The role of sphingosine-1-phosphate in inflammation and cancer progression
Abstract
Many inflammatory mediators are involved in the process of carcinogenesis and cancer progression. In addition to cytokines and chemokines, lipid mediators have recently attracted attention as signaling molecules associated with inflammatory diseases. Sphingosine-1-phosphate (S1P) is a pleiotropic lipid mediator that regulates cell survival and migration, immune cell recruitment, angiogenesis and lymphangiogenesis. S1P also plays a significant role in inflammation and cancer. The gradation of S1P concentration in the blood, lymph and tissue regulates lymphocyte trafficking, an important component of inflammation. Furthermore, cancer cells produce elevated levels of S1P, contributing to the tumor microenvironment and linking cancer and inflammation. Future technological advances may reveal greater detail about the mechanisms of S1P regulation in the tumor microenvironment and the contribution of S1P to cancer progression. Considering the critical role of S1P in linking inflammation and cancer, it is possible that the S1P signaling pathway could be a novel therapeutic target for cancers with chronic inflammation.
1 INTRODUCTION
The link between long-term chronic inflammation in the body and cancer development is well understood.1 One example is chronic inflammatory bowel disease, such as ulcerative colitis and Crohn's disease, in which chronic inflammation increases the risk of cancer development.2 Another example of chronic inflammation is obesity,3 which not only induces the production of insulin growth factors but also induces chronic inflammation that promotes cancer progression and worsens survival.4, 5 Obesity increases the risk of breast cancer and worsens the progression of the cancer in post-menopausal women.6, 7 Substantial research has been devoted to reveal the mechanisms by which chronic inflammation affects cancer development and progression, elucidating the involvement of inflammatory mediators and inflammatory cells in these processes.8
Multiple inflammatory cells and mediators, such as cytokines and chemokines, are involved in carcinogenesis and cancer progression and are affected by chronic inflammation8 For instance, the inflammatory cytokines interleukin-6 (IL-6) and tumor necrotic factor-alpha (TNF-alpha) promote the process of carcinogenesis aggravated by inflammation.9 Inflammatory cells, such as macrophages, myeloid-derived suppressor cells, eosinophils, mast cells and neutrophils, are recruited to the cancer microenvironment and contribute to inflammation.10 By inhibiting cancer-related inflammation, the risk of cancer decreases in some patients.8 For instance, aspirin reduces the risk of colorectal cancer.11 However, until now there have been no successful treatments targeting both inflammation and cancer.
In addition to cytokines and chemokines, lipid mediators have recently attracted attention as signaling molecules associated with inflammatory diseases.12-15 The role of sphingolipids in patients has been overlooked for a long time due to the difficulty of quantifying lipids in the human body.16 Recent technological advances in measuring lipids have progressed the field of lipid research and have revealed that lipid mediators play an important role in human disease.17 Of the lipid mediators, sphingosine-1-phosphate (S1P) is a bioactive sphingolipid mediator that regulates cell survival and migration, immune cell recruitment, angiogenesis and lymphangiogenesis.18, 19 S1P also plays multiple roles in a variety of disease processes,20-23 including inflammation and cancer.24-28 Considering its synergistic effects in both inflammation and cancer, S1P likely plays a role in inflammation-associated cancers, such as colitis-associated colorectal cancer and obesity-associated breast cancer. Furthermore, interference with S1P signaling might effectively suppress cancer progression in the context of inflammation.
In this review, we introduce the bioactive lipid mediator S1P and its role in inflammation and cancer progression. Moreover, we discuss the clinical utility of S1P as a future therapeutic target for patients with inflammation-related cancer.
