In This Issue
A vicious partnership between AKT and PHLDA3 to facilitate neuroendocrine tumors
Page 1101–1108
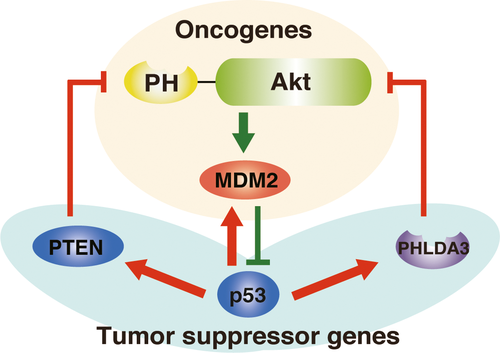
Pancreatic neuroendocrine tumors (PanNET) are a rare but deadly form of cancer that are poorly understood. In this study, Takikawa and Ohki focus on PHLDA3, a novel tumor suppressor gene that represses the activity of Akt. They suggest that progression of PanNET occurs through inactivation of PHLDA3, and discuss the putative relationship between the efficacy of everolimus, a drug that works to extend the lives of those individuals with PanNETs, and inactivation of the PHLDA3 gene in PanNET. They also describe their previous results, which have shown frequent loss of heterozygosity and increased DNA methylation at the PHLDA3 gene in PanNET samples. Finally, they speculate that more extensive investigation of PHLDA3 might reveal further information important to a more accurate diagnosis of PanNET.doi:10.1111/cas.13235
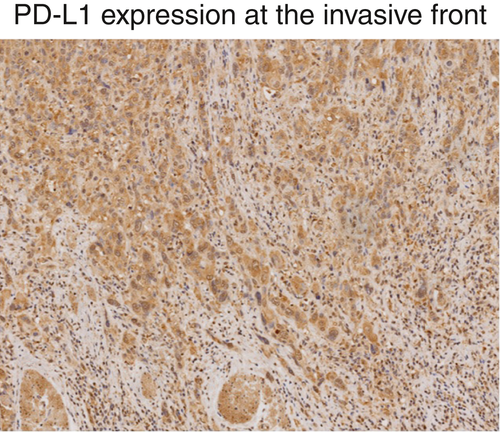
Programmed death-ligand 1 expression at the tumor invasive front is associated with epithelial–mesenchymal transition and poor prognosis in esophageal squamous cell carcinoma
Page 1119–1127
Programmed death-ligand 1 (PD-L1) has emerged as a critical component of tumor evasion of host immunity and is a prominent novel target of cancer treatment strategies. The promoter region of PD-L1 gene contains a binding site for a transcription factor, ZEB1, related to epithelial–mesenchymal transition, which could be important in the progression toward metastatic disease. This link between tumor immune resistance and metastatic potential has important implications for understanding cancer progression and the development of novel therapies. To better characterize this link, Tsutsumi and colleagues obtained tumor specimens from patients with esophageal cancer and showed that high PD-L1 expression at the invasive front of disease was associated with more aggressive tumor features. With further experimentation, the authors confirmed colocalization of PD-L1 and ZEB1 and showed that inhibition of ZEB1 might dampen epithelial–mesenchymal transition potential, which raises the exciting potential of new immunomodulatory targets.doi:10.1111/cas.13237
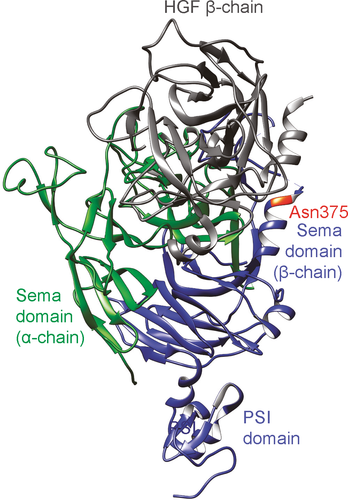
Exome sequencing deciphers a germline MET mutation in familial epidermal growth factor receptor-mutant lung cancer
Page 1263–1270
The vast majority of lung cancers are related to inhaled carcinogens in cigarette smoke and the disease continues to be the greatest cause of cancer mortality worldwide. In a subset of patients, particularly non-smoking Asian women, no link between lung cancer and a known carcinogen is established and these are more likely to be associated with particular oncogenic mutations. In particular, EGFR gene mutation can lead to hyperactivity of cellular growth pathways, but whether this anomaly directly drives carcinogenesis is unclear. To investigate this, Tode and others identified six siblings in a pedigree of familial EGFR-mutated lung cancer. The authors report that the four affected siblings all shared a MET proto-oncogene mutation. Additional intensive experimentation revealed that the MET mutation led to tumorigenicity that was synergistic with EGFR mutation. These findings provide a more comprehensive understanding of the mechanisms underlying an important subset of lung cancer patients.doi:10.1111/cas.13233