SLN124, a GalNac-siRNA targeting transmembrane serine protease 6, in combination with deferiprone therapy reduces ineffective erythropoiesis and hepatic iron-overload in a mouse model of β-thalassaemia
Summary
Beta-thalassaemia is an inherited blood disorder characterised by ineffective erythropoiesis and anaemia. Consequently, hepcidin expression is reduced resulting in increased iron absorption and primary iron overload. Hepcidin is under the negative control of transmembrane serine protease 6 (TMPRSS6) via cleavage of haemojuvelin (HJV), a co-receptor for the bone morphogenetic protein (BMP)-mothers against decapentaplegic homologue (SMAD) signalling pathway. Considering the central role of the TMPRSS6/HJV/hepcidin axis in iron homeostasis, the inhibition of TMPRSS6 expression represents a promising therapeutic strategy to increase hepcidin production and ameliorate anaemia and iron overload in β-thalassaemia. In the present study, we investigated a small interfering RNA (siRNA) conjugate optimised for hepatic targeting of Tmprss6 (SLN124) in β-thalassaemia mice (Hbbth3/+). Two subcutaneous injections of SLN124 (3 mg/kg) were sufficient to normalise hepcidin expression and reduce anaemia. We also observed a significant improvement in erythroid maturation, which was associated with a significant reduction in splenomegaly. Treatment with the iron chelator, deferiprone (DFP), did not impact any of the erythroid parameters. However, the combination of SLN124 with DFP was more effective in reducing hepatic iron overload than either treatment alone. Collectively, we show that the combination therapy can ameliorate several disease symptoms associated with chronic anaemia and iron overload, and therefore represents a promising pharmacological modality for the treatment of β-thalassaemia and related disorders.
Introduction
The β-thalassaemias are a group of inherited red blood cell (RBC) disorders caused by a reduction or absence of β-globin synthesis.1 The clinical presentations of these disorders appear soon after birth when the fetal γ-globin gene is progressively silenced and is replaced by the aberrant adult β-globin gene, contributing to atypical adult haemoglobin (HbA,α2β2) production.2 Affected individuals display extreme clinical heterogeneity, ranging from nearly asymptomatic to displaying life-threatening severe anaemia. Dyserythropoiesis is a constant finding, which is characterised by extensive erythroid expansion and maturation block at the polychromatophilic erythroblast stage, as well as increased apoptosis of late erythroblasts.3-5 The failures of erythropoiesis and chronic anaemia lead to abnormal iron metabolism and systemic iron overload, which contributes to cardiac, hepatic and endocrine dysfunction.6 Treatment options are limited, consisting mainly of RBC transfusions, which exacerbates iron loading leading to end-organ damage. If left untreated, iron overload can rapidly worsen and become life-threatening. Chelating agents allow for the active elimination of iron; however, chelation therapy is not sufficient to improve anaemia and to prevent iron overload-related complications.7, 8
In response to excess iron, the liver peptide hepcidin inhibits gastrointestinal iron absorption. However, in β-thalassaemia, hepcidin expression is suppressed contributing to uncontrolled absorption of dietary iron and insufficient iron retention by the reticuloendothelial system exacerbating ineffective erythropoiesis and anaemia.6 Thus, the manipulation of hepcidin and related pathways have become the target for development of novel therapies for iron overload disorders.9-12 Hepcidin is under negative control of the transmembrane serine protease 6 (TMPRSS6) via the inhibition of haemojuvelin (HJV) activity, a co-receptor for the bone morphogenetic protein (BMP)-mothers against decapentaplegic homologue (SMAD) signalling pathway, activated in a paracrine manner by BMP2 and BMP6 produced by liver sinusoidal endothelial cells.6, 10, 13 Notably, mutations that disrupt TMPRSS6 expression in both humans and mice result in elevated hepcidin expression and iron deficiency, characterised as iron-refractory iron deficiency anaemia (IRIDA).14, 15 Recent studies have shown that depletion of Tmprss6 expression using oligonucleotides such as anti-sense oligonucleotides (ASO) or small interfering RNA (siRNA) in β-thalassaemic mice not only decreased iron loading, but improved erythropoiesis and anaemia.16-20 Hence, the targeted reduction of TMPRSS6 expression represents a potential pharmacological modality to treat β-thalassaemia and other iron-loading anaemias associated with low hepcidin levels.16-21
