Oncogene swap as a novel mechanism of acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor in lung cancer
Funding Information:
IASLC Lung Cancer Young Investigator Award 2015–2016; Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology; Takeda Science Foundation and Uehara Memorial Foundation; Kaibara Morikazu Medical Science Promotion Foundation.
Abstract
Mutant selective epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs), such as rociletinib and AZD9291, are effective for tumors with T790M secondary mutation that become refractory to first-generation EGFR-TKI. However, acquired resistance to these prospective drugs is anticipated considering the high adaptability of cancer cells and the mechanisms remain largely obscure. Here, CNX-2006 (tool compound of rociletinib) resistant sublines were established by chronic exposure of HCC827EPR cells harboring exon 19 deletion and T790M to CNX-2006. Through the analyses of these resistant subclones, we identified two resistant mechanisms accompanied by MET amplification. One was bypass signaling by MET amplification in addition to T790M, which was inhibited by the combination of CNX-2006 and MET-TKI. Another was loss of amplified EGFR mutant allele including T790M while acquiring MET amplification. Interestingly, MET-TKI alone was able to overcome this resistance, suggesting that oncogenic dependence completely shifted from EGFR to MET. We propose describing this phenomenon as an “oncogene swap.” Furthermore, we analyzed multiple lesions from a patient who died of acquired resistance to gefitinib, then found a clinical example of an oncogene swap in which the EGFR mutation was lost and a MET gene copy was gained. In conclusion, an “oncogene swap” from EGFR to MET is a novel resistant mechanism to the EGFR-TKI. This novel mechanism should be considered in order to avoid futile inhibition of the original oncogene.
Activating mutations of epidermal growth factor receptor (EGFR) occur in approximately 40% and 20% of lung adenocarcinoma in East Asians and Caucasians, respectively.1 To date, several randomized trials have proven that EGFR-tyrosine kinase inhibitors (TKIs), such as gefitinib and erlotinib, yield longer progression-free survival of the patients with lung cancer harboring EGFR mutations than platinum-based chemotherapy.2-5 However, acquired resistance to EGFR-TKI inevitably develops in almost all patients. The secondary mutation of the EGFR resulting in threonine to methionine at codon 790 (T790M) accounts for approximately half these cases of resistance.6 T790M has been shown to dramatically increase the affinity between adenosine triphosphate (ATP) and EGFR and, at the same time, decrease the affinity between TKI and EGFR.7
Recently, third-generation EGFR-TKIs (3G-TKIs), such as CO-1686 (rociletinib) or AZD9291, have been designed to inhibit mutant EGFR, including T790M, while sparing wild-type EGFR.8, 9 The results of clinical trials for these agents obtained so far are quite encouraging. Response rates of patients with T790M-positive tumors treated with rociletinib and AZD9291 were reported to be 59% and 61%, respectively.10, 11 Moreover, progression free survival of patients with T790M treated with rociletinib and AZD9291 was 13.1 and 9.6 months, respectively.10, 11 However, it is anticipated that acquired resistance to these drugs will still emerge. Indeed, several mechanisms, such as ERK reactivation, maintained AKT phosphorylation, epithelial–mesenchymal transition, increased RAS dependence, loss of T790M and emergence of C797S have been reported, and the combination of 3G-TKI and various target inhibitors are under review.8, 12-18
In this study, we established cell lines with acquired resistance to 3G-TKI by chronic exposure of HCC827EPR cells harboring both exon 19 deletion and T790M to gain further insight into the mechanisms of resistance. Herein, we describe an “oncogene swap,” which is a novel mechanism of acquired resistance.
