Pembrolizumab monotherapy in Japanese patients with advanced ovarian cancer: Subgroup analysis from the KEYNOTE-100
Abstract
Interim results from the two-cohort, phase 2 KEYNOTE-100 study (NCT02674061) of 376 patients with previously treated advanced recurrent ovarian cancer (ROC) showed that pembrolizumab monotherapy was associated with an objective response rate (ORR) of 8.0% (95% CI, 5.4-11.2). We present outcomes for the Japanese patients (n = 21) enrolled in KEYNOTE-100. Patients with epithelial ROC had received either 1-3 prior chemotherapy lines and had platinum-free interval or treatment-free interval (PFI; TFI) of 3-12 months (cohort A) or 4-6 prior chemotherapy lines and had PFI/TFI of ≥3 months (cohort B). All patients received pembrolizumab 200 mg every 3 weeks as monotherapy for 2 years or until progression, death, unacceptable toxicity or consent withdrawal. Primary objectives were ORR per RECIST v1.1 for each cohort and higher programmed death ligand-1 (PD-L1) tumor expression. The relationship between PD-L1 expression (measured as combined positive score [CPS]) and ORR was assessed. Twenty-one Japanese patients (cohort A, n = 19; cohort B, n = 2) were treated. The median (range) age was 57 (37-78) years; 19 (90.5%) patients had ECOG status of 0 and 16 (76.2%) patients had stage III-IV disease. ORR was 19.0% (95% CI, 5.4-41.9) and seemed to increase with increasing PD-L1 expression. A total of 13 (61.9%) patients had treatment-related adverse events (TRAE), and 5 (23.8%) had grade 3-4 TRAE. There were no treatment-related deaths in this subpopulation. Pembrolizumab monotherapy was associated with antitumor activity in Japanese patients with ROC, with no new safety signals identified in this subpopulation. The data suggested a trend toward higher PD-L1 expression among some patients with higher ORR.
1 INTRODUCTION
Ovarian cancer is the third most commonly diagnosed gynecologic cancer worldwide and the second most common cause of gynecologic cancer deaths, with more than 184 000 women succumbing to ovarian cancer annually.1 In Japan, the estimated number of newly diagnosed cases of ovarian cancer was 10 438 in 2015; in 2017, 4745 deaths due to ovarian cancer marked the highest mortality rate among gynecologic cancers in Japan.2 Approximately 90% of malignant ovarian tumors are of epithelial origin. Epithelial ovarian cancer (EOC) is frequently diagnosed at an advanced stage.3 Despite response to initial platinum-based chemotherapy, most patients with advanced-stage disease will develop recurrent ovarian cancer (ROC). Subsequent lines of therapy are associated with progressively shorter disease-free intervals, and patients eventually die from their cancer.4 Treatment of ROC is based on platinum sensitivity; platinum-sensitive recurrence is defined as cancer progression at ≥6 months after platinum-based chemotherapy, and platinum-resistant recurrence is defined as progression at <6 months after platinum-based chemotherapy.5
The KEYNOTE-100 clinical trial (ClinicalTrials.gov identifier, NCT02674061) examined the antitumor activity and safety profile of pembrolizumab monotherapy in two cohorts of patients with advanced ROC.6 Patients in cohort A (n = 285) received 1-3 prior lines of treatment and had a platinum-free interval (PFI) or treatment-free interval (TFI) of 3-12 months based on the last regimen received; patients in cohort B (n = 91) received 4-6 prior lines of treatment and had a PFI/TFI of ≥3 months based on the last regimen received. The objective response rate (ORR) of single-agent pembrolizumab was 8.0% (95% confidence interval [CI], 5.4-11.2) in the combined cohorts (n = 376). Higher programmed death ligand-1 (PD-L1) expression was correlated with higher response. For patients who responded, responses were durable and typically lasted ≥6 months. The safety profile of pembrolizumab in patients with ROC was consistent with the safety profile observed in other single-agent pembrolizumab trials.
