Pattern and prognostic value of FLT3-ITD mutations in Chinese de novo adult acute myeloid leukemia
Abstract
FMS-like tyrosine kinase 3 (FLT3) is one of the most frequently mutated genes in hematological malignancies. FLT3 internal tandem duplication (FLT3-ITD) mutations located in juxtamembrane domain (JMD) and tyrosine kinase domain 1 (TKD1) regions account for two-thirds of all FLT3 mutations. The outcome of patients remains unsatisfactory, with low survival rates. It is not yet known whether the different mutations within the FLT3 gene are all associated with patient outcome. In addition, the cause of FLT3-ITD in-frame duplication events remains unknown. Although there are some published studies investigating the FLT3-ITD mutation and its clinical implications in Chinese acute myeloid leukemia (AML) patients, sample sizes tend to be small and detailed molecular profiles of FLT3 mutations are lacking in these studies. In our study, 227 FLT3-ITD sequences were analyzed from 227 Chinese de novo AML patients. ITD were next classified into 3 types based on molecular profiles of insertion DNA sequences: DNA complete duplication (type I), DNA partial duplication (type II) and complete random sequence (type III). From the 154 patients, we confirmed that high ITD allelic ratio (≥.5) and allogeneic stem cell transplant treatment under CR1 are independent prognostic factors. We also presented evidence that ITD integration sites in the hinge region or beta1-sheet region are an unfavorable prognostic factor in adult AML patients with FLT3-ITD mutations. These findings may help to decipher the mechanisms of FLT3-ITD in-frame duplication events and stratify patients when considering different therapeutic combinations.
1 INTRODUCTION
FMS-like tyrosine kinase 3 (FLT3) belongs to the class III receptor tyrosine kinase (RTK) family. FLT3 mutations have been found in 15%-35% of acute myeloid leukemia (AML) patients, making FLT3 one of the most frequently mutated genes in hematological malignancies.1, 2 FLT3 mutations consist of 2 major types: FLT3 internal tandem duplications (FLT3-ITD) and point mutations within the activation loop of the second tyrosine kinase domain (FLT3-TKD).2, 3 FLT3-ITD mutations located in juxtamembrane domain (JMD) and TKD1 regions account for two-thirds of all FLT3 mutations. Meanwhile, FLT3-ITD vary in mutant/wt ratio, number of clones and ITD insertion sites, which range from 3 to more than 400 base pairs.4-7 However, the cause of FLT3-ITD in-frame duplication events remains unknown.
FLT3-ITD mutation has been indicated as an independent variable in most of the retrospective data that confer a poor prognosis in AML.8-10 Furthermore, previous studies observed a prognostic role for the mutant/wt ratio, size, number of clones, and location of the ITD. Multiple retrospective studies consistently suggest a high mutant/wt ratio to be an independent prognostic factor.11-13 Kayser et al14 observed that patients with non-JMD ITD are associated with worse prognosis than those with JMD ITD. However, there is no published data confirming this result. Reports on the prognostic impact of ITD size or clone number are also controversial.5, 15-17 According to studies of the 2017 European Leukemia Net (ELN), NPM1-mutated AML with an FLT3-ITD allelic ratio (AR) of below .5 has a favorable prognosis, and allo-HSCT in CR1 is not actively recommended.18 In contrast, the guidelines of the National Comprehensive Cancer Network (NCCN) classify the FLT3-ITD gene mutation as a poor prognostic factor. Conflicting results are also reported in the literature about the role of FLT3-ITD gene mutation in prognosis.19-22 Thus, further studies are required to clarify these issues. Although there are some published studies investigating FLT3-ITD mutation and its clinical implications in Chinese AML patients, the sample sizes tend to be small and detailed molecular profiles of FLT3 mutations are lacking in these studies.
In the present study, we analyzed 227 ITD sequences from 227 AML patients and classified them into different types. In addition, from the 154 selected patients, we examined the insertion sites of ITD, the mutant/wt ratio of ITD, cytogenetic risk types and other related gene mutations, and correlated these variables with clinical outcome.
