Phase II trial of hypomethylating agent combined with nivolumab for acute myeloid leukaemia relapse after allogeneic haematopoietic cell transplantation—Immune signature correlates with response
Petya Apostolova, Stefanie Kreutmair, Cristina Toffalori, Marco Punta and Susanne Unger contributed equally to this work.
Burkhard Becher, Luca Vago and Robert Zeiser contributed equally to this work.
Summary
Acute myeloid leukaemia (AML) relapse after allogeneic haematopoietic cell transplantation (allo-HCT) is often driven by immune-related mechanisms and associated with poor prognosis. Immune checkpoint inhibitors combined with hypomethylating agents (HMA) may restore or enhance the graft-versus-leukaemia effect. Still, data about using this combination regimen after allo-HCT are limited. We conducted a prospective, phase II, open-label, single-arm study in which we treated patients with haematological AML relapse after allo-HCT with HMA plus the anti-PD-1 antibody nivolumab. The response was correlated with DNA-, RNA- and protein-based single-cell technology assessments to identify biomarkers associated with therapeutic efficacy. Sixteen patients received a median number of 2 (range 1–7) nivolumab applications. The overall response rate (CR/PR) at day 42 was 25%, and another 25% of the patients achieved stable disease. The median overall survival was 15.6 months. High-parametric cytometry documented a higher frequency of activated (ICOS+, HLA-DR+), low senescence (KLRG1−, CD57−) CD8+ effector T cells in responders. We confirmed these findings in a preclinical model. Single-cell transcriptomics revealed a pro-inflammatory rewiring of the expression profile of T and myeloid cells in responders. In summary, the study indicates that the post-allo-HCT HMA/nivolumab combination induces anti-AML immune responses in selected patients and could be considered as a bridging approach to a second allo-HCT.
Trial-registration: EudraCT-No. 2017-002194-18.
Abbreviations
-
- AE
-
- adverse events
-
- aGVHD
-
- acute graft-versus-host disease
-
- ALAT
-
- alanine aminotransferase
-
- allo-HCT
-
- allogeneic haematopoietic cell transplantation
-
- AML
-
- acute myeloid leukaemia
-
- ASAT
-
- aspartate aminotransferase
-
- BM
-
- bone marrow
-
- cGVHD
-
- chronic graft-versus-host disease
-
- CR
-
- complete response
-
- CRi
-
- complete response with incomplete recovery
-
- DLI
-
- donor lymphocyte infusions
-
- ECOG
-
- Eastern Cooperative Oncology Group
-
- ELN
-
- European Leukemia Net
-
- FAS
-
- full analysis dataset
-
- GVHD
-
- graft-versus-host disease
-
- HLA
-
- human leukocyte antigen
-
- HMA
-
- hypomethylating agents
-
- irAE
-
- immune-related adverse events
-
- MiHA
-
- minor histocompatibility antigen-specific
-
- MLFS
-
- morphologic leukaemia-free state
-
- NGS
-
- next-generation sequencing
-
- NR-FU
-
- non-responder follow-up
-
- NRM
-
- non-relapse mortality
-
- ORR
-
- overall response rate
-
- OS
-
- overall survival
-
- PD
-
- progressive disease
-
- PFS
-
- progression-free survival
-
- PP
-
- per-protocol dataset
-
- PR
-
- partial response
-
- r/r
-
- relapsed/refractory
-
- R-FU
-
- responder follow-up
-
- SAE
-
- severe adverse events
-
- SAF
-
- safety analysis dataset
-
- SD
-
- stable disease
-
- TCR
-
- T cell receptor
INTRODUCTION
Relapse of acute myeloid leukaemia (AML) after allogeneic haematopoietic cell transplantation (allo-HCT) is frequent and accounts for more than 50% of the mortality rate after allo-HCT.1 The mechanisms of immune escape are diverse2 and include impaired leukaemia cell recognition due to downregulation of human leukocyte antigen (HLA) class II or loss of mismatched HLA,3-6 the emergence of new mutations in oncogenes or tumour suppressor genes,7, 8 dysregulated production of cytokines and metabolites that promote an immunosuppressive microenvironment,9-11 and increased expression of immune checkpoint ligands.3, 12, 13 Donor lymphocyte infusions (DLI) for AML relapse result in remission rates of about 20%.14-16 Other approaches to prevent or treat AML relapse after allo-HCT comprise a second allo-HCT,17-19 FLT3-kinase inhibitors,20, 21 hypomethylating agents,22, 23 BCL-2 inhibitors,24-26 immune checkpoint inhibitors27, 28 and others.29 Despite the availability of these treatment options, the long-term prognosis of patients with AML relapse post-allo-HCT remains dismal.16, 18, 30
Several studies tested the safety and efficacy of anti-PD-1 antibodies in combination with other agents in patients with AML outside the allo-HCT setting.31-35 A recent meta-analysis reported an overall response rate (ORR) of 58% (95% CI: 33–81) as a first-line treatment and an ORR of 33% (95% CI: 27–39) in relapsed/refractory (r/r) disease.36 Apart from these trials outside of allo-HCT, several reports have provided a rationale for administering anti-PD-1 antibodies in the post-allo-HCT setting.37 High frequency of PD-1hi TIM-3+ T cells with signs of exhaustion was strongly associated with AML relapse post-allo-HCT.38 In patients with relapsed leukaemia, CD34+ malignant cells with high PD-L1 expression and PD-1 levels on CD8+ T cells increased.12 Blocking the PD-1/PD-L1 axis augmented the proliferation of minor histocompatibility antigen-specific (MiHA) T cells.12 Besides high levels of PD-1, MiHA-specific T cells from relapsed patients had elevated coexpression of the inhibitory receptors PD-1, TIGIT and KLRG1 compared to non-relapsed patients.39 Expression of PD-1 was exceptionally high early after allo-HCT with a progressive normalization at the CD8+ T cell surface, whereas levels at the CD4+ T cell surface remained elevated.40 Patients with multiple AML relapses had an increased frequency of CD8+ PD-1+ T cells expressing OX-40, TIM3, or LAG3 compared to patients with first AML relapse or newly diagnosed AML.41 A high proportion of early-differentiated memory stem and central memory bone marrow (BM) T cells expressing multiple inhibitory receptors was present in the BM of patients with AML relapse after allo-HCT.13 These studies suggest that engagement of anti-PD-1 might be targeted to prevent or treat post-allo-HCT relapse.