The efficacy and safety profiles of immune checkpoint inhibitors (ICI) in Japanese patients with ROC may differ from those of non–Japanese populations, potentially because of different characteristics of EOC between countries.7-10 In addition, only one clinical trial with a limited number of subjects (n = 20) describes the use of ICI in Japanese patients with ROC.11 To evaluate the potential impact of pembrolizumab monotherapy in the Japanese patient population, we performed a subgroup analysis of the 21 Japanese patients (cohort A, n = 19; cohort B, n = 2) enrolled in KEYNOTE-100.
2 MATERIAL AND METHODS
2.1 Study design and participants
The study design, patient eligibility, treatment schedule, and statistical analyses for KEYNOTE-100 were previously published.12 Patients were eligible if they were aged ≥18 years and had histologically confirmed ROC after receiving platinum-based frontline treatment and cytoreductive surgery, showed documented evidence of clinical response or disease stabilization to the last treatment regimen received, and had measurable disease based on Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1). The study enrolled two cohorts of patients: cohort A received 1-3 prior lines of treatment and had a PFI or TFI of 3-12 months based on the last regimen received, and cohort B received 4-6 prior lines of treatment and had a PFI or TFI of ≥3 months based on the last regimen received. Platinum-free interval was defined as the time that elapsed between the last dose of platinum-based treatment until documented evidence of disease progression per RECIST v1.1. Pembrolizumab 200 mg was administered intravenously every 3 weeks for 35 administrations (approximately 2 years) or until disease progression as assessed by the investigator, unacceptable toxicity or patient withdrawal of consent. Imaging-based disease assessment and testing of serum cancer antigen-125 (CA-125) were performed every 9 weeks for the first 54 weeks, and every 12 weeks thereafter.
2.2 Outcomes
The primary outcomes were the ORR as assessed per RECIST v1.1 by blinded independent central review (BICR) in both cohorts and by PD-L1 expression level. Secondary outcomes included progression-free survival (PFS), overall survival (OS), disease control rate (DCR), duration of response (DOR) and safety.
An immunohistochemistry assay (PD-L1 IHC 22C3 pharmDx; Dako North America) was used to assess PD-L1 expression from archival tumor tissue samples. The measure of expression was the combined positive score (CPS), defined as the number of PD-L1-stained cells (tumor cells, lymphocytes and macrophages) divided by the total number of viable tumor cells × 100.13
2.3 Statistical analyses
All efficacy and safety analyses were performed for all of the patients as the treated population. For ORR, the point estimate and 95% CI were estimated using an exact binomial distribution. Patients without response data were considered nonresponders. For PFS and OS, Kaplan-Meier curves, median estimates, and survival at 6, 12 and 18 months based on the Kaplan-Meier curves (95% CI based on Greenwood’s formula) were provided. Patients without efficacy or survival data were censored at day 1. Safety and tolerability were assessed by clinical review of all relevant parameters, including adverse events (AE), laboratory tests and vital signs. No statistical tests were performed for the Japanese subgroup analyses due to the nature of ad hoc analysis.
2.4 Ethical approval
Written informed consent was obtained from all participants before screening. The study protocol and informed consent form were reviewed by the independent ethics committee or the institutional review board for each center. This study was conducted according to the ethical principles of the Declaration of Helsinki.
3 RESULTS
Overall, 376 patients with ROC were enrolled in the KEYNOTE-100 trial, including 21 Japanese patients (5.6% of the study population). Nineteen Japanese patients were allocated to cohort A and 2 patients to cohort B. As of the data cutoff date of 2 February 2018, the median (range) follow up was 16.9 (8.5-18.5) months in the overall study population, in which 15 patients in cohort A and 6 patients in cohort B were still receiving study treatment, including 2 Japanese patients in cohort A and no Japanese patients in cohort B.