Materials and Methods
Cell lines and reagents
The human lung adenocarcinoma cell line HCC827 with exon 19 deletion of the EGFR gene (Del 19) was a kind gift from Dr A. F. Gazdar (Hamon Center for Therapeutic Oncology Research, University of Texas Southwestern Medical Center at Dallas, Dallas, TX, USA). HCC827EPR cells that are resistant to erlotinib harboring T790M and Del19 were established in our previous work.19 Cells were cultured in RPMI 1640 medium (Wako, Osaka, Japan) supplemented with 10% heat-inactivated FBS (Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a humidified incubator with 5% CO2. Erlotinib, afatinib, CNX-2006 (tool compound of rociletinib), AZD9291 and two MET-TKIs, PHA-665752 and crizotinib, were purchased from Selleck Chemicals (Houston, TX, USA). Afatinib is an irreversible inhibitor of pan-ERBB family, and CNX-2006 and AZD9291 are mutant EGFR-specific inhibitors.9, 20, 21
Establishment of in vitro CNX-2006-resistant cells
HCC827CNXR S1 and S4 cells were established by stepwise exposure of HCC827EPR cells to increasing concentration of CNX-2006 (50 nmol/L–1 μmol/L) for 4 months as described previously.19 Resultant cells were subsequently subcloned by limiting dilution in 96-well plates. As a result, only HCC827CNXR S1 and S4 cells were available for the present study. Cell identity of these cell lines were confirmed by cell line authentication service using short tandem repeat profiling (Promega, Madison, WI, USA).
Growth inhibition assay
Cell proliferation was measured using Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) as described previously.22 Briefly, cancer cells (3 × 103) were plated into each well of 96-well flat-bottomed plates and grown in RPMI with 10% FBS. After 24 h, test drugs were added to achieve indicated drug concentrations and the cells were incubated for an additional 72 h. A colorimetric assay was done after addition of 10 μL Cell Counting Kit-8 reagent in each well and the plates were incubated at 37°C for 2–4 h. Absorbance of 450 nm was read using a multiplate reader (Tecan, Männedorf, Switzerland). Percent growth was expressed relative to DMSO-treated controls. Experiments were performed in triplicate for each concentration.
DNA isolation and mutation analysis
Genomic DNA from cultured cells and frozen samples was extracted using a DNeasy Blood & Tissue kit (Qiagen, Venlo, the Netherlands) according to the manufacturer's protocol. Genomic DNA from formalin-fixed paraffin-embedded samples was extracted using DEXPAT Easy (TaKaRa, Shiga, Japan). Mutation analysis of exons 18–21 of the EGFR gene was done by direct sequencing method. Primers for PCR and sequence are listed in Table S1. PCR was performed as previously described.23 Direct sequencing was performed using a BigDye Terminator v3.1 Cycle Sequencing Kit (Life Technologies, Carlsbad, CA, USA) and ABI 3130XL (Life Technologies) according to the manufacturer's protocol.
Gene copy number analysis
The gene copy number of the EGFR and MET relative to LINE1 repetitive element was measured by the real-time PCR method using Power SYBR Green PCR Master Mix (Life Technologies) with StepOnePlus system (Life Technologies) as described previously.24 Primers for each gene are listed in Table S1. PCR experiments were performed in triplicate for each primer set. Genomic DNA from a healthy person was used as a control.
Phospho-receptor tyrosine kinase analysis
A human Phospho-RTK Array Kit (R&D Systems, Minneapolis, MN, USA) was used to detect the relative level of tyrosine phosphorylation of 49 receptor tyrosine kinases as described previously.19 Briefly, cells were lysed by NP-40 lysis buffer according to the manufacturer's protocol. Membranes with antibodies spotted against 49 phospho-RTK were treated with blocking buffer and incubated with 450 μg of cell lysate overnight at 4°C. The arrays were then washed, incubated with an HRP-conjugated phospho-tyrosine detection antibody, treated with ECL solution, and detected with EOS Kiss X6i (Canon, Tokyo, Japan).