A recent phase I trial using nivolumab for haematological malignancy relapse post-allo-HCT reported that anti-PD-1 immunotherapy was mainly effective in patients with lymphoid malignancies.42 At the same time, anti-CTLA-4 therapy has shown efficacy in AML relapse post-allo-HCT.27 So far, no study has explicitly investigated the hypomethylating agent (HMA)/nivolumab combination for haematological AML relapse post-allo-HCT. Here, we present clinical patient data, single-cell RNA sequencing, high-parametric spectral flow cytometry analysis and genome sequencing from a prospective, investigator-initiated phase II trial on the efficacy and safety of nivolumab in combination with HMA in patients with AML relapse after allo-HCT (Study Code: CA209-9P9, EudraCT-No. 2017-002194-18). Preclinical mechanistic studies complement the clinical data to identify how immune checkpoint inhibition might reinvigorate graft-versus-leukaemia immunity.
METHODS
Trial design
This prospective, open-label, non-randomized, investigator-initiated phase II trial assessed the efficacy and safety of nivolumab in patients with AML relapse after allo-HCT in combination with HMA and, in selected patients, DLI. For details on the trial design, see Table S1. Patients were enrolled and treated at the Medical Center—University of Freiburg, Germany. Treatment was performed intravenously with nivolumab 3 mg/kg body weight, infused over 1 h and repeated every 2 weeks. The planned duration of the intervention was 12 weeks (a total of 7 cycles of nivolumab) unless a relapse, disease progression, unacceptable toxic effects, or death occurred. The administration time could be extended to 60 weeks at the investigator's discretion in case the patient experienced a benefit from treatment. HMA were administered between the diagnosis of relapse and the first administration of nivolumab, but not after the patients had started nivolumab treatment. DLI could be given in combination with nivolumab after careful consideration of the risk for GVHD and discussion with the coordinating investigator. The planned follow-up per patient was 18 months after the permanent discontinuation of nivolumab.
Trial oversight
All patients provided written informed consent before participating in the trial, and approval was obtained from the University of Freiburg Ethics Committee. The trial was designed and conducted under the guidelines for Good Clinical Practice of the International Council for Harmonization, the principles of the Declaration of Helsinki and the local regulations. An independent data monitoring committee reviewed the safety data. The investigators designed the trial. Data were collected and analysed by the investigators and their research staff. Drafts of the manuscript were written by the first and last authors and revised in collaboration with all the authors.
Patients
Key eligibility criteria included age of 18 years and evidence of AML relapse after at least one previous allo-HCT provided by cytological or histological examination of a BM biopsy or an extramedullary site biopsy. Additional eligibility criteria were at least 10% of donor-derived chimerism, no administration of immune suppression for at least 2 weeks before the screening, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, and unavailability of a superior treatment approach. Key exclusion criteria were previous or currently active acute GVHD grade III–IV, chronic GVHD requiring immune suppression at the time of treatment, active autoimmune diseases, severely impaired pulmonary function (mechanical ventilation or resting O2 saturation < 90%), and severely impaired cardiac function (requirement for vasopressors, NYHA class III or IV heart failure, uncontrolled hypertension or ventricular arrhythmias). Patients who had received active treatment in a clinical study of any investigational agent within 30 days before the first administration of nivolumab were also excluded. Table S1 includes a complete list of inclusion and exclusion criteria.
Assessment of response and safety
Response definitions were established based on the recommendations of the International Working Group and the guidelines of the European Leukemia Net (ELN).43-45 Complete response (CR) was defined by a BM biopsy without clusters or collections of blast cells (<5%), an absence of extramedullary leukaemia, normal values for the absolute neutrophil count and platelet count, and independence from red blood cell transfusion. Complete response with incomplete recovery (CRi) was achieved when all CR criteria were fulfilled except for residual neutropenia or thrombocytopenia. A morphologic leukaemia-free state (MLFS) was recorded when the BM blasts were less than 5% with or without evidence of a haematological recovery. A partial response (PR) was defined as a decrease of blasts by at least 50% compared to the start of study treatment in the PB or BM, and BM blasts between 5% and 25%. A stable disease (SD) was considered when AML manifestations remained identical to treatment initiation and failed to fulfil the criteria for response. A progressive disease (PD) was defined as an increase of AML cells of at least 50% of the initial value in the PB or BM or the appearance of a new extramedullary disease. The ORR was defined as the proportion of patients achieving CR, CRi, MLFS, or PR.
Patients were monitored for clinical or laboratory signs of GVHD and immune-related adverse events (irAE) at each visit. Adverse events (AE) and serious adverse events (SAE) were scored according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Relationship to nivolumab was assessed for each AE/SAE by the investigator.
Endpoints
The primary endpoint was the ORR on days 42 and 84 after the start of nivolumab. The secondary endpoints concerning efficacy were: time to treatment response, duration of response, overall survival (OS), progression-free survival (PFS), non-relapse mortality (NRM), quality of life measured by EORTC QLQ-C30 and EORTC QLQ-HDC29, and changes in the alloreactive immune cell phenotype during treatment. The secondary endpoints concerning safety were: the incidence and severity of GVHD, the incidence of infectious complications, and the incidence and severity of irAE.
Statistical analysis of the clinical data
As this is a phase 2 trial without the intention of a confirmatory proof of efficacy, no formal sample size calculation was performed. Initially, it was planned to include 20 evaluable patients, mainly based on feasibility considerations. However, the precision of the ORR estimation is of interest, for example, when planning future trials. Therefore, we considered the size of a two-sided 90% confidence interval that can be calculated based on 20 patients and an expected ORR of 45%. When the sample size is 20, a two-sided 90.0% confidence interval for a single proportion using the large sample normal approximation will extend 0.183 from the observed proportion for an expected proportion of 0.450.
The data cutoff for the statistical analysis of clinical data was April 12, 2022. The full analysis dataset (FAS) and the safety set (SAF) included all patients who received at least one administration of nivolumab (n = 16). The per-protocol dataset (PP) had all patients who received at least two administrations of nivolumab (n = 11). Descriptive statistics were used to report continuous and discrete variables' frequency and percentages. Exact two-sided 95% confidence intervals for ORR were calculated based on the binomial distribution. OS and PFS were defined as the time from the start of treatment with nivolumab until death (OS)/death or disease progression (PFS), treating observation times where the event of interest did not occur as censored. Analyses of OS and PFS were performed with the Kaplan–Meier method. NRM was defined as the time from the start of treatment with nivolumab to the date of death not preceded by hematologic disease recurrence, which is considered a competing risk. For chronic graft-versus-host disease (cGvHD), death without prior observation of cGvHD was regarded as a competing risk. For these time-to-event endpoints with competing risks, cumulative incidence rates were calculated. The calculation of time to response and response duration was restricted to those patients where the response was related to nivolumab treatment, that is any responses after prior disease progression or after documentation of treatment failure were not counted. Due to the low number of responders, the data are reported descriptively.
