HSP90 inhibitor AUY922 can reverse Fulvestrant induced feedback reaction in human breast cancer cells
Funding Information
National Science and Technology Support Program (Grant/Award Number: ‘2015BAI12B15’), Tianjin Municipal Major Scientific and Technological Special Project for Significant Anticancer Development (Grant/Award Number: ‘12ZCDZSY15700’, ‘15JCYBJC28300’).
Abstract
Hormone therapy has become one of the main strategies for breast cancer, however, many estrogen receptor (ER) positive patients end in tumor collapse due to initial or acquired resistance to hormone treatment, which includes Fulvestrant. Here we report that ErbB receptors and downstream PI3K/AKT and ERK pathway have been reactivated after treatment of Fulvestrant in ER positive MCF-7 and T47D cells, which are related to Fulvestrant resistance. HSP90 is a universally expressed chaperone protein and plays a vital role in both normal and cancer cells, HSP90 inhibitor AUY922 can reverse this feedback reactivation effect of Fulvestrant by targeting multiple proteins related in ErbB receptors, PI3K/AKT and ERK pathway, which is much better than single targeting inhibitors. We also consolidate these effects in human fresh breast tumors. Combination of AUY922 and Fulvestrant may become a promising therapy strategy in breast cancer treatment.
Approximately 20% of breast cancer patients with stage I–III ER+/HER2- disease at the time of diagnosis will suffer from relapse within 10 years following surgery and 5 years of adjuvant anti-estrogen therapy to neutralize ER signaling.1 Anti-estrogen-resistant advanced breast cancer patients become an issue to be urgently solved. However, efforts to block the hormonal therapy resistance do not seem satisfied, largely because of the heterogeneity and complexity of the mechanisms underlying it, it seems impossible to try to solve this problem with one inhibitor that targeted one specific oncoprotein.
Previous research has found that combination therapies targeting both ER and PI3K pathway may overcome the acquired resistance to endocrine therapy in breast cancer,2 as they found that long-term estrogen deprivation cells (LTED cells; mimics resistance to aromatase inhibition) showed predominant tyrosine phosphorylation of HER family members (EGFR, HER2, HER3 and HER4), thus PI3K pathway became activated. Proteomic profiling also showed that LTED cells exhibit increased PI3K/AKT pathway activation. Other papers also showed that PI3K/AKT pathway has been activated in anti-estrogen resistance in breast cancer,3-5 and chronic anti-estrogen therapy induces the activation of the PI3K pathway in vitro,6, 7 which might contribute to the cancer cell growth and proliferation. However, the EGFR inhibitor Gefitinib fails to alter the AKT reactivation.8
In the presence of complex cross-talking network of signaling pathways, disruption of only one or two targets may not abrogate growth of cancer cells. Thus a simultaneous attack on many molecular targets involved in those pathways seems more likely to jeopardize the survival of cancer cells.
HSP90 is a universally expressed molecular chaperone, it plays an important role in the post-translational modulation and a large number of its client proteins are involved in oncogenesis. Moreover, cancer cells seem to have greater dependence on HSP90 for maintaining the homeostasis of essential proteins when compared to normal cells9 and HSP90 facilitates malignant progression in tumor cells. Expression of HSP90 in cancer cells is two- or three-folds higher than normal cells and is correlated to poor prognosis.9, 10
Hsp90 has been an alternative therapeutic target for cancer therapy. 17-AAG, the first HSP90 inhibitor tested in clinical trials; however, has several pharmacological limitations, including poor bioavailability and hepatotoxicity, which prevents its further clinical application. AUY922 is a second generation of HSP90 inhibitor that can inhibit the ATPase activity of HSP90, it has been found had a variety of antitumor effect in a wide range of human cancers.11
Feedback reaction of Fulvestrant can benefit cells of proliferation and survival; however, there is still no research concerns about if AUY922 can reverse this undesirable situation. In this study, we explore the effect of AUY922 in reversing this feedback activation after Fulvestrant treatment and hope this will become the molecular mechanism for a new therapeutic strategy.