2 METHODS
2.1 Patients and treatment
Between July 2010 and October 2015, 1101 consecutive patients were diagnosed with de novo AML based on WHO criteria at the Department of Hematology in the First Affiliated Hospital of Soochow University. Diagnostic bone marrow samples were collected and analyzed for genetic mutation in these patients. In the present study, 154 de novo adult AML patients (18-65 years, excluding APL) with FLT3-ITD mutations were included for prognostic analysis.
Standard induction chemotherapy comprising anthracycline and cytarabine, 3 + 7 regimen and consolidation therapies were given to all 154 patients. If patients failed to achieve complete remission after 2 courses of standard induction therapy, a priming regimen or a decitabine-based regimen with or without sorafenib was given for remission reinduction. Patients with a matched related donor (MRD), a matched unrelated donor (MUD) or a haploidentical related donor were referred for allogeneic stem cell transplant (Allo-SCT) (Figure S1).
This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (2014-048). Patients’ samples were collected after informed consent in accordance with the Declaration of Helsinki.
2.2 Detection of FLT3-ITD and other gene mutations
All samples investigated in this study were obtained at the time of diagnosis. Genomic DNA was extracted from bone marrow samples according to the manufacturer's protocols (QIAGEN, Hilden, Germany). FLT3-ITD detection was performed using the method described by Meshinchi et al.8 PCR products were sequenced using an ABI 3730 automated DNA sequencer (Applied Biosystems, Foster City, CA, USA).
To detect the ITD sequences at mRNA level, RT-PCR was performed for all samples with intron-contained ITD. Screening for NPM1, DNMT3A and CEBPA mutations was performed as described elsewhere.23-25
2.3 Genescan analysis
For Genescan analysis, an ABI 3730 automated sequencer was used. PCR setup and PCR conditions were the same as described above except that PCR primer “FLT3-F” was labeled with HEX and amplified for 25 cycles here. For allele ratio calculation, we used the value of mutant/wt ratio (Figure S2), while in the case of 2 or more different FLT3-ITD mutations presenting in 1 individual patient, the allelic loads were added up.
2.4 Statistical analysis
Statistical analysis was performed with SPSS Statistics for Macintosh, Version 20.0. The χ2 or the Fisher exact test was used to compare the categorical variables, while the Mann-Whitney test was used for continuous variables. The Kaplan-Meier method with the log-rank test was used in analyzing the differences in overall survival (OS) and disease-free survival (DFS). Cox regression models were used to analyze the correlation of OS and DFS with other variables. Variables that were significantly associated with prognosis or had a P-value <.05 were added to the multivariate analysis. P-values <.05 were considered statistically significant. When cross effects occured between variables, landmark analyses were performed according to a landmark point at 6 months, with the hazard ratio (HR) calculated separately for events that occurred up to 6 months after study initiation and events that occurred between 6 months and the end of the follow-up period. We then performed a test for the interaction between variable and time (first 6 months vs subsequent period). The last observation was 19 July 2016.
3 RESULTS
3.1 Molecular characteristics and classification of FLT3-internal tandem duplication
We screened 1101 de novo AML patients for the presence of FLT3-ITD mutations, among which 227 patients (20.6%) were found to harbor 1 or more ITD mutations. From all the 227 ITD mutated patients, 285 ITD were quantified by Genescan analysis and 227 ITD sequences were analyzed by PCR and automated DNA sequencer. Interestingly, the results showed that if the ITD contain the whole intron DNA sequences, the intron sequences would be removed after the DNA transcription process. However, if the ITD have partial intron DNA sequences, the intron would be retained after DNA transcription (Figure S3). Here, 72.7% (N = 165) of the ITD were inserted in the JMD and 27.3% (N = 62) of the ITD had the insertion sites in the TKD1. The FLT3-ITD mutant to wild type ratio ranged from .010 to 75.846, with a median of .568. Interestingly, FLT3-ITD size was strongly correlated with the insertion site: the more C-terminal the location of the insertion site, the longer the size of the inserted fragment (P < .001) (Table S1 and Figure S4).