All other methods are listed in the Supporting Information, Materials and methods section.
RESULTS
Patient characteristics and treatments
Between May 2018 and May 2020, 18 patients with AML relapse after allo-HCT signed written informed consent. One patient was excluded due to a screening failure, and one patient did not receive nivolumab. The remaining 16 patients received at least one nivolumab infusion and were included in the FAS. All patients were treated with 3 mg/kg body weight nivolumab every 2 weeks for up to 12 weeks unless a relapse, disease progression, unacceptable toxic effects, or death occurred. An additional treatment phase of up to 60 weeks was allowed if patients had benefited from the treatment. A per-protocol (PP) dataset was defined as the patients who received at least two doses of nivolumab (n = 11). An overview of the patient cohorts and trial design is provided in Figure S1. The primary end-point was the ORR on days 42 and 84. The ORR was determined as the summed percentage of patients achieving a CR, a CRi, a MLFS, or a PR defined in line with the recommendations of the International Working Group and the guidelines of the ELN 2017.43-45 A study synopsis describing the inclusion and exclusion criteria and endpoints is reported in Table S1.
The clinical characteristics are presented in Table 1 and Table S2. The median patient age was 58 years (range 23–73), and seven patients (43.75%) were female. The majority of patients (75%) had an adverse genetic risk (according to the 2017 ELN risk stratification by genetics43). The median time between allo-HCT and relapse was 409 days (range 45–2724 days), and the median time between relapse and study inclusion was 20 days (range 2–366 days). Treatment between diagnosis of relapse and the start of nivolumab was as follows: most patients (10/16, 62.5%) received DLI in combination with decitabine or azacytidine. Five patients (31.25%) were treated with HMA alone, and one patient (6.25%) received DLI alone (Table S3). The median number of nivolumab applications on trial until week 12 was 2 (range 1–7). The most frequent reason for discontinuing nivolumab was disease progression or lack of response (8/16 patients, 50%). Other reasons were intolerable toxicities, death, CRi achieved, or patient wish. One patient had a SD with long-term benefits from nivolumab and continued treatment after week 12 with an additional 14 doses of nivolumab (Table S3).
| Variable | Value |
|---|---|
| Number of patients | 16 |
| Median age (range)—years | 58 (23–73) |
| Sex—no. (%) | |
| Female | 7 (43.75%) |
| Male | 9 (56.25%) |
| ECOG performance status—no. (%) | |
| 0 | 6 (37.5%) |
| 1 | 8 (50%) |
| 2 | 2 (12.5%) |
| AML type—no. (%) | |
| De novo | 8 (50%) |
| Secondary | 8 (50%) |
| AML genetic risk classification—no. (%) | |
| Favourable | 2 (12.5%) |
| Intermediate | 1 (6.25%) |
| Adverse | 12 (75%) |
| Unknown | 1 (6.25%) |
| Initial treatment for AML | |
| Cytarabine-based chemotherapy | 11 (68.75%) |
| HMA | 3 (18.75%) |
| Upfront allo-HCT | 2 (12.5%) |
| Allo-HCT donor sex—no. (%) | |
| Female | 5 (31.25%) |
| Male | 11 (68.75%) |
| Median donor age (range)—years | 28 (20–49) |
| Donor type—no. (%) | |
| Unrelated donor | 14 (87.5%) |
| Sibling | 2 (12.5%) |
| Graft source—no. (%) | |
| Peripheral blood stem cells | 14 (87.5%) |
| Bone marrow | 1 (6.25%) |
| Unknown | 1 (6.25%) |
| Best response to allo-HCT—no. (%) | |
| Complete remission | 15 (93.75%) |
| Refractory disease | 1 (6.25%) |
| Median time from allo-HCT to relapse (range)—days | 409 (45–2724) |
| Median time from relapse to study inclusion (range)—days | 20 (2–366) |
| Extramedullary manifestation of relapse | |
| No | 13 (81.25%) |
| Yes | 1 (6.25%) |
| Unknown | 2 (12.5%) |
| Median bone marrow blasts at screening (range)—% | 25 (11–96) |
- Note: Demographic and disease characteristics of the full analysis dataset (FAS) with n = 16 patients.
- Abbreviations: allo-HCT, allogeneic haematopoietic cell transplantation; AML, acute myeloid leukaemia; ECOG, Eastern Cooperative Oncology Group; HMA, hypomethylating agents.
Efficacy, survival and NRM
The course of disease during nivolumab treatment for each patient is shown in Figure 1A. Response evaluations were performed on days 42 and 84 after the start of nivolumab. The ORR on day 42 was 25% (95% CI 7.3–52.4) (Table 2; Figure 1B). Two patients achieved CRi (12.5%), two patients achieved PR (12.5%), four patients had SD (25%) and two patients had PD (12.5%). Five patients (31.25%) had died or had disease progression before day 42, leading to salvage therapy, and for one patient (6.25%), the evaluation was missing (Figure 1B). On day 84, two patients were evaluated for response, with one having an SD (6.25%) and the other having PD (6.25%). Nine patients (56.25%) had died or had disease progression before day 84, and for five patients (31.25%), the evaluation was missing due to discontinuation of the treatment (Table 2). The percentage of donor-derived chimerism at screening and week 6 is shown in Table S4. The median follow-up time was 18.8 months (95% CI 18.1–22.5 months). The median PFS was 1.8 months (95% CI 0.76–6.9 months, Figure 1C). The median OS was 15.6 months (95% CI 0.85–not reached, Figure 1C). In a subgroup of patients who received at least two doses of nivolumab (PP dataset), the response on day 42 was 18.2% (95% CI 2.3–51.8) (Table 2). In this subgroup, the median PFS was 4.7 months (95% CI 0.85–11.3 months), and the median OS was not reached (Figure 1D). After nivolumab discontinuation, 10/16 patients (62.5%) underwent a second allo-HCT. NRM was 25% (95% CI 7.2–48.2).