3.1 Demographics and baseline characteristics
The median (range) age was 61 (25-89) years for the overall population and 57 (37-78) years for Japanese patients (Table 1). More than half of the Japanese patients received ≥2 prior lines of advanced-stage treatment, although 10 out of 21 patients had just 1 prior line of therapy. Most patients had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0. The breakdown of histology was different in the Japanese and overall study populations, with the Japanese population having fewer cases of high-grade serous ROC and more cases of endometrioid and clear-cell ROC. Platinum sensitivity profiles and PFI/TFI were generally consistent with those of the overall study population, providing the modest shift to longer PFI/TFI in the Japanese subpopulation (Table 1).
| Characteristic, n (%)a | Overall study population (N = 376) | Japanese subpopulation (n = 21) |
|---|---|---|
| Age, median (range), y | 61 (25-89) | 57 (37-78) |
| <65 y | 140 (37.2) | 17 (81.0) |
| ECOG performance status | ||
| 0 | 242 (64.4) | 19 (90.5) |
| 1 | 134 (35.6) | 2 (9.5) |
| FIGO cancer stage | ||
| I-IIIB | 71 (18.9) | 5 (23.8) |
| IIIC-IV | 299 (79.5) | 16 (76.2) |
| Histology | ||
| High-grade serous | 283 (75.3) | 14 (66.7) |
| Endometrioid | 28 (7.4) | 3 (14.3) |
| Low-grade serous | 21 (5.6) | 1 (4.8) |
| Clear cell | 19 (5.1) | 3 (14.3) |
| Otherb | 25 (6.6) | 0 (0) |
| PD-L1 expression | ||
| CPS < 1 | 141 (37.5) | 3 (14.3) |
| CPS 1 to <10 | 115 (30.6) | 6 (28.6) |
| CPS ≥ 10 | 82 (21.8) | 2 (9.5) |
| Unknown | 38 (10.1) | 10 (47.6) |
| Number of lines of prior therapy | ||
| 1 | 85 (22.6) | 10 (47.6) |
| 2 | 121 (32.2) | 4 (19.0) |
| 3 | 79 (21.0) | 5 (23.8) |
| 4 | 42 (11.2) | 1 (4.8) |
| ≥5 | 49 (13.0) | 1 (4.8) |
| PFI/TFI | ||
| 1-3 mo | 31 (8.2) | 1 (4.8) |
| 3-6 mo | 176 (46.8) | 7 (33.3) |
| 6-12 mo | 150 (40.0) | 12 (57.1) |
| >12 mo | 19 (5.1) | 1 (4.8) |
| Platinum sensitivity | ||
| Platinum refractory | 4 (1.1) | 0 (0) |
| Platinum resistant | 141 (37.5) | 7 (33.3) |
| Partial platinum sensitive | 128 (34.0) | 10 (47.6) |
| Platinum sensitive | 18 (4.8) | 1 (4.8) |
| Other | 85 (22.6) | 3 (14.3) |
- CPS, combined positive score; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Obstetrics and Gynecology; PD-L1, programmed death ligand-1; PFI, platinum-free interval; TFI, treatment-free interval.
- a Unless otherwise noted.
- b Not specified as low-grade or high-grade serous or listed as papillary serous; unclassified or listed as adenocarcinoma or carcinoma; transitional.