1.1 S1P, a bioactive lipid mediator
A quarter of a century ago, Sarah Spiegel discovered S1P as a lipid mediator, which acts as a signaling molecule in the cell.29, 30 Since then, molecules that regulate S1P have been described, including S1P synthetic enzymes, receptors and degrading enzymes, all of which regulate S1P concentration and its signaling inside and outside of cells.31 These discoveries of S1P and related molecules have driven lipid research forward.32
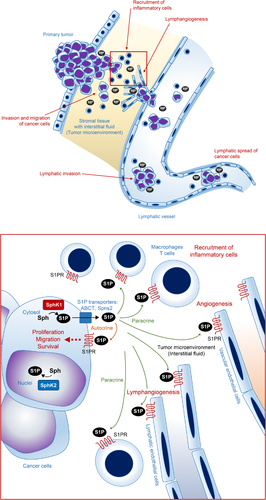
S1P is produced by two different sphingosine kinases, SphK1 and SphK2.33 SphK1 exists mainly in the cytosol close to the cell membrane and is found in nearly every cell type. S1P is exported from the cell by S1P-specific transporters, such as ATP-binding cassette (ABC) transporters and Spns2, which will be described later in this review. Outside the cell, S1P binds to S1P-specific G-protein-coupled receptors, S1PR1-5, which further evoke cell signaling in an autocrine, paracrine or endocrine manner. The “inside-out signaling” process refers to the signaling pathway by which S1P synthesized in the cell is transported out of the cell to activate S1PR on the cell surface in an autocrine and paracrine manner (Figure 1).34
1.2 Sphingosine kinases, S1P transporters and receptors
Whereas SphK1 exists in the cytosol close to the cell membrane and contributes to the production of S1P, SphK2 has been reported mainly in the nuclei and mitochondria (Figure 2).35, 36 Although S1P does not differ structurally when produced by SphK1 or SphK2, it carries out different functions in the body depending on where it is produced.31 S1P in the nuclei works as a histone deacetylase (HDAC) inhibitor to epigenetically regulate gene transcription.36 Accordingly, SphK2 is predominantly expressed in highly differentiated organs, such as the brain, liver and kidney, all of which exhibit complicated functions.37, 38 In these organs, S1P in the nuclei and mitochondria epigenetically controls the transcription of key genes. For instance, we found that S1P produced by SphK2 regulates the expression of genes that maintain lipid metabolism in the liver.38 In contrast, high levels of SphK2 in the mitochondria may be associated with beta-oxidation,35 indicating the importance of S1P in these organelles as well.
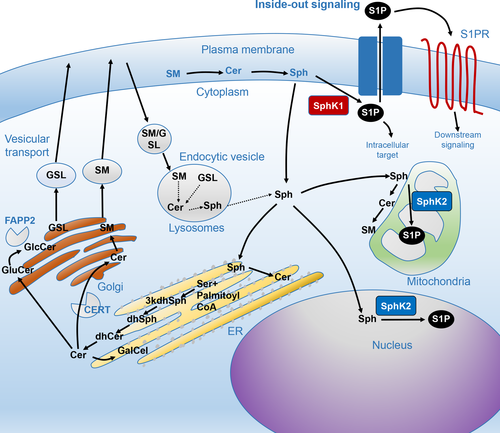
Transporters that export S1P from the cell have been identified over the past decade.34, 39, 40 Some of the ATP-binding cassette (ABC) transporters have been found to transport S1P out of the cell, including ABCC1.39, 40 The function of ABCC1 as a S1P transporter was initially discovered in mast cells.39 It has been previously reported that ABCC1 and ABCG2 export S1P from ER-positive breast cancer cells in response to nongenomic effects of estrogen.40 More recently, Spinster 2 (Spns2) has been identified as an S1P transporter.41-43 Spns2 is a member of the major facilitator superfamily, which does not have a typical ATP-binding motif.41, 42, 44 Two research groups have observed Spns2 as a S1P transporter in zebrafish. A dysfunction of the Spns2 gene causes abnormal development, resulting in cardia bifida (two hearts).41, 45 Notably, the cardia bifida phenotype was also observed in S1PR2 knockout zebrafish, suggesting a close relationship between Spns2 and S1PR2.41, 45 Spns2 exists in vascular and lymphatic endothelial cells,46, 47 where it is crucial to the development of lymphatic vessels. Based on current research, we can assume that Spns2 plays an important role in regulating the concentration of S1P in the blood and lymph.43, 46