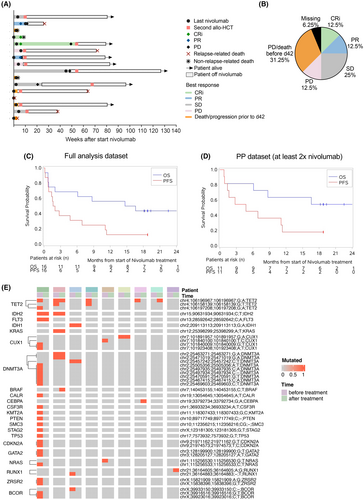
| Variable | Value |
|---|---|
| Response evaluation FAS on day 42—no. (%) | 16 (100%) |
| CR | 0 (0%) |
| CRi | 2 (12.5%) |
| MLFS | 0 (0%) |
| PR | 2 (12.5%) |
| SD | 4 (25%) |
| PD | 2 (12.5%) |
| Progression/death before day 42 | 5 (31.25%) |
| Missing evaluation | 1 (6.25%) |
| Response evaluation FAS on day 84—no. (%) | |
| CR | 0 (0%) |
| CRi | 0 (0%) |
| MLFS | 0 (0%) |
| PR | 0 (0%) |
| SD | 1 (6.25%) |
| PD | 1 (6.25%) |
| Progression/death before day 84 | 9 (56.25%) |
| Missing evaluation | 5 (31.25%) |
| Response evaluation PP on day 42—no. (%) | 11 (100%) |
| CR | 0 (0%) |
| CRi | 1 (9.1%) |
| MLFS | 0 (0%) |
| PR | 1 (9.1%) |
| SD | 4 (36.4%) |
| PD | 2 (18.2%) |
| Progression/death before day 42 | 2 (18.2%) |
| Missing evaluation | 1 (9.1%) |
| Response evaluation PP on day 84—no. (%) | 11 (100%) |
| CR | 0 (0%) |
| CRi | 0 (0%) |
| MLFS | 0 (0%) |
| PR | 0 (0%) |
| SD | 1 (9.1%) |
| PD | 1 (9.1%) |
| Progression/death before day 84 | 4 (36.4%) |
| Missing evaluation | 5 (45.5%) |
- Note: Response evaluation on days 42 and 84 after the start of nivolumab treatment using response definitions based on the recommendations of the International Working Group and the guidelines of the European Leukemia Net.43-45
- Abbreviations: CR, complete response; CRi, complete response with incomplete recovery; FAS, full analysis dataset; MLFS, morphologic leukaemia-free state; PD, progressive disease; PP, per-protocol dataset; PR, partial response; SD, stable disease.
We sought to determine whether the response to HMA/nivolumab was dependent on the presence of specific genomic mutations. To this end, we performed a targeted genomic next-generation sequencing (NGS) for hotspot mutations frequently found in AML patients. The panel included 15 full-length genes (exons only) and 39 additional genes, where oncogenic hotspots were covered (Table S5). DNA extracts from peripheral blood leukocytes directly before the start of nivolumab and after nivolumab discontinuation were available for nine patients (Table S6). We observed a TET2 mutation loss after therapy in four patients and a loss of mutations in CUX1 and NRAS in two patients (Figure 1E). New mutations were found for BRAF, CEBPA and RUNX1 (Figure 1E). Response varied between the patients with TET2-mutant clone loss, with one each having CRi, PR, SD and PD on day 42 after initiating nivolumab treatment. These data suggest that AML clones might differ in their sensitivity to HMA/nivolumab, although further studies are warranted.
Safety
The safety analysis dataset (SAF) included all 16 patients who received at least one dose of nivolumab. All patients were monitored weekly for AEs development. Fifteen patients (93.75%) had at least one AE. The mean number of AEs per patient was 7.13 (range 0–20). The most frequent AEs were disorders of the blood and lymphatic system, such as febrile neutropenia (6/16 pts, 37.5%), thrombocytopenia (5/16 pts, 31.25%), anaemia (3/16 pts, 18.75%) and neutropenia (3/16 pts, 18.75%). Frequent non-hematologic AEs were pyrexia (4/16 pts, 25%), fatigue (3/16 pts, 18.75%), peripheral oedema (3/16 pts, 18.75%), pneumonia (3/16 pts, 18.75%) and increased transaminases (3/16 pts, 18.75%). Fourteen patients (87.5%) experienced at least one AE ≥ grade 3. Frequent AEs (defined as occurring in at least two patients) are reported in Table 3. In 7 patients (43.75%), AEs related to nivolumab were reported (Table S7). The most frequent nivolumab-related AEs were increased transaminases, fatigue and pyrexia. In one case each, pericardial effusion, pleural effusion, pneumonitis, arthralgia, myalgia and encephalopathy were reported. Nivolumab-related AEs grade 3 or higher were reported in 4 patients (25%) (Table S7). In two patients, severe adverse events (SAEs) led to death. These were sepsis and cerebral haemorrhage; none was attributed to nivolumab (Table S8).
| AE | Any grade | Grade ≧3 |
|---|---|---|
| Absolute number (%) | Absolute number (%) | |
| Any | 15 (93.75%) | 14 (87.5%) |
| Hematologic AE | ||
| Febrile neutropenia | 6 (37.5%) | 6 (37.5%) |
| Thrombocytopenia | 5 (31.25%) | 3 (18.75%) |
| Anaemia | 3 (18.75%) | 2 (12.5%) |
| Neutropenia | 3 (18.75%) | 3 (18.75%) |
| Non-hematologic AE | ||
| Pyrexia | 4 (25%) | 1 (6.25%) |
| Fatigue | 3 (18.75%) | 1 (6.25%) |
| Peripheral oedema | 3 (18.75%) | 0 (0%) |
| Pneumonia | 3 (18.75%) | 1 (6.25%) |
| Transaminases increased | 3 (18.75%) | 1 (6.25%) |
| ALAT increased | 2 (12.5%) | 1 (6.25%) |
| ASAT increased | 2 (12.5%) | 1 (6.25%) |
| Blood bilirubin increased | 2 (12.5%) | 1 (6.25%) |
| C-reactive protein increased | 2 (12.5%) | 0 (0%) |
| Weight loss | 2 (12.5%) | 0 (0%) |
| Polyneuropathy | 2 (12.5%) | 0 (0%) |
| Pleural effusion | 2 (12.5%) | 0 (0%) |
| Urinary retention | 2 (12.5%) | 0 (0%) |
| General physical health deterioration | 2 (12.5%) | 2 (12.5%) |
- Note: AEs were recorded weekly according to CTC-AE Version 4.0. Frequent AEs defined as those occurring in at least two patients are reported.
- Abbreviations: ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase.