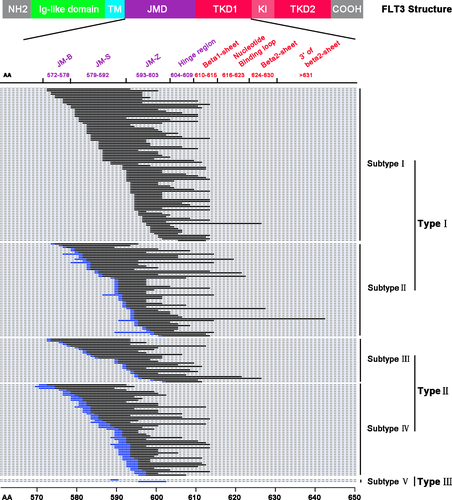
Based on the profiles of ITD sequences (genomic DNA and transcripted mRNA sequences), all the 227 ITD base sequences were divided into 3 types: DNA complete duplication (type I), DNA partial duplication (type II) and complete random sequence (type III) (Figure 1). A total of 145 ITD (63.9%) were classified into type I mutations, and they can be subdivided into triplet codon complete duplication (subtype I) and triplet codon partially duplication (subtype II) due to changed triplet codon in the insertion site or mRNA sequences containing partial intron sequences. Type II mutations consisted of 80 ITD and can also be subdivided into 2 subgroups according to mRNA similarity with template sequences: 1 codon difference (subtype III) and more than 1 codon difference (subtype IV). Interestingly, 2 ITD showed no similarity with adjacent DNA template sequences, which were categorized as type III or subtype V. Y591-Y599 tyrosine-rich amino acid stretch plays a particular role in intracellular signaling pathways;26 among the 227 analyzed ITD sequences, 206 of them (90.3%) involved at least 1 residue in this specific motif and 68 (30%) of them including this whole motif; 21 ITD sequences were not involved in any amino acid of this specific motif (Figure 1).
3.2 Clinical significance of FLT3-internal tandem duplication mutant level
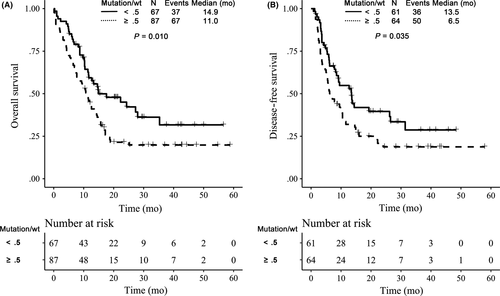
The clinical data of the 154 de novo adult AML patients (18-65 years, excluding APL) with FLT3-ITD mutations are given in Table 1. The average duration of follow-up time was 22.4 months, and the minimum follow-up time was 5.2 months, respectively. The last observation was 19 July 2016. To analyze the impact of the FLT3 mutant/wt ratio, patients were grouped according to a FLT3 allelic ratio (AR) below or above the cut-off value of .5, identified as low (<.5) and high (≥.5) mutant level, respectively. Based on the analysis, patients with high mutant level had significantly worse OS than those with a low mutant level (19.8% vs 31.7%) (P = .010) (Figure 2A). After induction therapy, 125 of 154 patients achieved complete remission (CR), and were included in the analysis for DFS. The estimated DFS rates at 3 years for high and low mutant level groups were 18.7% vs 28.7%, respectively (P = .035) (Figure 2B).