3.2 Efficacy
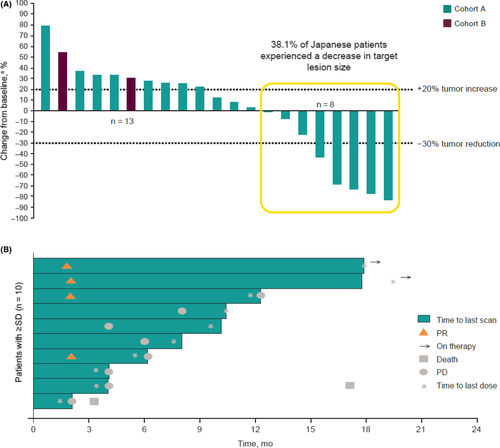
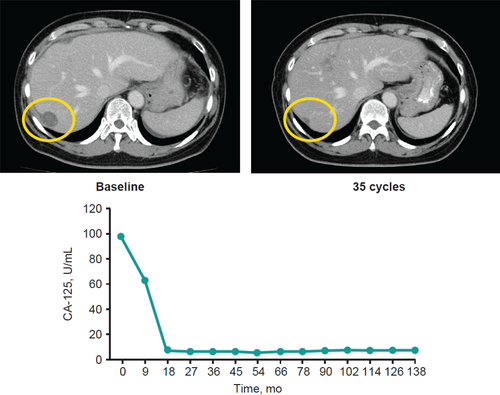
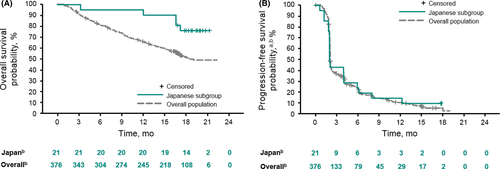
In the Japanese subgroup, the ORR was 19.0% (95% CI, 5.4-41.9) and the DCR was 47.6% (95% CI, 25.7-70.2) with no complete responses (Table 2). Figure 1A shows the best percentage change from the baseline in target lesion size per patient. Out of 21 patients, 8 (38.1%) experienced a decrease in target lesion size. For responders in the Japanese subgroup (n = 4), the median (range) time to response was 2.1 (1.9-2.1) months, and the median DOR was not reached at the data cutoff date on 2 February 2018. Three of four responses lasted >6 months (Figure 1B). On the data cutoff date, 2 of the 4 responses were ongoing, both of which were partial responses (PR) for more than 16 months. One of the two durable responders was a 71-year-old patient with high-grade serous ROC who had 1 prior line of therapy. The measurable target lesion was located on the chest wall with metastases to liver, intestine and lymph nodes as nontarget lesions. The patient has maintained a PR for >2 years without experiencing a tumor relapse. The other long-term responder was a 56-year-old woman who underwent 3 lines of prior therapy; at baseline, she had clear-cell ROC along with para-aortic lymph node metastasis, multiple liver metastases and peritoneal dissemination with ascites. The ascites disappeared 27 weeks after pembrolizumab administration with a dramatic decrease of CA-125 (Figure 2). The patient was followed as an outpatient for more than 3 years after the administration of pembrolizumab and has maintained a PR. For the Japanese subpopulation, median OS was not reached (NR) at the data cutoff date (95% CI, NR-NR; Figure 3A) and median PFS was 2.1 months (95% CI, 2.1-4.1; Figure 3B).
| Overall study population (N = 376) | Japanese subpopulation (n = 21) | |
|---|---|---|
| ORR, % (95% CI) | 8.0 (5.4-11.2) | 19.0 (5.4-41.9) |
| DCR, % (95% CI) | 37.2 (32.3-42.3) | 47.6 (25.7-70.2) |
| Best overall response, n (%) | ||
| Complete response | 7 (1.9) | 0 (0.0) |
| Partial response | 23 (6.1) | 4 (19.0) |
| Stable disease | 110 (29.3) | 6 (28.6) |
| Progressive disease | 215 (57.2) | 11 (52.4) |
| Responders, n (%) | 30 (7.9) | 4 (19.0) |
| Time to response, median (range), mo | 2.1 (1.8-12.3) | 2.1 (1.9-2.1) |
| Duration of response, median (range), mo | 8.2 (3.3+ to 18.6) | NR (4.1 to 16.1+) |
- DCR, disease control rate; NR, not reached; ORR, objective response rate.