Development of GVHD/irAE and infections are particularly interesting in a cohort of allo-HCT recipients treated with a checkpoint inhibitor and reported as a secondary endpoint in our study (Table S9). Until day 84, eight patients (50%) had immune-related complications after nivolumab. Five patients (31.25%) developed acute graft-versus-host disease (aGVHD), with the skin affected in four patients and the liver in one. The maximal overall aGVHD grade was II. Four patients (25%) had manifestations of cGVHD. One of them developed severe multiple-organ cGVHD, which led to death. Six patients (37.5%) developed infections (Table S9). Overall, our study's frequency and type of AEs align with published literature, with GVHD remaining frequent toxicity of checkpoint inhibitor treatment.
High-parametric cytometry reveals a correlation between CD8+ effector memory T cell phenotype and response
Immune checkpoint inhibition induces clinical responses by boosting the T cell-driven anti-tumour response. We next asked whether responders displayed specific phenotypic patterns in their T cell compartments. To address this question, we first interrogated samples from the peripheral blood collected at screening and at serial time points after the start of nivolumab (Figure 2A) using high-parametric spectral flow cytometry (Table S10). Specimens were collected only as long as patients did not receive an AML treatment other than HMA, nivolumab and DLI. An overview of the samples analysed at each time point is presented in Table S11. The patients were stratified according to the response at week 6 after the start of nivolumab treatment, where patients with CRi or PR were classified as responders, and patients with SD or PD were classified as non-responders. We found that the frequencies of B, T, NK and myeloid cells at screening and during treatment with HMA/nivolumab were comparable between responders and non-responders (Figure S2). We then focused on the T cell compartment (Figure S3A,B) and analysed the expression of activating and inhibitory cell surface molecules. We found that CD8+ effector memory T cells (CD8 Tem) of responders showed a signature of higher activation and co-stimulation marker expression (HLA-DR, ICOS, CD27 and CD38) and lower senescence marker expression (KLRG1 and CD57) than CD8 Tem from non-responders (Figure 2B–D). This signature was detectable 1 week after the first administration of nivolumab and was statistically significant at week 6 when we assessed the clinical response (Figure 2B–D). Comparable results in CD4+ T cells (Figure S3C) and lower frequencies of CD8+ terminal effector T cells (Temra, Figure S3D) in responders suggest that terminal T cell differentiation/senescence associates with inferior response rates. Transfer of responder patient T cells into a xenograft mouse AML model resulted in prolonged survival compared to the transfer of non-responder patient T cells, indicating an improved leukaemia control (Figure 2E).
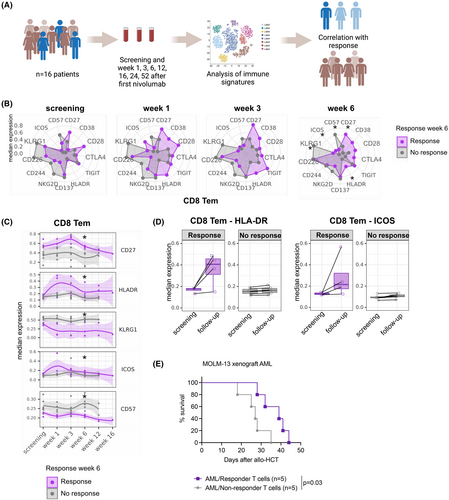
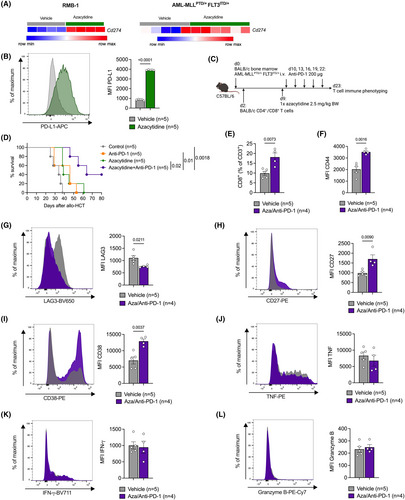
Surprisingly, the longitudinally obtained cytokine polarization profile of CD8 Tem and other T cell subsets, as well as NK cells, did not significantly correlate with the response (Figure S4), suggesting that activation/senescence marker expression might be a better predictive marker for response than cytokine production. To expand on this finding, we studied the response to azacytidine and anti-PD-1 in an experimental mouse model resembling the human setting. We observed that treatment with azacytidine increased the expression of the PD-1 ligand PD-L1 (encoded by Cd274) on AML cells (Figure 3A,B). We next used a mouse model, in which mice received AML cells (bearing MLL-PTD and FLT3-ITD mutations), followed by a transfer of allogeneic T cells and treatment with one dose of azacytidine and five doses of an anti-PD-1 antibody, either alone or in combination (Figure 3C). The combination treatment resulted in significantly prolonged survival, compared to no or single-agent treatment (Figure 3D). We found a higher percentage of CD8+ T cells within the CD3+ population of mice treated with azacytidine and anti-PD-1 than vehicle-treated animals (Figure 3E). CD44 expression on CD3+ T cells was elevated, indicating an enhanced memory phenotype (Figure 3F). Furthermore, we observed lower expression of LAG3 (Figure 3G) and higher expression of the activation markers CD27 and CD38 on T cells (Figure 3H,I) following treatment. The production of the pro-inflammatory cytokines IFN-γ, TNF and Granzyme B remained unchanged (Figure 3J–L). These data suggest—in line with our observations in patients—that surface activation markers, rather than cytokine production, correlate with response to HMA/nivolumab treatment and should be further explored as biomarkers of response.
The transcriptional profile of the BM immune microenvironment has patient- and response-specific features
Next, we performed single-cell RNA sequencing of available BM aspirates from two responders (patients 01-02 and 01-05) and two non-responders (patients 01-08 and 01-11, Figure 4A). Disease characteristics for these patients are provided in Table S12. For patient 01-02, we analysed samples at screening, week 6 (in PR), and week 12 (in PD) after the first nivolumab administration. For patient 01-05, we analysed a sample obtained 24 weeks after the first nivolumab administration (in CRi). For patient 01-08, we analysed samples obtained at screening and week 6 after the start of nivolumab (in SD). Finally, for patient 01-11, we analysed samples obtained at screening and 12 weeks after nivolumab administration (in PD). The BM samples were accordingly classified as “screening”, “responder follow-up (R-FU)” (defined as PR or CRi and termed as R-FU), or “non-responder follow-up (NR-FU)” (defined as SD or PD and termed as NR-FU). We confirmed patients' association with these categories by flow cytometry analysis of the BM composition (Figure 4A).