| Variables | Median (Min-Max)/N(%) |
|---|---|
| Mutation/wt | .54 (.01-75.85) |
| Localization of ITD integration | |
| JM-S or JM-Z | 83 (53.90%) |
| Hinge region | 27 (17.53%) |
| Beta1-sheet | 35 (22.73%) |
| Other-TKD1 | 9 (5.84%) |
| Median age, y (range) | 46 (19-65) |
| Sex | |
| Female | 77 (50.0%) |
| Male | 77 (50.0%) |
| Marrow blast (range, %) | 73.0 (21.5-97.5) |
| Median WBC count, ×109/L (range) | 52.7 (1.0-441.5) |
| Median HB level, g/L (range) | 84 (36-147) |
| Median PLT count, ×109/L (range) | 48 (4-587) |
| Cytogenetic risk group | |
| Favorable risk | 12 (7.79%) |
| Intermediate risk | 129 (83.77%) |
| High risk | 13 (8.44%) |
| NPM1 | |
| Negative | 81 (52.60%) |
| Positive | 73 (47.40%) |
| CEBPA | |
| Negative | 136 (88.31%) |
| Single | 9 (5.84%) |
| Double | 9 (5.84%) |
| DNMT3A | |
| Negative | 120 (77.92%) |
| Positive | 34 (22.08%) |
| Allo-HSCT under CR1 | |
| No | 109 (70.78%) |
| Yes | 45 (29.22%) |
- CEBPA, CCAAT/enhancer binding protein alpha; DNMT3A, DNA methyltransferases 3A; HB, hemoglobin; ITD, internal tandem duplication; NPM1, nucleophosmin 1; PLT, platelet; WBC, white blood cell.
3.3 Clinical significance of FLT3-internal tandem duplication insertion site
Internal tandem duplication insertion sites were divided into JMD and TKD1 subgroups to evaluate the impact of the FLT3-ITD insertion site on clinical outcome. JMD subgroup patients (29.5%) achieved a better OS rate at 3 years compared to the TKD1 subgroup (16.6%) (P = .038) (Figure 3A) (Table S2). The difference of DFS rate at 3 years for JMD and TKD1 subgroups was not statistically significant (P = .130) (Figure 3B). Because the DFS curves crossed, we selected 6 months as a cut-off point and ran landmark analysis. From the results, JMD subgroup patients (66.0%) achieved a better DFS rate within 6 months compared to the TKD1 subgroup (43.8%) (P = .006). However, after 6 months, the difference of DFS rate for JMD and TKD1 subgroups was not statistically significant (P = .430) (Figure 3C).
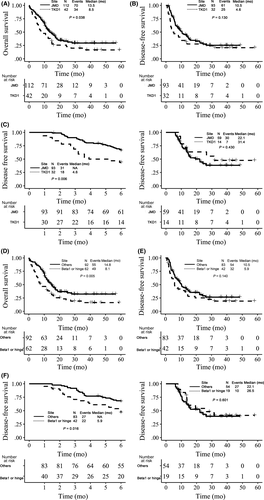
Then, based on functional region, ITD insertion sites were divided into 4 subgroups: JM-S or JM-Z, hinge region, beta1-sheet, and other TKD1. The results showed that patients with ITD insertion sites in the hinge region or the beta1-sheet region had a significantly worse OS rate at 3 years than patients with ITD in other regions (P = .005), with estimated OS rates of 16.4% and 32.4%, respectively (Figure 3D). Although patients with ITD insertion sites in the hinge region or the beta1-sheet region had an inferior DFS rate at 3 years compared with patients with ITD in other regions (19.2% vs 26.6%); this difference is not statistically significant (P = .140) (Figure 3E). From the landmark analysis, within 6 months, hinge region or beta1-sheet subgroup patients (46.50%) achieved a worse DFS rate compared to the other region subgroup (67.2%) (P = .016), and after 6 months, the difference was not statistically significant (P = .601) (Figure 3F).
3.4 Efficacy of allogeneic stem cell transplant in FLT3-internal tandem duplication mutated acute myeloid leukemia
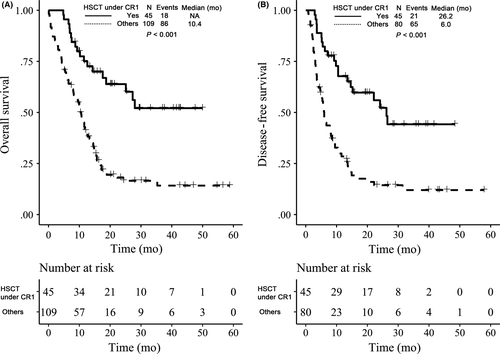
To determine the efficacy of Allo-SCT in patients with FLT3-ITD, patients were divided into Allo-SCT under CR1 (N = 45) and others subgroups (N = 109). The results revealed that patients with Allo-SCT under CR1 achieved significantly better OS and DFS rates at 3 years compared to the others subgroup (P < .001; P < .001), with the estimated OS and DFS 52.1% vs 14.2% and 44.2% vs 12.0%, respectively (Figure 4A, B).