Materials and Methods
Cell lines, culture condition and reagents
The estrogen receptor positive(ER+) human breast cancer cell lines MCF-7, T47D were maintained in our lab and kept at 37°C in 5% CO2 in RPMI-1640 containing 10% fetal bovine serum (Corning, NY, USA), 1% penicillin-streptomycin (Corning, NY, USA). HSP90 inhibitor NVP-AUY922 was provided by NOVARTIS (Basel, Switzerland) and dissolved in phosphate-buffered saline (PBS) to a final concentration of 10 mmol/L, Fulvestrant was purchased from Selleck (Houston, TX, USA) and dissolved in dimethylsulfoxidedimethylsulfoxide (DMSO) to a final concentration of 1 mmol/L, all the drugs were stored at −20°C.
Western blotting analysis
Cells were harvested after indicated treatment and suspended in RIPA lysis buffer (Solarbio, Beijing, China) with protease inhibitor and phosphatase inhibitors (1 mM sodium fluoride and 2 mM sodium orthovanadate and 1 mM phenylmethylsulfonyl fluoride [PMSF]) on ice for 30 min, centrifuged at 13 500 g for 10 min to collect the whole cell lysates. The proteins (20 μg for most proteins and 40–50 μg for ErbB receptors) were separated on an 8% SDS–PAGE and transferred onto a polyvinylidene fluoride (PVDF) membrane (Milipore, Bedford, MA, USA). Specific antibodies were used in the immunoblotting process. HSP70, HSP90, ER, EGFR, HER2, HER3, HER4, pEGFR(Tyr1068), pHER2(Tyr1221/1222), pHER3(Tyr1289), pHER4(Tyr1284), p110α, AKT, p-AKT(Ser473), p-AKT(Thr308), p-p70S6(Thr389), p-S6(Ser235/236), ERK1/2 were purchased from Cell Signaling Technology (Danvers, MA, USA), p70S6 and β-actin were purchased from Immunoway (Plano, TX, USA). p-ERK1/2 (Thr202/Tyr204) was purchased from Santa Cruz Biotechnology (CA, USA). Proteins were detected using an ECL kit according to the manufacturer's protocol.
Human tumor assay
Breast tumor samples were obtained after patients provided written informed consent for research use and the research was approved by the Ethics Committee of our institution. We performed this experiment according to Dr Jennifer R. Bean's paper.3 Untreated primary tumor samples from four patients with early-stage ER+/HER2-breast cancer were collected by using core needle biopsy, later confirmed by pathological examination. Within 1 h post-biopsy, samples were put into DMEM medium (Corning, NY, USA) with 10% fetal bovine serum (FBS) after several brief washes with 1XPBS, 100 nM Fulvestrant and 30 nM AUY922 was added in the medium for each single group and kept for 48 and 24 h, respectively. 100 nM Fulvestrant was added at first and then 30 nM AUY922 was added 24 h later and kept for another 24 h for the combination group, after ex vivo culture, tumor sample were frozen and stored at −80°C.
Cell growth assay
MCF-7 and T47D cells were seeded in 96-well plates and treated in triplicate with indicated drugs at desired concentrations at 37°C for 72 h. Cell proliferation was assessed by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Solarbio, Beijing, China) assay.
Cell cycle analysis
We used Flow cytometric analysis to evaluate cell cycle changes. MCF-7 and T47D cells were harvested after treatments and fixed in 70% ethanol overnight. After washing with 1XPBS twice, cells were treated with RNase (final concentration: 50 μg/mL) and then stained with propidium iodide (PI, final concentration: 50 μg/mL). The samples were loaded into a flow cytometer, and the cell cycle distributions were obtained.