The objective response rate was assessed per RECIST v1.1 by BICR in subgroups of Japanese patients (Figure S1). Based on PD-L1 status, ORR was 0.0% (95% CI, 0.0-70.8) in patients with CPS < 1 (n = 3), 16.7% (95% CI, 0.4-64.1) in patients with CPS 1 to <10 (n = 6) and 50.0% (95% CI, 1.3-98.7) in patients with CPS ≥ 10 (n = 2). Based on histology subgroup, ORR was 33.3% (95% CI, 0.8-90.6) in patients with the clear-cell subtype (n = 3), 0% (95% CI, 0.0-70.8) in patients with the endometrioid subtype (n = 3), 21.4% (95% CI, 4.7-50.8) in patients with the high-grade serous subtype (n = 14), and 0.0% (95% CI, 0.0-97.5) in the patient with the low-grade serous subtype (n = 1).
3.3 Safety
Table 3 summarizes the safety profile in the Japanese subgroup. A total of 13 patients (61.9%) experienced ≥1 treatment-related AE (TRAE) of any grade that was observed in at least 2 patients, and 5 patients (23.8%) experienced ≥1 grade 3 or 4 TRAE. There were no treatment-related deaths in the Japanese subgroup. At the time of the data cutoff date, no Japanese patients discontinued because of a TRAE. Immune-mediated AE occurred in 4 Japanese patients (19.0%); the most common were grade 1 hyperthyroidism in 3 patients (14.3%) and grade 1 hypothyroidism in 2 patients (9.5%). The most common immune-mediated AE of grade 3 to 4 severity, observed in 1 patient each, were grade 3 severe skin toxicity and grade 4 nephritis (Table S1).
| Adverse events, n (%) | Any grade | Grade 3-4 |
|---|---|---|
| Any | 13 (61.9) | 5 (23.8) |
| Nausea | 3 (14.3) | 0 |
| Pyrexia | 3 (14.3) | 0 |
| Rash | 3 (14.3) | 0 |
| Alanine aminotransferase increased | 2 (9.5) | 0 |
| Amylase increased | 2 (9.5) | 2 (9.5) |
| Deep vein thrombosis | 2 (9.5) | 0 |
| Hyperthyroidism | 2 (9.5) | 0 |
| Hypothyroidism | 2 (9.5) | 0 |
| Malaise | 2 (9.5) | 0 |
| Peripheral sensory neuropathy | 2 (9.5) | 0 |
| Pulmonary embolism | 2 (9.5) | 0 |
| Rash maculo-papular | 2 (9.5) | 1 (4.8) |
| Weight decreased | 1 (4.8) | 1 (4.8) |
| Proteinuria | 1 (4.8) | 1 (4.8) |
| Embolism venous | 1 (4.8) | 1 (4.8) |
| Vena cava embolism | 1 (4.8) | 1 (4.8) |
- a n = 21.
4 DISCUSSION
The present study evaluated the efficacy and safety of pembrolizumab monotherapy in a Japanese subgroup of patients with advanced ROC enrolled in the KEYNOTE-100 clinical trial, which is currently the largest study of a single-agent ICI for ROC reported. Although we could not draw a definite conclusion because this was an ad hoc analysis with a limited sample size, the antitumor activity and safety profile observed in the Japanese subgroup appeared to be comparable with those of the overall study population.
The distribution pattern of histological subtypes in the Japanese subgroup was potentially distinct from the overall study population. Clear-cell carcinoma (CCC) of the ovary accounts for 5%-25% of all EOC cases across geographic locations; in North America and Europe, 1%-12% of EOC cases are of the CCC subtype.8 In contrast, in Japan, ovarian CCC accounts for more than 25% of EOC cases and has increased significantly in the last few decades.8-10, 14, 15 This may be correlated with the increased incidence of endometriosis in Japan.10, 16 Although advanced stage/recurrent CCC is reported to have a worse prognosis than the more common serous adenocarcinoma due to an intrinsic resistance to platinum-based chemotherapy,7-9 a series of clinical trials, including KEYNOTE-100, suggest the potential efficacy of ICI for CCC.11, 12, 17, 18 In the KEYNOTE-100 study, there was a trend toward improved response rates in the clear-cell histology subtype, with an ORR of 15.8%.12 Similar to the overall population, the clear-cell subtype appeared to be associated with a higher ORR (33.3%) in the Japanese subpopulation, although the results of subgroup analyses in the Japanese population should be interpreted with caution because of the small sample size and widely overlapping confidence intervals. Hamanishi et al examined the safety and antitumor activity of single-agent nivolumab in a phase 2 clinical trial that enrolled 20 Japanese patients with platinum-resistant ovarian cancer11; the ORR was 15%, with 2 patients experiencing a durable complete response, including 1 who had clear-cell histology and was followed up with no evidence of disease (written communication with J. Hamanishi, MD, PhD, July 2019). In the present subgroup analyses, 3 Japanese patients had histologically clear-cell subtype, including 1 patient who maintained a PR more than 12 months after the completion of pembrolizumab administration; the remaining 2 patients with clear-cell ROC had progressive disease within 10 weeks of starting pembrolizumab.