3.5 Efficacy of NPM1 mutation on FLT3-internal tandem duplication mutated acute myeloid leukemia
To analyze the prognosis of NPM1-mutated AML with FLT3-ITD low AR, patients were divided into 3 subgroups according to the 2017 ELN recommendations: a low-risk subgroup (NPM1MUT with FLT3-ITD low AR), a medium-risk subgroup (NPM1WT with FLT3-ITD low AR or NPM1MUT with FLT3-ITD high AR) and a high-risk subgroup (NPM1WT with FLT3-ITD high AR). The OS and DFS rates at 3 years were 23.1% vs 34.1% vs 8.7% and 22.8% vs 28.8% vs 13.1%, respectively, and the difference was statistically significant (P = .014, P = .013) (Figure 5A, B). However, the prognosis of NPM1-mutated AML with FLT3-ITD low AR (low-risk subgroup) was similar to that of the medium-risk subgroup (NPM1WT with FLT3-ITD low AR, or NPM1MUT with FLT3-ITD high AR).
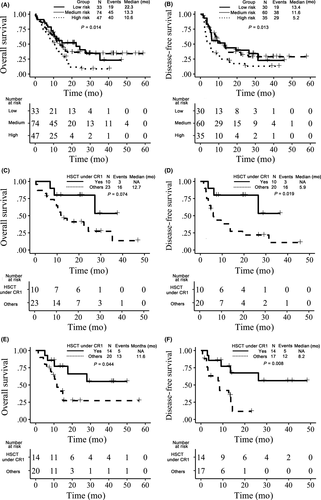
To further analyze the effect of Allo-SCT under CR1 for the NPM1-mutated AML patients with FLT3-ITD low AR, 67 patients with FLT3-ITD low AR were picked out, 33 of which were mutated on NPM1 gene. From the analysis, NPM1-mutated patients who achieved Allo-SCT under CR1 had better DFS rates (53.3% vs 10.9%) at 3 years (P = .019); however, the difference of OS rates (53.3% vs 13.6%) was not statistically significant (P = .074) (Figure 5C, D). The NPM1WT patients achieved better OS (55.1% vs 27.3%) and DFS (56.3% vs 11.7%) than other patients (P = .044, P = .008) (Figure 5E, F).
3.6 Prognostic factors
Prognostic factors were evaluated for OS and DFS using Cox regression models. In the univariate model, age was another significant factor for worse OS rate with HR of 1.02 (P = .027). High cytogenetic risk was a significant factor for worse OS rate with an HR of 4.17 (P = .009), but it was not statistically significant for DFS rate (P = .064). The presence of NPM1 mutations or CEBPA mutations or DNMT3A, Sex, and LogWBC were not associated with clinical outcome in this univariate model (Table S3). In the multivariate model, a high FLT3-ITD mutant/wt ratio and high cytogenetic risk group were still independent prognostic factors for an inferior outcome for OS and for DFS in different models (P < .05). Furthermore, Allo-SCT under CR1 was an independent prognostic factor for better OS and DFS rates in different models (P < .05). For OS and DFS rates, FLT3-ITD located in beta1-sheet or hinge regions was worse than in other regions; however, the differences were not significant (P > .05, P > .05) (Table 2).