Statistical analysis
Data were presented as the mean ± SD. Differences between groups were analyzed by one-way ANOVA using the (GraphPad Prism 6.0 Software, Inc., La Jolla, CA, USA). The ratio of immunoblot bands were calculated using ImageJ software. For all experiments, a P-value less than 0.05 (two-tailed) was considered significant. An asterisk indicates that the P-value is less than 0.05.
Results
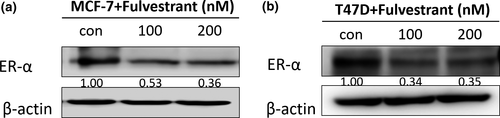
Fulvestrant decreases ER-α in breast cancer cells
First, we checked the effect of Fulvestrant on ER-α in MCF-7 and T47D cells. Cells were harvested after 48 h of Fulvestrant treatment, ER was significantly decreased; however, there was no difference between 100 and 200 nM of Fulvestrant (Fig. 1).
Feedback reactivation of Fulvestrant on ErbB receptors and downstream PI3K/AKT and ERK pathway
Next, we checked the ErbB receptors changes after Fulvestrant treatment in both MCF-7 and T47D cells. EGFR, HER2, HER3 and HER4 total protein were increased significantly in MCF-7 cells, and the same as the phosphorylation types (Fig. 2a,b). EGFR total proteins didn't change a lot in T47D cells; however, HER2, HER3 and HER4 total protein were significantly increased (Fig. 2c), and all the phosphorylation type proteins went up comparing with control group (Fig. 2d).
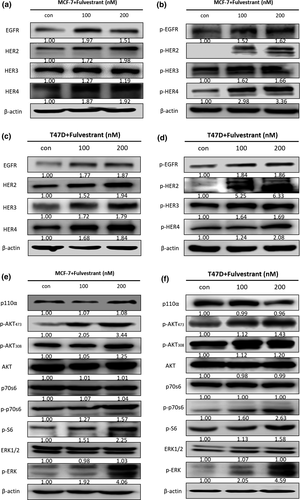
We also tested the downstream PI3K/AKT and ERK pathway. As expected, we found that p-AKT473, p-AKT308, p-p70S6, p-S6 and p-ERK were increased while their total protein didn't change in both MCF-7 (Fig. 2e) and T47D cells (Fig. 2d). There was no difference between 100 and 200 nM of Fulvestrant treatment, so we chose 100 nM Fulvestrant in the following experiments.
HSP90 inhibitor AUY922 inhibits PI3K/AKT and ERK pathway
HSP90 was a chaperone protein and played a vital role in proliferation of cancer cells, so we used western blotting to check the influence of its inhibitor AUY922 on the proliferation related PI3K/AKT and ERK pathway. Expression of HSP70 was a surrogate marker of Hsp90 inhibition. HSP70 increased in both MCF-7 and T47D cells after treatment of AUY922 for 24 h, while HSP90 didn't change (Fig. 3a), which indicated that AUY922 worked out well in both cell lines. We also checked if Fulvestrant had any influence on HSP90, results showed that there was no change of HSP70 or HSP90 (Fig. 3b). Moreover, downstream PI3K/AKT and ERK pathway activities were being blocked after AUY922 treatment. p-AKT473, p-AKT308, p-p70S6, p-S6 and p-ERK were universally decreased at about the concentration of 30 nM in both MCF-7 and T47D cells (Fig. 3c), so we chose 30 nM AUY922 in the following combination experiments.