S1P receptors are found on the surface of cancer cells and on surrounding cells in the tumor microenvironment, such as stromal cells. There are five S1P-specific G-protein-coupled receptors,31 each with its own signaling pathway and expression on the cell surface.48 S1PR1 is one of the most prominent receptors in S1P signaling. S1PR1 is expressed on many immune cells given that S1P signaling regulates immune cell trafficking.49 S1P signaling through S1PR1 also plays a pivotal role in cell egress from the secondary lymphatic system, including the lymph nodes, thymus and spleen.49-51 If S1PR1 is blocked, lymphocytes cannot egress from the secondary lymphatic system, and lymphocytes in the blood decrease dramatically.49, 52 Both S1PR1 and S1PR3 may play important roles in cancer progression, and S1PR2 may work oppositely depending on the cancer cell type and its environment.53 S1PR4 and S1PR5 have not been as well investigated.54, 55
1.3 S1P concentration gradient and roles of degrading enzymes
The concentrations of S1P in the blood and lymph are higher than in tissue.49, 56, 57 In blood, S1P concentration ranges from several hundred nanomolar to several micromolar, whereas in the peripheral tissues, where degrading enzymes are more active, the S1P concentration ranges from several nanomolar to several tens of nanomolar. S1P in the blood is derived mainly from hematopoietic cells, particularly red blood cells, which play a major role on S1P storage.58, 59 Lymphatic endothelial cells are the major source of S1P in lymphatic fluid.46 Interestingly, mice with SphK1 and SphK2 knockout in endothelial cells showed a loss of S1P in lymphatic fluid while maintaining normal plasma S1P, suggesting that SphK in the endothelial cells are the major source of S1P in lymph but not in the blood.58
S1P degrading enzymes play an important role in maintaining low levels of S1P in the tissue.49, 57 S1P lyase is an enzyme that decomposes S1P irreversibly.60, 61 S1P phosphatase is an enzyme that removes phosphate from S1P to produce sphingosine,62 which can then be converted to ceramide by ceramide synthase.63 Whereas S1P acts as a “cell survival signal” for cell proliferation and survival, ceramide acts as a “cell death signal” for cellular apoptosis.63 Consequently, it is thought that the life or death of a cell is regulated by the balance between S1P and ceramide.63 However, further research is needed to determine how the balance between S1P and ceramide is regulated to maintain cell homeostasis. Together, SphK, S1P transporters, S1P receptors and degrading enzymes all regulate S1P gradation and signaling, which control normal physiological function as well as inflammation and cancer progression.
1.4 S1P and inflammation
As mentioned previously, the concentration gradient of S1P in the blood and peripheral tissues plays a major role in controlling immune cell dynamics.49 The S1P concentration gradient regulates lymphocyte trafficking, which is important for the pathology of inflammation, and it may play a role in many inflammatory diseases.25, 26, 64, 65 Key cytokines and chemokines, such as IL-6 and TNF-alpha, are also linked with S1P signaling in inflammation.25, 26 Indeed, we and the others have found that the S1P-Stat3-S1PR1 amplification loop plays an important role in amplifying chronic inflammation.24, 66, 67 TNF-alpha, interleukin 1-beta (IL1-beta), and other cytokines can activate SphK1, which can then induce cyclo-oxygenase2 (COX2) in cells.68 Importantly, COX2 suppresses the anti-tumor response by CTL-, Th1-, and NK cell-mediated type-1 immunity. Considering that chronic inflammation evoked by SphK1 and S1P promotes COX2 induction, which suppresses the anti-tumor immune response, these molecules may be key players in cancer progression in the context of chronic inflammation.