The main challenge in oligonucleotide therapeutics is achieving efficient and targeted delivery to tissues and cells. The N-acetylgalactosamine (GalNAc) ligand is a well-defined liver-targeting moiety benefitting from its high affinity to the asialoglycoprotein receptor (ASGPR).22, 23 GalNAc-conjugation of therapeutic oligonucleotides has led to development of a large number of drug candidates targeting liver hepatocytes for a diverse range of conditions, including SLN124, which is composed of a trimeric GalNAc ligand conjugated to TMPRSS6-siRNA.22, 24 We aimed to further investigate the therapeutic utility of SLN124, by treating β-thalassaemic mice (Hbbth3/+) to study the effect of Tmprss6 suppression.25 Our results indicate that two subcutaneous (s.c.) injections of SLN124 (3 mg/kg) in Hbbth3/+ mice, alone or in combination with the oral iron chelator deferiprone (DFP),26 not only suppressed Tmprss6 expression, but also normalised serum hepcidin levels, significantly improved erythropoiesis and reduced splenomegaly. Notably, the combination treatment achieved a significantly greater reduction in liver iron content when compared with each agent alone. Collectively, we show that the combination treatment can ameliorate several indicators of chronic anaemia and iron overload in Hbbth3/+ mice and therefore may provide a significant health benefit to patients with β-thalassaemia.
Materials and methods
Treatment of β-thalassaemia mice
The β-thalassaemic mice (Hbbth3/+) were bred on the C57BL/6 background, at the Monash Animal Research Platform, Monash University, under approved protocols. Mice were housed under a constant light–dark cycle and maintained on a standard mouse diet containing 200 parts per million (ppm) iron (Specialty Feeds, Glen Forrest, WA, Australia) with ad libitum access to food and water. The 6-month-old female mice were treated with either two doses of TMPRSS6 GalNAc-siRNA (SLN124) at 3 mg/kg, or the TMPRSS6 siRNA without GalNAc targeting ligand (CTRL) or the vehicle control [phosphate-buffered saline (PBS)], by s.c. injections on days 1 and 14. The oral iron chelator DFP was administered through the drinking water at 1·25 mg/ml, and maintained on standard commercial diet. The mice were humanely killed 21 days after the second dose for analysis.
Oligonucleotides
SLN124 comprises a double-stranded 19mer RNA oligonucleotide with 2′-O-methyl,2′-fluoro-2′-deoxy modifications and phosphorothioate bonds targeting TMPRSS6, which is linked to a tri-antennary GalNAc unit at the 5′ end of the sense strand. The non-targeting control oligonucleotide (CTRL) contains the same siRNA and modifications but lacks the tri-antennary GalNAc unit. The SLN124 and the CTRL oligonucleotide used in this study were produced by Silence Therapeutics GmbH (Berlin, Germany), by chemical synthesis using standard solid phase technology for oligonucleotides.27 Briefly, the anti-sense strand and the sense strand were individually synthesised, purified by anion-exchange (AEX) chromatography and desalted. After hybridisation of the single strands, the final double-stranded molecules were obtained as lyophilised powder. The identity of the single strands was confirmed by denaturing ion-pair reversed-phase high-performance liquid chromatography mass spectrometry (IP-RP-HPLC-MS), whereby the recorded single strand masses complied with the calculated molecular weights. The purity of the singe strands was analysed by AEX-HPLC with ultraviolet detection (AEX-HPLC-UV) and resulted in purities ≥85%. Purity of the final double strands was analysed by native IP-RP-HPLC-UV and resulted in values of ≥90%.
