral vector and lentiviral vector to suppress both endogenous TCR and HLA. The introduction rate of retroviral vectors was 30%–40% and that of lentiviral vectors was 10%–20%, whereas gene-modified cells containing both vectors showed a low transduction rate of 3%–5%. Although the enriched cells deficient for both endogenous TCR and HLA class I retained anti-cancer efficacy, the low rate of co-transduction is a limitation of this approach; thus, studies are needed to produce gene-modified cells more efficiently for clinical application as an “off-the-shelf” product. We are currently developing a retrovirus vector that encodes siRNAs for both endogenous TCR and β-2 microglobulin for future study.
HLA class I was successfully knocked down in TCR-T cells in this study. However, one concern of this approach is that these TCR-T cells may trigger natural killer (NK) cell activation, resulting in rejection of the gene-modified TCR-T cells in the host. We are also currently working on inducing ligands for inhibiting the receptor of NK cells to reduce the sensitivity to NK cells for future study.
In conclusion, our study suggests that TCR-T cell therapy using allogeneic lymphocytes with suppressed endogenous TCR and HLA class I may be effective for treating cancer, and siTCR vector is a potent approach of this therapy. Evaluation of this concept in patients awaits the results from future clinical trials using allogeneic TCR-T cells with siTCR vectors. Moreover, additional modification of TCR-T cells on other molecules such as MHC class II or NK inhibitory ligand may further improve the safety and usefulness of this therapy.
AUTHOR CONTRIBUTIONS
Satomi Okada, Ayumi Kawamura, and Hiroaki Ikeda conducted the experiments. Satomi Okada and HI wrote the manuscript. Daisuke Muraoka, Kiyoshi Yasui, Isao Tawara, Satomi Okada, Junichi Mineno, Hiroshi Shiku, Hiroaki Ikeda, and Susumu Eguchi contributed to the study concept and design.
ACKNOWLEDGMENTS
Ms. Sayuri Yamaguchi (Nagasaki University) provided technical assistance with the experiments.
FUNDING INFORMATION
This research was supported by the Project for Cancer Research and Therapeutic Evaluation (P-CREATE) from the Japan Agency for Medical Research and Development (AMED, grant number 21cm0106351h0003), Grants-in Aid for Scientific Research (JSPS KAKENHI, grant number 21H02990), and a Joint Research Grant from Takara Bio Inc.
CONFLICT OF INTEREST STATEMENT
HI was provided a research grant from Takara Bio Inc. HI is an editorial board member of Cancer Science. The other authors have no conflicts of interest to declare.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: The study protocol was approved by the clinical research ethics committee of Nagasaki University Hospital.
Informed Consent: All participants provided informed consent for blood collection.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: The animal study protocol was approved by the ethics committee of animal experiments in Nagasaki University.