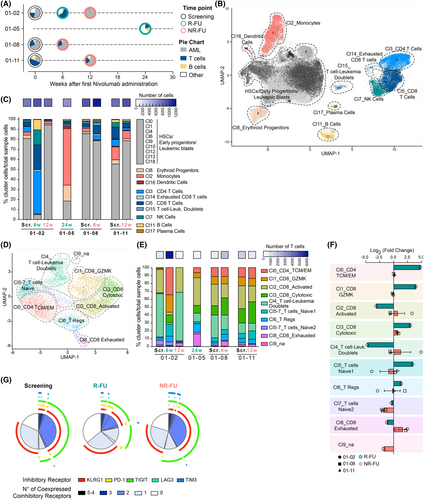
Analysis of single-cell transcriptomic data was performed using our customized bioinformatic pipeline (see the Supporting Information, Materials and method section). In total, 76 375 cells were captured and sequenced. After merging duplicates of the same sample, the number of cells per sample ranged from 6104 to 12 606 (median 9675). Details on the different metrics for the dataset and for each sample are reported in the Supporting Information, Materials and method section and Table S13. When merging cells from all samples into a single dataset, we could identify 19 different clusters that we annotated as follows: HSCs, Early Progenitors and Leukaemic Blasts (clusters 0, 1, 4, 6, 9, 10, 12, 13, 18), Erythroid Progenitors (cluster 8), Monocytes (cluster 2), Dendritic Cells (cluster 16), CD4 T cells (cluster 3), CD8 T cells (cluster 5), Exhausted CD8 T cells (cluster 14), T cell-leukaemia doublets (cluster 15), NK cells (cluster 7), B cells (cluster 11) and Plasma cells (cluster 17) (Figure 4B; Figure S5). Of note, one of the clusters (number 15) showed unusual features, including a high proportion of cell doublets (Figure S6A,B). While doublet-rich clusters are usually not considered in downstream scRNAseq analyses, it is intriguing to observe that this one contained clonally expanded TCR+ T cells (Figure S6C,D) and cells expressing the immature-myeloid CD34 and FLT3 genes (Figure S6D), possibly indicating it could be comprised of CD8+ T cells bound to their leukaemic targets (this cluster is mainly composed of cells from non-responder patient 01-08).
We first observed that the BM composition of patients that responded to therapy appeared to be associated with a patient-specific immune signature. In particular, the R-FU sample of patient 01-05 was strongly enriched in monocytes, while the R-FU sample of patient 01-02 featured large populations of T cells, natural killer cells and B cells (Figure 4C), both in line with flow cytometry data (Figure 4A). Next, we focused on the whole T-cell compartment. We re-ran our bioinformatic pipeline on cells belonging to the CD4, CD8 and T cell-Leukaemia doublets clusters to obtain a more fine-grained characterization of T lymphocyte cellular subtypes. We could distinguish nine subtypes (Figure 4D; Figure S7). The higher resolution of the re-clustered T cell map revealed a marked expansion of CD4+ T and CD8+ GZMK cells in the 01-02 R-FU compared to screening, which was not documented in non-responder patients (Figure S4E,F). Moreover, we observed that NR-FU samples respective to their precedent time points increased the proportion of cells belonging to the CD8 exhausted cluster (Figure 4F).
We next characterized further the BM T cell compartment at the phenotypic level. Multiparametric immunophenotypic analysis evidenced that at the time of response, a higher fraction of PD-1+ T cells infiltrated the BM (median among samples: Scr.: 6.9%, R-FU: 10.4%; NR-FU: 4.1%), with a higher fraction of CD4+ compared to CD8+ T cells, and in particular in the long-term responder patient 01-05 (Figure 4G; Figure S8A–D). Furthermore, in non-responder samples, we observed a higher proportion of T cells infiltrating the BM co-expressing more than two inhibitory receptors compared to responder ones (Figure 4G), which was more pronounced in CD8+ T cells from patient 01-08 (Figure S8A–D). These data suggest that the response to nivolumab treatment is possibly associated with the presence of T cells positive for the PD-1 receptor and with a lower fraction of lymphocytes infiltrating the BM co-expressing more than two inhibitory receptors.
Single-cell protein- and RNA-based analyses indicate that a pro-inflammatory myeloid immune compartment might facilitate response to HMA/nivolumab
To complement our studies of immune phenotypes in responders and non-responders, we next focused on the myeloid/dendritic cell compartment. First, we analysed the expression of activating and inhibitory cell surface molecules on circulating cells by spectral flow cytometry (Figure S9A,B). We observed a trend towards lower expression of CD155 in classical monocytes of responders compared to non-responders (Figure 5A). Previous studies have shown that tumour-derived CD155 inhibits the anti-tumour activity of natural killer cells.46 Additionally, CD155 mediates CD8+ T cell suppression through TIGIT, CD9647 and the degradation of the activating receptor CD226.48 The lower levels of CD155 were also seen in intermediate and non-classical monocytes of responders (Figure S9C). There was also a trend towards an increased HLA-DR expression on classical monocytes of responders compared to non-responders indicating improved antigen presentation through MHC-II by these cells (Figure 5B). Downregulation of HLA class II was connected to AML relapse post-allo-HCT.6 Conversely, increased PD-L1 expression was found in conventional DCs of responders compared to non-responders (Figure 5C). High PD-L1 levels on DCs may render the AML relapse more susceptible to anti-PD-1 immunotherapy. Taken together, we detected immunosuppressive features on myeloid cells in the peripheral blood of non-responders.
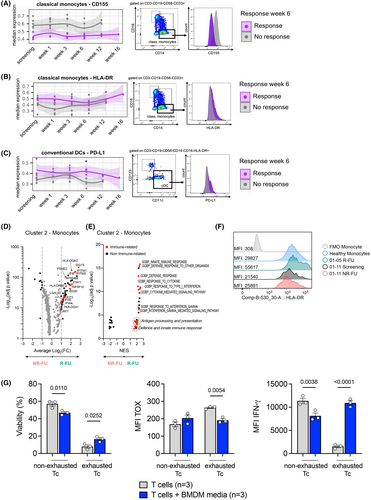
Moreover, in the single-cell transcriptomic analysis of BM aspirates, we observed that the immune environment in patient 01-05 (who had the longest CRi) was characterized by a large mature monocyte compartment (Figure 4C). In-depth analysis of the monocyte cluster 2 showed that compared to non-responders, monocytes in responder samples (the latter overwhelmingly from patient 01-05) expressed higher levels of genes such as HLA-DQA2 and DQB1, PSMB9 and IFITM1 that were enriched in processes involved in antigen processing and presentation and response to interferons (Figure 5D,E; Tables S14 and S15). IRF7, in particular, was previously shown to be connected to GVL effects.9 Consistent with this observation, 01-05 R-FU monocytes showed high surface expression of HLA-DR, even compared to monocytes from the peripheral blood of a healthy individual (Figure 5F). By combining data from routine diagnostic chimerism analysis, immune phenotype and computational genotyping (see Methods), we could identify the (patient or donor) origin of monocytes in each of the samples, and this led us to conclude that the transcriptional differences that we observed in this cluster between responders and non-responders were not linked to the patient or donor origin. In particular, monocytes from both sample 01-05 R-FU and sample 01-11 NR-FU were determined to be of likely donor origin (Figure S10A,B). These data show that a pro-inflammatory, actively antigen-presenting monocyte population dominated the BM microenvironment of patient 01-05, who remained in CRi for 11 months after discontinuing nivolumab.