Western blot analysis
Cells were cultured in RPMI 1640 with 10% FBS until subconfluency and media was changed to new media with indicated concentration of test drugs. After 24 h, cells were rinsed with PBS, lysed in SDS buffer, and homogenized using a scraper. Approximately 20 μg to total cell lysates were subjected to SDS polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA). After blocking with Western BLoT Blocking Buffer Protein Free (TaKaRa) or PBS containing 2.5% skimmed milk and 2.5% BSA, membranes were incubated with primary antibodies (1:2000) overnight, washed with PBS containing tween-20 (PBS-T), reacted with secondary antibody (1:5000), and washed with PBS-T again and treated with Western BLoT Quant HRP Substrate (TaKaRa). Chemiluminescence was detected with by EOS Kiss X6i (Canon). Anti-phospho-EGFR, anti-EGFR, anti-phospho-MET, anti-MET, anti-phospho-AKT, ant-AKT, anti-phospho-ERK, anti-ERK, anti-PARP, anti-cleaved PARP, anti-GAPDH and anti-beta-actin antibodies were all purchased from Cell Signaling Technologies (Danvers, MA, USA).
Autopsy samples
Autopsy samples of EGFR-mutated lung adenocarcinoma were obtained from a patient who passed away with multiple gefitinib-refractory lung tumors after initial good response. Approval for the use of the tumor tissue specimens was obtained from the institutional review board of Higashi-Hiroshima Medical Center and Kinki University Faculty of Medicine.
Target sequencing analysis
Target sequencing analysis was performed as previously described.25 Briefly, we used 10 ng of DNA for the multiplex PCR amplification using Ion AmpliSeq Library Kit 2.0 (Life Technologies) and the Ion AmpliSeq Cancer Hotspot panel v2 (Life Technologies). The Ion Xpress Barcode Adapters (Life Technologies) were ligated into the PCR products and purified with Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, USA). The purified libraries were then pooled and sequenced on an Ion Torrent PGM device (Life Technologies) using the Ion PGM 200 Sequencing Kit v2 (Life Technologies) and the Ion 318 v2 Chip Kit.
DNA sequencing data were accessed through the Torrent Suite v4.0 software program (Life Technologies). Reads were aligned against the hg19 human reference genome, and variants were called using the variant caller v4.0. Raw variant calls were filtered out using the following annotations: homozygous and heterozygous variants, quality score of <100, and depth of coverage <19. Germline mutations were excluded using the Human Genetic Variation Database (http://www.genome.med.kyoto-u.ac.jp/SnpDB).26
Results
Generation of CNX-2006 resistant HCC827EPR cells
By exposing HCC827EPR cells with T790M to increasing concentrations of CNX2006 (50 nmol/L–1 μmol/L) for 4 months, we were able to establish two resistant clones to CNX-2006 (HCC827CNXR S1 and S4). These cells are approximately 44 times and 68 times resistant to CNX-2006 compared with their parental cell line HCC827EPR, respectively (Table 1 and Fig. 1a,b). As expected, these cell lines are also highly resistant to any of erlotinib (1G-TKI), afatinib (2G-TKI) and AZD9291 (another 3G-TKI; Table 1).
| Cell lines | IC50 (nM) | |||
|---|---|---|---|---|
| Erlotinib | Afatinib | CNX-2006 | AZD9291 | |
| HCC827 | 10 | <4.6 | 25 | <4.6 |
| HCC827EPR | 7100 | 36 | 62 | <4.6 |
| HCC827CNXR S1 | >10 000 | 680 | 2700 | 1400 |
| HCC827CNXR S4 | 9100 | 1200 | 4200 | 2100 |
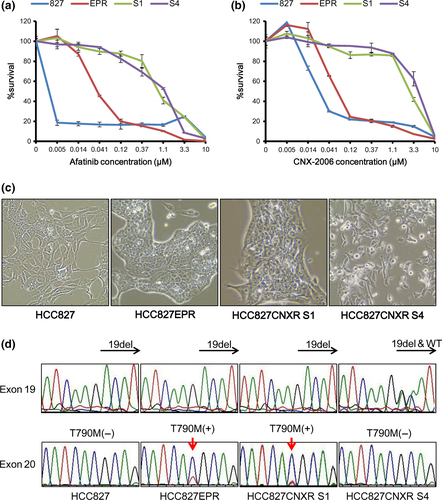
Morphologically, HCC827CNXR S1 cells exhibited an epithelial shape that resembled HCC827 and HCC827EPR cells (Fig. 1c). However, HCC827CNXR S4 cells tended to grow in a scattered fashion with fewer cell to cell contacts compared with HCC827CXR S1 (Fig. 1c).