Clear-cell carcinoma is distinct from other histological types of EOC, with frequent genetic/epigenetic alterations of genes such as PIK3CA and ARID1A. Indeed, CCC has a specific immune-related molecular profile and epidemiologic associations with ethnicity and endometriosis.10, 19 Intriguingly, CCC is a major histologic subtype of renal cell carcinoma, with remarkable multifaceted mutational similarities between endometrial and renal CCC.20-22 It is reported that loss-of-function mutations of PBRM1, a subunit of the switch-sucrose nonfermentable (SWI/SNF) chromatin-remodeling complex, was associated with clinical benefit for ICI in clear-cell renal carcinoma; this is supported by other reports showing that lower expression of PBRM1 and ARID2 was correlated with higher cytotoxic activity contributed by CD8 T cells in human cancers and that Pbrm1-deficient murine tumor cells were more sensitive to T-cell-mediated cytotoxicity.23, 24 Furthermore, ARID1A deficiency was reported to be a potential predictor of response to ICI because ARID1A interacts with mismatch repair protein MSH2, which suggests that the impaired interaction of ARID1A with MSH2 would result in increased microsatellite instability seen in a variety of cancer genomes.25 Taken together, because ARID1A is also a key component of the SWI/SNF complex, one may expect a potential benefit in treating ovarian CCC with ICI, even in the frontline setting, considering the promising results from immune checkpoint blockade monotherapy in renal CCC.26
It is important to recognize the limitations of pembrolizumab monotherapy. Single-agent immune checkpoint blockade trials for patients with ROC with the majority of other histological subtypes have demonstrated only modest ORR, including in KEYNOTE-028 and KEYNOTE-100.11, 27-30 A number of clinical trials are exploring ICI in combination with other ovarian cancer therapies, including chemotherapy, poly (ADP-ribose) polymerase inhibitors, antiangiogenics and other biologics, to enhance the effect of ICI in a variety of settings.30 Even focusing on the CCC subtype, combinations of ICI with agents targeting angiogenesis will be of high interest considering the favorable outcomes observed in phase 3 clinical trials of patients with previously untreated advanced renal-cell carcinoma.31, 32
Similar to the overall population, there appeared to be a potential trend for higher ORR in Japanese patients with greater PD-L1 expression as measured by CPS, although definitive conclusions cannot be made because only 11 Japanese patients had samples evaluable for PD-L1 expression. The expression of PD-L1 on tumor-infiltrating lymphocytes and tumor cells is reportedly linked with clinical outcomes in high-grade serous ovarian cancer and ovarian CCC.18, 33, 34 This might partially explain the results of the ORR analyses from KEYNOTE-100 for the entire population, in which there was a trend of higher response rates in both the high-grade serous (ORR, 8.5%) and clear-cell carcinoma (ORR, 15.8%) subtypes, whereas the endometrioid carcinoma and low-grade serous subtypes both had poor ORR of 0%.12 Although the level of PD-L1 expression by CPS can be a predictive biomarker of ORR, in light of a dualistic model of epithelial ovarian carcinogenesis (type I and type II tumors), which has become more complex in the era of next-generation sequencing, further analyses with larger samples are warranted to elucidate biomarkers that may be responsive to immune checkpoint blockade therapy.35, 36 In the overall population, clinical features such as number of lines of prior treatment, PFI/TFI and level of platinum sensitivity did not appear to influence the ORR of single-agent pembrolizumab6; we observed similar trends in the Japanese subpopulation, although no definite conclusion can be drawn due to the small sample size (Figure S1). There was a favorable trend for OS in the Japanese subpopulation, which might reflect the fewer prior lines of therapy and slightly longer PFI/TFI profiles (6-12 months) as well as the better ECOG performance status compared with the overall population.