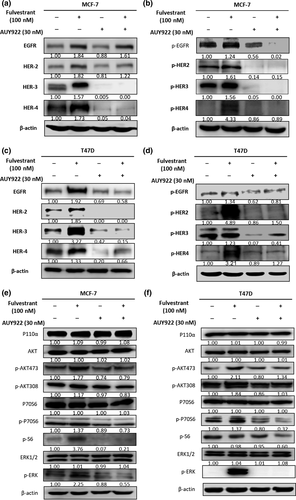
AUY922 can reverse the feedback reactivation effect of Fulvestrant in breast cancer cells
Since Fulvestrant treatment could induce the feedback reactivation of ErbB receptors and their downstream PI3K/AKT and ERK pathway, AUY922 could inhibit these pathways, so we tested if combination use of AUY922 could reverse this feedback effect. As shown in the results, both MCF-7 and T47D cells with Fulvestrant treatment alone induced activation of ErbB receptors and downstream proliferation pathway; however, when combined with AUY922 after 24 h treatment of Fulvestrant, both total and phosphorylated type of ErbB receptors were downregulated compared with Fulvestrant treatment alone (Fig. 4a–d). As for the downstream pathways, the feedback reactivated phosphorylated proteins in PI3K/AKT pathway and p-ERK were all be inhibited after combined with AUY922 (Fig. 4e,f). According to these results, AUY922 can reverse the feedback effect of Fulvestrant in both PI3K/AKT and ERK pathway.
Combination use of AUY922 and Fulvestrant inhibits cell proliferation and causes cell cycle arrest in breast cancer cells
To further evaluate the potential synergy of AUY922 and Fulvestrant, the effect of drug combination on cell viability was analyzed. 100 nM Fulvestrant alone had really limited effects in growth inhibition in MCF-7 and T47D cells after 72 h treatment, 22.8% and 18.1%, respectively. Combination use of AUY922 and Fulvestrant were more efficient than AUY922 alone at 20–30 nM; however, this combination effect was covered by AUY922 when the concentration was more than 40 nM (Fig. 5a), which suggested a desired combination effect could be reached with lower dosage of AUY922 (20–30 nM), which could limit the adverse effects of AUY922 in clinical use.
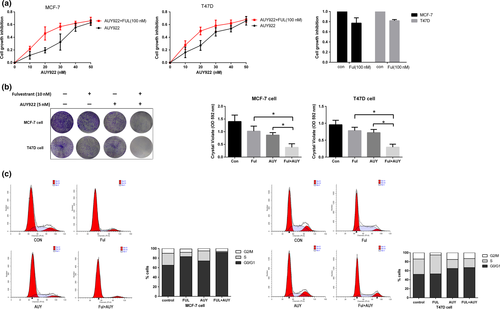
Moreover, to evaluate the efficacy of combination of Fulvestrant and AUY922 on colony forming, we stained cell cultures with crystal violet, as a surrogate for cell mass,12 after 10 days treatment with indicated concentration of each drug alone or combination. Colony forming rates were significantly inhibited under treatment of Fulvestrant and AUY922 compared with each alone in MCF-7 cells (Ful+AUY: 27.5% vs Ful: 72.9% and AUY: 61.9%) and T47D cells (Ful+AUY: 30.8% vs Ful: 81.9% and AUY: 75.7%) (Fig. 5b).
Next, we performed cell cycle analysis, Fulvestrant combined with AUY922 had an advantage in G0/G1 arrest in MCF-7 cells (combination: 91.92% vs Fulvestrant: 83.55%, AUY922: 73.65% and control: 64.86%); however, this combination effects was much limited in T47D cells (combination: 66.79% vs Fulvestrant: 52.71%, AUY922: 64.85% and control: 52.35%) (Fig. 5c).