Epidermal growth factor receptor mutation and MET gene amplification
We sequenced exons 18–21 of the EGFR gene. In HCC827CNXR S1 cells, Del19 and T790M remained unchanged from the parental HCC827EPR cells (Fig. 1d). However, in HCC827CNXR S4 cells, the wild-type sequence that was not detectable in other subclones reappeared as well as Del19 (Fig. 1d). Furthermore, T790M was no longer detectable in exon 20 (Fig. 1d). C797S, which was reported to induce acquired resistance to 3G-TKI, was not seen in both resistant cells.15-17 Quantitative genomic PCR revealed that while the other three cell lines other than HCC827CXR S4 had high levels (30–50 fold) of EGFR amplification, HCC827CNXR S4 cells lost amplified-EGFR to levels comparable to the DNA from a healthy volunteer (Fig. 2a).
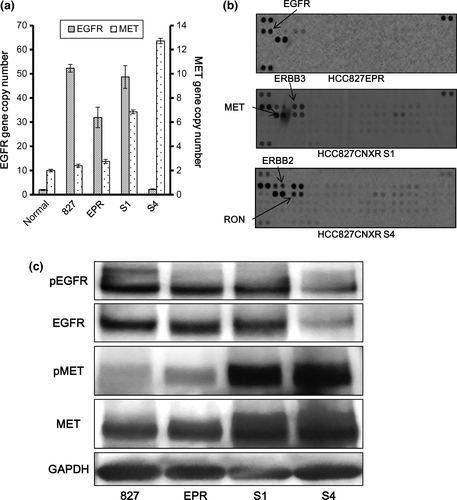
We then searched for possible activation of other RTK signals bypassing the EGFR using a phospho-RTK array. Compared with parental cells, ERBB3 and MET were phosphorylated in both HCC827CXR S1 and S4 cells (Fig. 2b). This result was similar to that in gefitinib-resistant HCC827 cells with MET amplification.27 In fact, quantitative PCR demonstrated that HCC827CNXR S1 and S4 cells had moderate (approximately 6.9×) and high (approximately 12×) MET gene copy number gain, respectively (Fig. 2a). Western blot also confirmed that MET was overexpressed and activated compared to HCC827 or HCC827EPR cells in these resistant cells (Fig. 2c). In contrast, both expression and phosphorylation of EGFR were decreased in HCC827CNXR S4 cells (Fig. 2c).
Effect of MET-tyrosine kinase inhibitors with or without epidermal growth factor receptor-tyrosine kinase inhibitors on HCC827CNXR cells
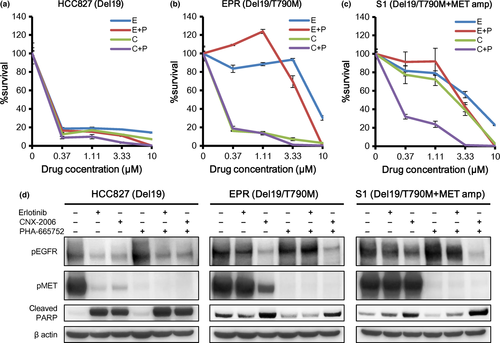
We then treated HCC827CNXR S1 cells with the combination of EGFR-TKI and MET-TKI. As expected, CNX-2006 with PHA-665752 inhibited cell proliferation and survival in HCC827CNXR S1 cells, although erlotinib with PHA-665752 did not (Fig. 3a–c). Induction of apoptosis by this combination was confirmed by western blot analysis (Fig. 3d).