1.5 S1P and cancer progression
S1P promotes cancer progression in multiple ways by contributing to cell proliferation, migration, invasion, and cell survival.28, 47, 69 SphK1 is highly expressed by many cancer cell types and at higher levels than in normal cells.19 We measured S1P concentrations in cancer tissues and normal tissues using laboratory animal specimens and surgical specimens by mass spectrometry, and we confirmed that S1P concentrations in cancer tissues were significantly higher than that in normal tissues, both in animals and in human patients.19, 70
The higher concentrations of S1P produced by cancer cells may also affect the tumor microenvironment, which is a critical factor in cancer biology and progression.71-73 For instance, it was observed that significantly higher S1P concentrations in cancer interstitial fluid than in the interstitial fluid of surrounding normal tissue, both in laboratory animals and in human cancer patients.73 Interstitial fluid bathes the tumor and stromal cells and is considered an essential part of the tumor microenvironment, not only as the initial route of metastasis but also as a supplier of factors that promote tumor metastasis.73 S1P released into the cancer interstitial fluid acts on vascular/lymphatic endothelial cells, immune cells, and interstitial cells in the tumor microenvironment, and it induces angiogenesis, lymphangiogenesis, immune response, and interstitial reaction. Supporting this concept, we have found that S1P promotes lymphangiogenesis in breast cancer in animal models, and the inhibition of S1P reduces the metastasis of cancer and prolongs the survival of animals with implanted tumors.19 Moreover, we clarified that angiogenesis and lymphangiogenesis caused by breast cancer in an animal model can be suppressed by the inhibition of S1P production through SphK1 inhibition using a SphK1-specific inhibitor.19
We have recently revealed that S1P concentrations in cancer tissue are almost always higher than in surrounding non-cancerous tissue in a variety types of cancer, including breast, gastric, and pancreatic cancers in human patients.70, 74-76 Notably, accumulating evidence suggests that SphK1 and S1P seem to be associated with lymphatic spread based on the analysis of clinical samples from human cancer patients,74, 75 validating our findings in the experimental system. Based on the available findings, we hypothesize that the involvement of S1P in cancer prior to metastasis is as follows (Figure 1): S1P is released into the interstitial fluid surrounding cancer cells, where it promotes the invasion and migration of cancer cells. The cancer interstitial fluid with a high concentration of S1P is then drained into the lymphatic vessels, promoting lymphangiogenesis and lymphatic metastasis of cancer cells. Cancer cells that have entered the lymph duct survive in the lymphatic vessels, primarily stimulated by S1P supplied from the lymphatic endothelial cells. Eventually, the cancer cells in the lymph duct reach the lymph nodes and cause lymph node metastases. Further clinical and translational studies are needed to validate this hypothesis and to clarify the role of S1P in cancer progression.
1.6 Roles of S1P in inflammation and cancer
In the interaction between cancer and the host, S1P is an important factor linking cancer and inflammation. It has been previously shown that S1P is the missing link between chronic inflammation and cancer progression in colitis-associated cancer.24 S1P plays an indispensable role in the production of IL-6 regulated by NF-κB, the constitutive activation of transcription factor STAT3, and the resulting increase in expression of S1PR1.24 The feed-forward amplification loop of the S1P/S1PR1/Stat3 system is key to amplifying chronic inflammation in the disease process.24 Notably, treatment with FTY720 significantly reduces not only chronic inflammation but also the development of colon tumors. More recently, we have shown that the amplification loop is important for chronic inflammation evoked by obesity, which stimulates breast cancer progression in both syngeneic and spontaneous breast cancer mouse models.77 FTY720 treatment significantly reduced inflammatory cytokines IL-6 and TNF-alpha and tumor progression in the both mouse models.77 In these models, it has been shown that S1P plays a definitive role in the formation of premetastatic niches, which is exacerbated by obesity. Human breast cancer patients who were obese showed higher levels of S1P compared with normal weight patients.77 Together these findings support the important role of S1P in cancer with chronic inflammation, not only in the animal models but also in human disease, which might direct treatment strategies in the near future.