| Overall survival | PFS time (≤6 months) | PFS time (>6 months) | P for interaction | ||||
|---|---|---|---|---|---|---|---|
| Hazard ratio (95%CI) | P-value | Hazard ratio (95%CI) | P-value | Hazard ratio (95%CI) | P-value | ||
| Model 1 | |||||||
| Mutation/wt (≥.5 vs <.5) | 1.70 (1.13-2.56) | .011 | 1.98 (1.09-3.58) | .025 | 1.30 (.67-2.51) | .438 | |
| Localization of ITD integration (TKD1 vs JMD) | 1.26 (.82-1.92) | .294 | 2.05 (1.13-3.71) | .018 | .50 (.21-1.20) | .122 | .011 |
| Age (continuous variable) | 1.01 (.99-1.02) | .616 | — | — | |||
| Cytogenetic risk group | |||||||
| Favorable risk | 1.0 | 1.0 | 1.0 | ||||
| Intermediate risk | 1.82 (.73-4.55) | .201 | 4.44 (.61-32.59) | .143 | 1.69 (.58-4.89) | .335 | |
| High risk | 3.52 (1.16-10.64) | .026 | 3.39 (.34-34.01) | .300 | 5.65 (1.30-24.47) | .021 | |
| HSCT under CR1 (yes vs no) | .34 (.20-.58) | <.001 | .27 (.13-.59) | .001 | .35 (.18-.71) | .003 | |
| Model 2 | |||||||
| Mutation/wt (≥.5 vs <.5) | 1.72 (1.14-2.59) | .010 | 1.92 (1.06-3.49) | .032 | 1.30 (.67-2.53) | .442 | |
| Localization of ITD integration (beta1-sheet and/or hinge region vs others) | 1.48 (1.00-2.20) | .052 | 1.66 (.94-2.93) | .083 | .73 (.34-1.56) | .415 | .085 |
| Age (continuous variable) | 1.01 (.99-1.03) | .498 | — | — | |||
| Cytogenetic risk group | |||||||
| Favorable risk | 1.0 | 1.0 | 1.0 | ||||
| Intermediate risk | 1.67 (.66-4.21) | .276 | 4.53 (.62-33.29) | .138 | 1.52 (.53-4.39) | .439 | |
| High risk | 3.37 (1.12-10.11) | .030 | 4.14 (.42-41.02) | .224 | 4.71 (1.11-20.08) | .036 | |
| HSCT under CR1 (yes vs no) | .35 (.21-.59) | <.001 | .28 (.13-.59) | <.001 | .39 (.20-.77) | .007 | |
- ITD, internal tandem duplication; PFS, progression-free survival.
4 DISCUSSION
As most frequently mutated gene in AML, FLT3 and its activated mutations have been systematically studied over the past 20 years. Studies related to FLT3-ITD are focused on the cause of these duplication events, signaling pathways involved in pathogenesis, molecular characterization and correlation with clinical outcome and the potential as a drug target. In the present study, we analyzed 227 ITD sequences from 227 AML patients and classified them into different types. In addition, from the 154 patients, we confirmed that high mutant/wt ratio and Allo-SCT treatment under CR1 were independent prognostic factors. Moreover, we identified that ITD integration sites in the hinge region or beta1-sheet region appear to be an unfavorable prognostic factor in adult AML patients with FLT3-ITD mutations.
The cause of FLT3-ITD in-frame duplication events remains unknown. To decipher the mechanisms, it is important to characterize the ITD molecular profiles, such as the duplication pattern, the size and the insertion site). In this paper, we found that when ITD contained the whole intron in genomic DNA, the whole intron was removed after transcription, which was confirmed by PCR and sequencing detection. However, when ITD contains part of the intron, the partial intron would remain during DNA transcription (Figure S3). Although some of study groups also used cDNA as a detection template,7, 27, 28 they did not claim to attain different results compared with genomic DNA template. For the first time, we observed this intron transcription rule during ITD duplication events. Moreover, we found that there are 3 patterns of ITD. Based on the profiles of ITD sequences, we divided all the identified ITD into 3 types and 5 subtypes (Figure 1).