No new safety signals were identified in the Japanese subgroup, with an overall safety profile consistent with that of the overall population of KEYNOTE-100. However, there were immune-mediated AE that warrant consideration. Although the use of pembrolizumab in Japan for treating advanced/recurrent microsatellite instability-high (MSI-H) solid tumors was granted in December 2018,37 MSI-H tumors have been observed in less than 2% of ovarian cancers (no samples with MSI-H were detected out of 319 paired samples tested for MSI status in the KEYNOTE-100 study12).38 Thus, a comprehensive assessment of immune-mediated AE in women with ovarian cancer has not been established together with the limited number of clinical trials. As such, gynecologic oncologists should follow published algorithms and consensus guidelines to ensure there is vigilant monitoring for the management of immune-mediated AE.28, 30
In the overall KEYNOTE-100 study, 6 patients experienced pneumonitis (1.6%) by the data cutoff date, 1 of which was grade 3-4 in severity. Although no cases of pneumonitis were reported in the Japanese subgroup, it is a life-threatening immune-mediated AE that requires immediate action by healthcare providers. The results from the phase 1b KEYNOTE-025 study (ClinicalTrials.gov identifier, NCT02007070) regarding efficacy and safety of pembrolizumab in 38 Japanese patients with previously treated PD-L1-expressing advanced NSCLC were recently reported.39 The most common immune-mediated AE in this study was pneumonitis (n = 4, 10.5%), including 1 fatal case (the remaining three cases resolved). It is difficult to definitively determine whether pneumonitis occurs more frequently in Japanese patients. However, given the potential risks and insufficient evidence in the gynecological field regarding immune-mediated AE, it is essential to recognize symptoms and risk factors of pneumonitis.40 Because of the multifaceted nature of immune-mediated AE and their overlap with other organ-specific toxicities, a multidisciplinary approach with specialty consultation is strongly recommended, along with patient and caregiver education for facilitating early identification of immune-mediated AE.30, 41
In summary, pembrolizumab monotherapy was associated with antitumor activity in some Japanese patients with advanced ROC, consistent with what was observed in the overall population of the KEYNOTE-100 study. There were no new safety signals in the Japanese subgroup. Given the potential for greater antitumor activity of ICI in patients with higher PD-L1 expression and in patients with specific histological subtypes of ovarian cancer (such as ovarian CCC), further investigations are warranted to clarify the role of ICI in controlling tumor progression and metastasis of ovarian cancer. Combination therapies with other agents such as antiangiogenics or PARP inhibitors should continue to be assessed in frontline and recurrent settings of advanced ovarian cancer.
ACKNOWLEDGMENTS
Support for this work was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc, Kenilworth, NJ, USA. There is no grant associated with this trial. Editorial assistance was provided by Jenna Lewis, MA, ELS, of MedThink SciCom. This assistance was funded by Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc, Kenilworth, NJ, USA.
DISCLOSURE
These authors report financial relationships for themselves: KY and MA are employees of MSD KK, and KS and SK are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc, Kenilworth, NJ, USA. KS and SK hold stock/stock options in Merck & Co., Inc, Kenilworth, NJ, USA. The study was designed under the responsibility of Merck & Co.; The study was funded by Merck & Co., Inc; pembrolizumab was provided by Merck & Co., Inc, who collected and analyzed the data and contributed to the interpretation of the study. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.