1.7 Clinical impact of S1P
Technological advances now allow for the accurate measurement of S1P in surgical specimens,70 thus enabling a greater understanding of the clinical impacts of this lipid mediator. We have measured levels of S1P in surgical specimens and have found that S1P levels in breast cancer, gastric cancer, colorectal cancer, liver cancer, and pancreatic cancer are almost always higher than in normal tissue.74, 75 Immunohistochemistry of phosphorylated-SphK1 (pSphK1) is an easy and effective way to estimate S1P production in clinical samples.74, 75 It has been revealed that elevated expression of pSphK1 is significantly associated with lymph node metastasis in breast cancer74 and with lymphatic invasion and lymph node metastasis in gastric cancer.75 Moreover, gastric cancer patients with high SphK1 show significantly worse overall survival compared with patients with low pSphK1 expression.75
The S1P gradient in the tumor microenvironment may play an important role in cancer progression. Considering that conditions with chronic inflammation evoke cytokine production, S1P is expected to be upregulated by the inflammatory mediators that stimulate SphK1 in cancer cells and stromal cells in the tumor microenvironment. S1P has a strong action of recruiting immune cells by its concentration gradient and can also recruit cancer cells toward the vasculature. Advances in technology may reveal more about the mechanisms of S1P regulation in the tumor microenvironment and its contribution to cancer progression.
1.8 Clinical utility of therapies targeting S1P
Given that S1P plays an important role in inflammation and cancer progression, targeting S1P signaling might be a promising therapy for cancer patients, especially patients with chronic inflammation. Potential therapeutic strategies targeting the S1P signal transduction system could include novel agents that inhibit S1P production enzymes, receptors, or S1P itself.
Inhibition of the enzymes that produce S1P is a promising strategy to treat cancer patients.28 For instance, we have previously demonstrated that SK1-I, a SphK1 specific inhibitor, suppresses angiogenesis and lymphangiogenesis that lead to reduction of tumor burden and metastases in murine breast cancer model.19 PF-543 is another inhibitor of SphK1 with much stronger potency.78 However, the effect of PF-543 in vitro has been controversial due to some negative results.79, 80 Importantly, the effect of PF-543 has not been assessed in vivo settings of cancer with inflammation. Considering the important role of S1P in the inflammation and cancer, it is worth investigating the effect of PF-543 in these settings.
ABC compound, a SphK2 inhibitor, and sphingomab, an anti-S1P antibody are other S1P signal-targeted drugs,28, 81 and they were investigated in phase I and II clinical trials. Sphingomab has been demonstrated to neutralize extracellular S1P and angiogenesis was suppressed.82 Alternatively, S1P signaling pathway can be inhibited by existing drugs, such as FTY720. FTY720 is an FDA-approved drug for multiple sclerosis.83 Although many studies have shown the anti-cancer activity of FTY720 in vitro and in vivo, clinical trials using FTY720 for cancer patients have not yet been conducted, possibly because of its side effects on the immune system. In order to verify the effect of S1P inhibitors in inflammatory-associated cancers, it is desirable to conduct clinical trials after appropriate preclinical studies. Furthermore, the possibility of combining an S1P signal inhibitor with anticancer drug therapy and/or molecular targeted drugs should be explored.
Considering the close functions of S1P in chronic inflammation and in cancer reviewed herein, we would like to emphasize that therapies targeting S1P signaling are expected to have a significant positive effect in cancer patients in the context of chronic inflammation. Further studies are needed to validate this concept, but it is worth carrying out clinical trials for cancer patients with chronic inflammation by targeting S1P signaling, as this may provide better outcomes for those patients.
2 CONCLUSION
Given the critical role of S1P in linking inflammation and cancer, it is possible that cancer with chronic inflammation may be best treated by targeting the S1P signaling pathway as a novel therapeutic approach. Although further investigation will be needed to develop the targeted therapies, accelerated technological innovation could greatly improve the possibility of cancer treatments targeting S1P.
ACKNOWLEDGMENT
This work was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research Grant Number JP18K19576 for M. Nagahashi and JP16K15610 for T. Wakai and K. Takabe is supported by NIH/NCI grant R01CA160688 and Susan G. Komen Investigator Initiated Research Grant IIR12222224.
CONFLICT OF INTEREST
The authors have no conflict of interest.