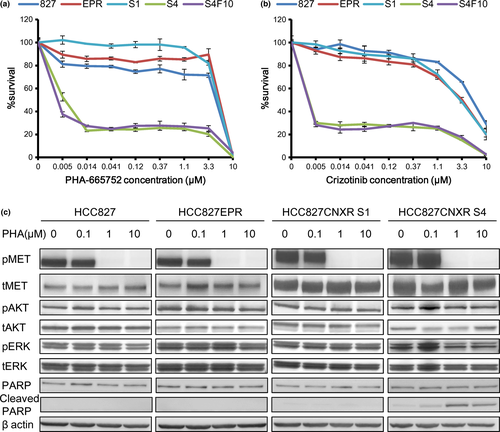
Because direct sequencing, copy number analyses and western blot analysis all suggested that the driver oncogene of the HCC82CNXR S4 swapped from EGFR to MET, we tested whether that MET-TKI alone was effective in HCC827CNXR S4 cells (Fig. 2c). Indeed, only HCC827CNXR S4 cells were sensitive to MET-TKI (PHA-665752 and crizotinib) alone, while other cell lines were highly resistant to this treatment as expected, probably because their EGFR signaling was still active (Fig. 4a,b). This sensitization of HCC827CNXR S4 to MET inhibition was not transient because HCC827CNXR S4F10 cells that were cultured without CNX-2006 for 10 passages showed identical sensitivity to HCC827CNXR S4 and MET-TKI (Fig. 4a,b). Immunoblotting demonstrated that phosphorylation of MET was inhibited by PHA-665752 in all cells. However, ERK phosphorylation was inhibited only in HCC827CNXR S4 cells, although AKT phosphorylation was slightly inhibited. In addition, cleaved PARP was induced at lower concentrations only in HCC827CNXR S4 cells (Fig. 4c).
Clinical case of an oncogene swap from epidermal growth factor receptor to MET
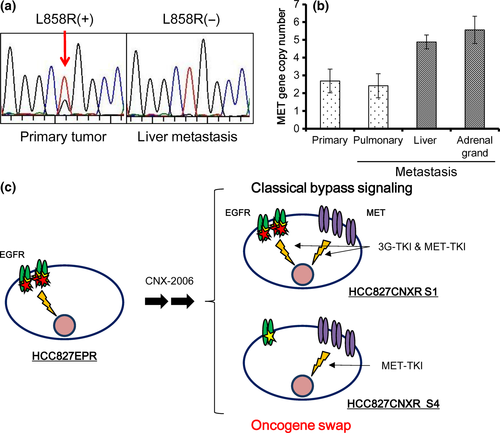
To see whether an oncogene swap is a novel mechanism of acquired resistance that actually occurs in patients, we examined several specimens obtained from the autopsy of patients with lung cancer harboring EGFR mutations who died of acquired resistance after an initial good response to EGFR-TKI. Of the four gefitinib-refractory lesions developed in a female patient, the primary tumor had L858R (Fig. 5a). However, the liver metastasis lost this mutation (Fig. 5a) and T790M was absent in this tumor. Pulmonary and adrenal grand metastases were not available for direct sequence analysis. This mutation status in each site was confirmed by target sequencing analysis. Quantitative PCR revealed that metastases in the liver and adrenal glands had moderate MET copy number gain (4.9 and 5.6 copies, respectively), while the primary and metastatic pulmonary lesions did not show increased MET copy numbers (Fig. 5b). This finding may represent a clinical example of the oncogene swap observed in our in vitro resistant model.
Discussion
In the present study, we established CNX-2006 resistant cells from HCC827EPR harboring Del19/T790M. One of the subclones, HCC827CNXR S4, lost dependence on EGFR activation while gaining MET pathway dependence, which we call an “oncogene swap,” and propose as a novel mechanism of acquired resistance.