Based on these findings, we hypothesized that myeloid cells could improve the cytotoxic capacity of exhausted CD8+ T cells. We generated exhausted T cells in vitro using a well-established model based on repetitive antigenic stimulation.49 We co-cultured non-exhausted and exhausted T cells with media harvested from a bone marrow-derived macrophage (BMDM) culture. As expected, chronic antigen stimulation resulted in reduced viability, higher expression of the exhaustion marker TOX and lower capacity to produce IFN-γ (Figure 5G, “non-exhausted Tc” vs. “exhausted Tc”). These effects were partially reversed upon culture with BMDM media (Figure 5G, “exhausted T cells + BMDM media” vs. “exhausted T cells”). Our results indicate that a pro-inflammatory myeloid population might boost the T cell immune response.
DISCUSSION
AML relapse after allo-HCT is marked by a high mortality rate and remains a therapeutic challenge with no gold standard. Several trials have tested the efficacy of HMA combined with DLI. A phase II prospective trial reported an ORR of 30%.50 A retrospective study from our institution found a CR rate of 9% with a median OS of 108 days.51 Decitabine with DLI induced a CR/CRi in 15% of the patients and PR in 4% in another retrospective study, and the median OS was 4.7 months.52 We found that HMA increased anti-CD123 CAR T cell cytotoxicity against AML.22 Based on these results, we hypothesized that HMA and immune checkpoint inhibition might synergize to potentiate graft-versus-leukaemia immunity. The efficacy and safety of the HMA/nivolumab combination in allo-HCT recipients have not been explicitly studied in a prospective trial. We observed an ORR (CR, CRi, PR) at day 42 after the initiation of nivolumab treatment of 25%, comparable with an earlier study that reported an ORR of 21% (4/19 patients) with nivolumab as monotherapy for myeloid malignancy relapse post-allo-HCT.42 In comparison, CTLA-4 blockade with ipilimumab induced a CR in 5/12 (42%) patients with myelodysplastic syndrome/AML.27 Decitabine combined with ipilimumab resulted in a CR/CRi in 5/25 (20%) patients with post-allo-HCT relapse.53 Our data align with the literature and demonstrate that long-lasting responses to PD-1 blockade, even in combination with HMA and DLI, are seen primarily in individual patients. We propose that HMA/nivolumab can be applied as a “bridging” approach to the subsequent treatment, such as a second allo-HCT. It may be essential to intervene earlier than at haematological relapse as a recent case report showed that the combined application of tislelizumab (an anti-PD-1 antibody) and azacytidine in an AML patient with molecular relapse was effective at preventing haematological relapse.54 An earlier treatment might also be beneficial as it might prevent T cells from reaching a terminal effector/senescence stage through chronic antigen stimulation.
One primary clinical concern in the context of immunotherapy is the risk of developing immune-related complications. We observed these in 50% of the patients in our cohort. In 1/16 treated patients (6.25%), GVHD was fatal despite immunosuppressive treatment. These data align with previous studies reporting severe acute GVHD grade III–IV in 29% of the patients,55 total GVHD rates of 30% in patients with Hodgkin lymphoma treated with nivolumab after allo-HCT,56 and 39% in patients with lymphoid and myeloid malignancies.42 Overall, GVHD remains a significant toxicity for this combination and warrants close monitoring of the patients.
We questioned whether patients who responded to HMA/nivolumab had characteristic changes in the BM or peripheral blood immune compartment. We combined single-cell RNA sequencing and multidimensional spectral flow cytometry to profile T cells and myeloid immune cells extensively. First, we set out to determine whether the response to HMA/nivolumab could be predicted by analysis of the peripheral immune compartment. Defining biomarkers of response would be necessary for the early identification of patients who might benefit from the treatment. We found that the CD8 Tem of responders showed a signature of higher activation and costimulation marker expression and lower senescence marker expression than CD8 Tem from non-responders. This signature was detectable already 1 week after the first administration of nivolumab. Our data align with a recently published phase II trial of pembrolizumab after high-dose cytarabine in r/r AML outside of the allo-HCT setting.35 In this study, the frequency of senescent T cells (CD45RA+ KLRG1+CD57+) in the BM and PB of non-responders was significantly higher compared to patients who achieved a CR.35 Notably, the presence of such senescent-like CD3+ CD8+ KLRG1+ CD57+ cells in the BM of newly diagnosed AML patients was associated with significantly worse OS after treatment with chemotherapy.57 Our findings that HLA-DR and ICOS increased in CD8 Tem in the peripheral blood of responders are different from a study using an anti-CTLA-4 treatment that showed that ipilimumab increased expression of T cell activation and costimulation markers such as PD-1, HLA-DR and ICOS, irrespective of response.58 It is conceivable that changes in the immune compartment are specific to the type of checkpoint inhibitor used.
Furthermore, we observed that response in one patient was associated with a substantial expansion of T cells in the BM, particularly CD4+ central memory/effector memory T cells and Granzyme K+ T cells. Using single-cell RNA sequencing analysis from paired BM samples pre/post azacytidine and nivolumab treatment outside of the allo-HCT setting, it was shown that the disease-related T cell subsets were highly heterogeneous, and their abundance changed following PD-1 blockade-based treatment.59 The authors found that a BM-residing memory CD8+ T cell subset with stem-like properties, which expressed granzyme K was enriched in responders.59 Of note, an important difference between the two studies lies in the fact that all of our patients had undergone an allo-HCT, whereas the study by Abbas et al. was performed outside the allo-HCT setting. Our data indicate that the expansion of activated, low-senescence T cells is associated with response to HMA/nivolumab.