The molecular characteristics of ITD, such as mutant/wt ratio and insertion sites, are considered to correlate with clinical outcome in previous reports. In a large retrospective study of adult AML patients with FLT3-ITD mutations, patients with a high mutant/wt ratio had significantly shorter OS and DFS.4 Subsequent retrospective studies of pediatric de novo AML patients showed worse prognosis with mutant/wt ratio higher than .4 or .51.12,13,28 In this paper, we confirmed that patients with mutant/wt ratio of higher than .5 had significantly worse OS and DFS based on multivariate models. Therefore, the mutant/wt ratio as an independent variable is recommended for use in clinical trials for adult AML patients in the future. It is worth mentioning that there is another allele ratio calculation method used by other groups (such as Gale et al15), mutant/(mutant+wt), caution should be used in comparing the results. Following Breitenbuecher and colleagues demonstrating that nearly one-third of ITD integrate into TKD1 but not all in JMD, the functional and clinical relevance of ITD locations were determined by study groups. Kayser et al14 observed that adult AML patients with an ITD in the beta-1 sheet had significantly inferior OS and DFS compared to those with ITD located in other regions. However, Blau's group29 did not find significantly worse clinical outcomes in patients with non-JMD ITD mutations. According to our results, 27.3% of ITD were located in TKD1, and patients with ITD in TKD1 showed obvious worse OS than ITD in JMD group (P = .038). This result was consistent with results of previous studies. When we performed a landmark analysis, we found that the impact of different ITD insertion sites (JMD vs TKD1) on DFS was more different during the 6 months after study initiation, with interactions between time and insertion site, suggesting that the benefit of JMD on DFS or longer remission duration may disappear with an increasing duration of follow-up.
After we divided JMD and TKD1 domains into different functional regions, we found that ITD located in the hinge region or the beta-1 sheet region achieved inferior OS in analysis models. By landmark analysis, although there was no statistic difference, a worse trend of DFS or lower remission duration could be seen in patients with FLT3-ITD located in beta1-sheet or hinge regions, with an HR of 1.66 and .73 for 2 time periods (≤6 months vs >6 months, P for interaction = .085). It appeared that when the duration of follow-up was increased, the different effects disappeared too.
FLT3-ITD-mutated AML patients are often referred for allo-SCT in first complete remission, which is becoming the preferred therapeutic option.30 From our data, Allo-SCT under the CR1 subgroup achieved significant better OS and DFS survival and this approach can be considered as an independent favorable factor in multivariate models. Mutations of NPM1 and CEBPA are generally associated with a trend for better clinical outcomes; however, most studies indicate that this benefit is lost in the presence of FLT3-ITD.31-34 In our study, the presence of NPM1 or CEBPA mutation is not significant for OS and DFS rates. For AML cases, both with NPM1 mutant and FLT3-ITD low AR, although DFS can be improved by Allo-SCT under CR1 (P = .014), these advantages did not translate into eventual survival benefits (P = .074), which may be due to higher transplantation-related mortality (TRM) in patients after Allo-SCT. For another group of patients both with NPM1WT and FLT3-ITD low AR, not only DFS but OS benefited from Allo-SCT under CR1. Therefore, Allo-SCT under CR1 is recommended for FLT3-ITD low AR AML with NPM1WT, and decisions of transplant under CR1 need to be taken with great care for NPM1MUT AML with FLT3-ITD low AR. These results are in line with previous studies.15, 19 Because FLT3-ITD mutation causes ligand-independent constitutive kinase activation and cell survival, there has been significant interest in targeting the FLT3 receptor tyrosine kinase with small molecule inhibitors for many years.34, 35 A number of FLT3 inhibitors (like quizartinib, midostaurin or sorafenib) have been evaluated or undergone evaluation in clinical trials, and exciting responses have been achieved; on 28 April 2017, the U.S. Food and Drug administration approved midostaurin for the treatment of FLT3-mutated adult AML patients.36, 37 In our study, FLT3 inhibitors were not recommended to the patients, so we did not analyze the effects of FLT3 inhibitors on FLT3-ITD mutation-positive patients.
In summary, the results of this study demonstrate that ITD can be classified into 3 types: DNA complete duplication (type I), DNA partial duplication (type II) and complete random sequence (type III). High ITD allelic ratio (≥.5) and Allo-SCT treatment under CR1 are independent prognostic factors. ITD integration sites in the hinge region or beta1-sheet region are an unfavorable prognostic factor in adult AML patients with FLT3-ITD mutations. Taken together, these findings may help to decipher the mechanisms of FLT3-ITD in-frame duplication events and stratify patients when considering different therapeutic combinations.
5 ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (2014-048). Patients’ samples were collected after informed consent in accordance with the Declaration of Helsinki.
CONFLICT OF INTERESTS
The authors declare no competing financial interests.