AUY922 can reverse the feedback effect of Fulvestrant in human breast tumor
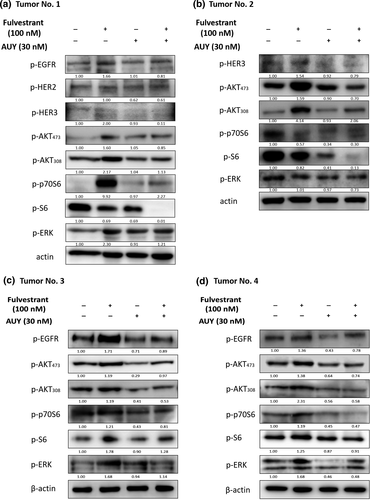
In order to validate the results showed in the MCF-7 and T47D cell lines, we tested the effect of AUY922 and Fulvestrant on fresh ER+/HER2- human breast tumor samples. Since the phosphorylation type was the active status of proteins and we didn't have enough sample protein, so we just tested the phosphorylation type of ErbB receptors. In all the four patients’ samples, p-AKT308 and p-AKT473 were significantly upregulated after Fulvestrant treatment alone and downregulated when combined with AUY922, this was the same trend with p-HER3 in both tumor No.1 and No.2, and p-EGFR in tumor No.1, No.3 and No.4. In Tumor No.1, there seemed to be no change of p-HER2 after Fulvestrant treatment but we could see a combination effect of two drugs. Unfortunately, we did not get images of other phosphorylation status of ErbB receptors, and there was no signal of them. Moreover, there was a significant increase of p-p70S6 and p-ERK after Fulvestrant treatment and downregulation after combined with AUY922 in Tumor No.1, No.3 and No.4. This was the same trend of p-S6 in tumors No.3 and No.4. The results of tumor samples were roughly in line with those in breast cancer cell lines. The ErbB receptors were activated after Fulvestrant treatment due to feedback effect and caused activation of downstream PI3K/AKT and ERK pathway, this upregulation could be abolished after combination use of AUY922 in breast tumor (Fig. 6a–d).
Discussion
Hormone therapy has improved the survival of many hormone receptor positive breast cancer patients; however, hormone resistance is becoming a great challenge for oncologists. Fulvestrant has been widely used in ER+ patients, it functions to downregulate ER by binding to ER dimers and causing immobilization of ER, which is accompanied by degradation via the ubiquitin-proteasome pathway.13 Identification of molecular mechanisms underlying Fulvestrant resistance may give new opportunities to overcome the treatment failure and to discover new strategies for antiestrogen resistance.
Our results showed ErbB receptors and downstream PI3K/AKT and ERK pathways were reactivated after treatment of Fulvestrant in ER+ breast cancer cells, which was in line with other people's results.4, 14 There are also clinical results that provide insights into possible mechanisms of Fulvestrant resistance through growth factor receptor cross-talk.15 Moreover, ER+ breast cancer patients with a protein expression/phosphorylation signature of PI3K activation have a shorter recurrence-free survival, as determined using reverse-phase protein arrays (RPPA).2 So ErbB receptors and downstream PI3K/AKT and ERK pathways play a vital role in Fulvestrant resistance.
HSP90 has been found to be overexpressed in breast cancer patients, and is related to poor prognosis.16 Moreover, HSP90 empowers evolution of resistance to hormonal therapy in human breast cancer,17 so inhibition of HSP90 is really important in conquering hormonal resistance. AUY922 is a second-generation synthetic HSP90 inhibitor that can influence many HSP90 client proteins. It can inhibit phosphorylation status of ErbB receptor proteins and PI3K/AKT, ERK pathway proteins alone after 24 h treatment in our results, this is in line with the results also in lung cancer.18 It is an inhibitor that can target more than only one signal pathway, which benefits it more efficiency in tumor inhibition. In other papers, it showed that inhibition of HER-2 alone had no effect on the HER-2 over expressing T47D fulvestrant resistant cell lines,19 which suggests that single inhibition of one target may not be enough.
Since Fulvestrant's feedback effect involves more than one growth factor receptor and multi downstream proliferation proteins, so we test if AUY922 can reverse feedback reactivation of these important proteins. As expected, AUY922 can efficiently eliminate these feedback effects of Fulvestrant. Combination use of AUY922 and Fulvestrant is also more efficient in inhibiting cell proliferation and colony forming. However, this drug combination effect in growth inhibition is much more obvious when AUY922 is around 20–30 nM plus 100 nM Fulvestrant, and covered by AUY922 when its concentration is more than 40 nM, which may be because endocrine therapies are cytostatic rather than cytotoxic, leading to mild and limited reduction of growth rate, and AUY922 is harsher in causing cell death. In the cell cycle analysis, combination use of these two drugs are more effective than each alone. However, when compared with cell growth and colony forming assay, the effect in cell cycle arrest is limited. This may be because of different time intervals of treatment, and there are also other factors that could influence cell growth.