We further focused on profiling the BM and peripheral blood myeloid compartment. We found that the patient who had the longest CRi on the trial (>11 months) had a remarkable monocyte population with pro-inflammatory gene signature in the BM. Pro-inflammatory macrophage infiltration predicted the response to atezolizumab in urothelial cancer with a robustness was comparable with tumour mutational burden and tumour neoantigen burden and superior to CD8+ T cell infiltration and PD-L1 expression.60 Consistent with our findings, a study performed in non-small-cell lung cancer patients found that “M1-like” macrophage and high peripheral T cell signature were associated with a significantly longer PFS.61 Interestingly, AML blasts can re-educate monocytes and macrophages towards a leukaemia-supporting “M2-like” phenotype62 through the secretion of arginase and other mechanisms.63 We speculate that response to HMA/nivolumab is more likely to occur in a pro-inflammatory BM microenvironment shaped by inflammatory macrophages. Similarly, there were differences in the peripheral myeloid compartment of responders and non-responders, such as a trend towards increased PD-L1 expression in conventional DCs of responders versus non-responders. In a study of breast cancer patients receiving anti-PD-1 treatment combined with neoadjuvant chemotherapy, the relative frequency of PD-L1+ immunoregulatory DCs correlated positively with T cell expansion.64 Additionally, we observed reduced expression of CD155 on classical monocytes of responders. CD155 inhibits the anti-tumour activity and activation of both T cells and NK cells,46, 48 once again reinforcing our hypothesis that the phenotype of the myeloid compartment shapes the response to HMA/nivolumab. This concept is further supported by our data showing that macrophage-conditioned cell culture media can reverse impaired cytokine production in T cells caused by chronic antigen exposure.
In summary, we show that the HMA/nivolumab combination has limited anti-AML activity post-allo-HCT and could be considered as a bridging approach to a second allo-HCT. Response correlates with confined terminal effector T cell differentiation/senescence and pro-inflammatory myeloid profiles in a patient-dependent manner. Although further investigation in a larger cohort of patients is needed, we believe that the myeloid compartment might be an important predictor of response of AML to HMA/checkpoint inhibitor treatment and should be investigated in future studies. Additionally, we propose analysis of surface markers of T cell activation (HLA-DR, ICOS) and senescence (KLRG1, CD57) as a potential approach for early identification of patients who might benefit from treatment.
AUTHOR CONTRIBUTIONS
Developed the overall concept and designed the study protocol: Petya Apostolova, Juergen Finke and Robert Zeiser. Participated in patient treatment, collected data, performed experiments and data analysis: Petya Apostolova, Stefanie Kreutmair, Cristina Toffalori, Marco Punta, Susanne Unger, Ann-Cathrin Burk, Claudia Wehr, Kristina Maas-Bauer, Wolfgang Melchinger, Eileen Haring, Rouven Hoefflin, Khalid Shoumariyeh, Sandra Duquesne, Theresa Lowinus, Nicolás Gonzalo Núñez, Chiara Alberti, Sara da Costa Pereira, Carla Helena Merten, Laura Power, Matthias Weiss, Caroline Böke, Dietmar Pfeifer, Reinhard Marks, Hartmut Bertz, Ralph Wäsch, Bernhard Gentner, Justus Duyster, Melanie Boerries, Geoffroy Andrieux, Luca Vago, Burkhard Becher. Provided help with the trial protocol design, statistical analysis and graphical visualizations: Gabriele Ihorst. Wrote the first version of the manuscript: Petya Apostolova, Stefanie Kreutmair, Cristina Toffalori, Luca Vago, Robert Zeiser. All authors edited, discussed and agreed to the final version of the manuscript.
ACKNOWLEDGEMENTS
The authors would like to thank Barbara Sauer for technical assistance and Irina Surlan, Dr. Alexandra Schulz, Tatja Dopatka, Rainer Lohmüller, Katrin Wiegmann and Philipp Zart from the Early Clinical Trial Unit of the Department of Medicine I, Medical Center—University of Freiburg, for their assistance with planning and the documentation of this trial. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
This study was funded by the German Cancer Consortium (DKTK, FR01-375 to P.A.), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation Project 492259164 to P.A.), the Faculty of Medicine, University of Freiburg Germany (to P.A., J.F. and R.Z.), the Italian Ministry of Health (GR-2018-12367860 to L.V.), the Associazione Italiana per la Ricerca sul Cancro (IG #22197 to L.V.) and the CARIPLO Foundation (Giovani Ricercatori 2019-1708 to C.T.). R.Z. is supported by the DFG: CRC 1479 (Project ID 441891347), CRC 1160 (Project ID 256073931), TRR167 (Project ID 259373024) and CRC 850, the INTERREG V European regional development fund (European Union) program (project 3.2 TRIDIAG), the European Union: EU Proposal n° ERC-2022-ADG Project: 101094168—AlloCure (ERC Advanced grant to R.Z.), the Deutsche Krebshilfe (grant number 70114655), the Jose-Carreras Leukemia Foundation (grant number DJCLS 09R/2022). S.K. is a recipient of a research fellowship (442457282) of the German Research Foundation (DFG). G.A. and M.B. are supported by the DFG: CRC 1479 (Project ID 441891347-S1), CRC 1160 (Project ID 256073931-Z02), CRC 1453 (Project ID 431984000—S1), TRR 167 (Project ID 259373024-Z01), TRR 359 (Project ID 491676693-Z01), and the German Federal Ministry of Education and Research by MIRACUM within the Medical Informatics Funding Scheme (FKZ 01ZZ1801B for M.B. and EkoEstMed–FKZ 01ZZ2015 for G.A.). This project has further received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme grant agreement No 882424, the Swiss National Science Foundation (733 310030_170320, 310030_188450 and CRSII5_183478 to B.B.) and the Loop Zurich project INTERCEPT.
CONFLICT OF INTEREST STATEMENT
Bristol Myers Squibb provided nivolumab for patient treatment in this study. R.Z. has received honoraria from Novartis, Sanofi and Mallinckrodt.
ETHICS STATEMENT
Approval was obtained from the Ethics Committee of the University of Freiburg. The trial was designed and conducted in accordance with the guidelines for Good Clinical Practice of the International Council for Harmonization, the principles of the Declaration of Helsinki and the local regulations.
PATIENT CONSENT STATEMENT
All patients provided written informed consent before participating in the trial.
CLINICAL TRIAL REGISTRATION (INCLUDING TRIAL NUMBER)
The clinical trial was registered with the European Union Drug Regulating Authorities Clinical Trials Database, EudraCT-No. 2017-002194-18.
Open Research
DATA AVAILABILITY STATEMENT
Original data are available from the corresponding authors upon reasonable request.