Acquired resistant mechanisms to EGFR small-molecule inhibitors are grouped into several categories28: (i) secondary EGFR mutation, mainly T790M; (ii) alternative pathway activation or bypass track mechanisms; and (iii) histologic transformation, including small cell lung cancer transformation. We previously reported that resistant mechanisms can be heterogeneous among different lesions in a single patient probably depending on the microenvironment of each tumor, such as differences in drug concentrations or differences in concentration of growth factors, such as hepatocyte growth factor (HGF) and insulin-like growth factor (IGF).19
The molecules involved in alternative pathway activation are extensive. MET amplification is the first reported alteration belongs this category.27 However, it is now known that numerous pathways trigger resistance, including activation of HER2, AXL, IGF1R, BRAF and CRKL, or loss of PTEN and RAS.14, 29-34 Recently, Crystal et al.35 developed cell culture models derived from biopsy samples and then subjected these cells to pharmacological screening for 76 compounds in the presence of original TKI. As a result, they were able to identify a combination of EGFR and fibroblast growth factor receptor (FGFR) inhibitors that was active in an EGFR mutant resistant cancer with a mutation in FGFR3. In these cases, the original EGFR pathway is still active; therefore, EGFR-TKI need to be combined with inhibitors of novel pathways.
In HCC827CNXR S1 cells harboring T790M, MET inhibitor alone did not affect ERK and AKT phosphorylation. In contrast, HCC827CNXR S4 cells are quite unique in that they have lost amplified-EGFR, including the T790M allele, and the MET gene is amplified instead, resulting in change of impact of MET inhibition. In HCC827CNXR S4 cells, ERK phosphorylation was effectively suppressed by MET inhibition alone, leading to marked growth inhibition, while neither ERK nor AKT phosphorylation was inhibited by MET-TKI monotherapy in HCC827CNXR S1 cells. From these results, we conclude that amplified-MET works as a bypass signaling of EGFR in HCC827CNXR S1 cells, while amplified-MET works as a major oncogenic signaling instead of EGFR in HCC827CNXR S4 cells, of which we coined the term, “oncogene swap” (Fig. 5c). Loss of amplified EGFR gene as a mechanism of acquired resistance has been reported previously36; however, the authors were not able to identify the alternative driver oncogene.
Furthermore, we suggest that an oncogene swap is not just an artifact in vitro; rather, this phenomenon may account for at least some of the resistant mechanism in patients. The liver metastasis of the patient we presented lost the EGFR mutation but had MET copy number gain, strongly suggesting the presence of an oncogene swap. Recently, Planchard et al.37 report the loss of T790M with MET or HER2 amplification in 3G-TKI resistant cases.
When combination therapy is considered for treatment of resistant tumors with alternative pathway activation, overlapping toxicities of agents that make up the combination will limit the efficacy of the treatment. If the “oncogene swap” is identified in patients, original TKI (in the present case, EGFR-TKI) is no longer necessary and a new TKI (in the present case, MET-TKI) is all that is needed; this is expected to lead to higher tolerability as well as efficacy. However, resistant mechanisms in multiple metastatic sites in one patient are sometimes heterogeneous. Thus, indication of treatment with single agents should be carefully considered.
In conclusion, we found that an oncogene swap from EGFR to MET might be a novel mechanism of acquired resistance to EGFR-TKI. We should keep in mind this can happen to avoid not just futile but potentially harmful inhibition of the original oncogene.
Acknowledgments
This work was partly supported by the IASLC Lung Cancer Young Investigator Award 2015–2016 (K.S.) and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (25830119 and 15K18450 to K.S.), Takeda Science Foundation and Uehara Memorial Foundation (to T.M.) and Kaibara Morikazu Medical Science Promotion Foundation to (K.S).
Disclosure Statement
Tetsuya Mitsudomi received a lecture fee from AstraZeneca, Boehringer Ingelheim, Chugai, Taiho and Pfizer and research funding from AstraZeneca, Boehringer Ingelheim, Chugai and Taiho. The other authors have no conflict of interest to declare.