This is the first study to systematically test if AUY922 can reverse the feedback reaction of fulvestrant, and these results are in line with Ganetespib, another HSP90 inhibitor,17 our results provide further positive evidence of HSP90 inhibitors that can be used in combination with fulvestrant, and furthermore another mechanism of research to support related clinical results. Ganetespib is also an HSP90 inhibitor that can compete in the ATP binding domain. When compared with AUY922, the latter seems more promising in clinical application as the results of clinical studies have shown on Pubmed until now. There were four phase II clinical trial reported results of treatment of Ganetespib alone or combined with other drugs. These clinical trials were carried out in metastatic castrate-resistant prostate cancer,20 advanced non-small cell lung cancer,21 metastatic breast cancer,22 chemotherapy-refractory metastatic colorectal cancer,23 but none of them showed expected clinical activity or improvement in PFS. There were only two phase II clinical trials of AUY922 that had reported their results online. AUY922 produced a median PFS in patients with refractory to treatment with Imatinib.24 The other one was a Phase I/II Study about EGFR-Mutant lung cancer with acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors, where partial responses were observed, but this phase II study of AUY922 and Erlotinib did not meet its primary end point.25 However, more clinical trial results are needed to assess the effects of these two drugs before their wider use in clinical application.
There is clinical evidence that the overexpression or amplification of HER2 or EGFR is responsible for resistance to endocrine therapy in metastasis breast cancer patients.26-28 It was also found that there are modest HER2 increases and loss of EGFR phosphorylation and MAP kinase (ERK1/2) phosphorylation in the patients tumor samples after 6 months treatment of Fulvestrant.29 We consolidated our results of cell lines in fresh tumor samples. Not all phosphorylated ErbB receptor proteins are activated, because there were no signals of some phosphorylated receptors, and some of the downstream PI3K/AKT pathway proteins and p-ERK were increased after Fulvestrant treatment and inhibited after adding AUY922.
It is interesting that in the human tumor assay we got a signal of p-HER2 in tumor No. 1. The reason why p-HER2 could be tested in Luminal A breast tumor could be explained by heterogeneity of each tumor and the criterion of HER2 negative that we used in clinical treatment. We mashed all the tumor tissue to extract total protein, which was different from clinical testing, which only used a few pieces of sample slices. The criterion for HER2 negative definition was 0–1+ by immunohistochemistry or FISH-negative (ratio < 2.2) according to ASCO/CAP update HER2 Testing Guidelines, 2013.30 HER2 negative does not mean there is absolutely no expression of HER2. Those small parts of HER2 overexpressing cells may become the origin of drug resistant clones.
Moreover, different tumors have different feedback responses to Fulvestrant, suggesting that different patients have different mechanisms of Fulvestrant resistance. It is a pity that we could not obtain enough fresh tumor samples to test all of the related total proteins, we just tested the phosphorylated ones. Patient samples are more important and convincible than just tumor cell lines, so it is necessary to combine them to analyze problems.
Generally speaking, we demonstrate that ErbB receptors and downstream PI3K/AKT and ERK pathway are reactivated after treatment of Fulvestrant, and AUY922 can reverse these feedback effects in both human breast cell lines and human fresh tumor samples, which provides a new mechanism of combination use of Fulvestrant and AUY922.
Acknowledgments
The authors thank Novartis for providing AUY922. This project was supported by National Science and Technology Support program (2015BAI12B15) and Tianjin municipal Major Scientific and Technological Special Project for Significant Anticancer Development (12ZCDZSY15700 and 15JCYBJC28300).
Disclosure Statement
No competing financial interests exist.