Gene expression analysis
Approximately 10 mg of liver tissue was disrupted and homogenised in a Mixer Mill MM 400 (Retsch GmbH, Haan, Germany) using tungsten carbid beads (Qiagen, Hilden, Germany) and total RNA was prepared with InviTrap® Spin Tissue RNA Mini Kit (Stratec, Berlin, Germany) according to the manufacturer’s instruction (InviTrap Protocol). In all, 100 ng total RNA was used for real-time quantitative polymerase chain reaction (RT-qPCR) with the following amplicon sets/sequences for Tmprss6, hepcidin anti-microbial peptide (Hamp) and Actin (mACTB) (Eurogentec, Seraing, Belgium) respectively Table SI. The RT-qPCR reactions were carried out with a QuantStudio 6 Flex (Applied Biosystems part of Thermo Fisher Scientific Inc., Waltham, MA, USA) using Takyon™ One-Step Low Rox Probe 5X MasterMix dTTP (Eurogentec). The data were calculated by using the comparative CT method also known as the 2−ΔΔCt method.28, 29
Hepcidin assay
Serum hepcidin was quantified using the ‘Hepcidin Murine-Compete ELISA Kit’ (Intrinsic Lifesciences, La Jolla, CA, USA) according to manufacturer’s instructions.
Full blood examination
Blood was collected from the submandibular vein in heparinised tubes. Full blood examination was performed using an automated Roche Applied Science Cobas Helios haematological analyser at the Walter and Eliza Hall Institute, Melbourne, Australia.
Reactive oxygen species measurements
The reactive oxygen species (ROS) level was assessed as described before.30 Briefly, RBCs were washed and re-suspended in PBS supplemented with 2% fetal bovine serum and incubated with 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) stain (5 μmol/l) in the dark for 5 min. The oxidative conversion of CM-H2DCFDA to its fluorescent product by ROS was measured immediately by flow cytometry. The ROS fluorescence signals (median fluorescence intensity) were recorded in these gated RBC populations. For all the analyses, cells were acquired using the LSR Fortessa X-20 using fluorescence-activated cell sorting (FACSDiva) software (BD Biosciences San Jose, CA, USA) and the results analysed with FlowJo software (Tree Star Inc., Ashland, OR, USA).
Erythroid differentiation in bone marrow and spleen
Erythroid cells were analysed from both the spleen and bone marrow (BM) by flow cytometry using fluorescein isothiocyanate (FITC)-conjugated anti mouse-CD71, allophycocyanin (APC)-conjugated anti-mouse Ter119 and phycoerythrin (PE)-conjugated anti-mouse CD44 antibodies (BD Biosciences). This assay allows the separation of erythroid cells into distinct populations corresponding to (i) pro-erythroblasts, (ii) basophilic, (iii) polychromatic, (iv) reticulocytes and (v) RBCs.31 For all the cytometric analyses 7-amino-actinomycin D (7-AAD) was used as viability dye and immunophenotyping was carried out on 7-AAD−ve cells. A minimum of 10 000 events were recorded for erythrocytes in BM and spleen. For all the analyses, cells were acquired using the LSR Fortessa X-20 using FACSDiva software (BD Biosciences) and the results analysed with FlowJo software (Tree Star Inc.).
Tissue iron, magnesium and zinc measurements
Tissue sections were cut using the Microm HM330 at 10 µm and put onto Superfrost slides. Laser ablation-inductively coupled plasma-MS (LA-ICP-MS) was used to quantitate the distribution of iron in liver and spleen in formalin fixed tissue sections using external reference standards.32 Iron (Fe), magnesium (Mg) and zinc (Zn) measurements were performed using the New Wave Research UP213 (Kennelec Scientific, Mitcham, Victoria, Australia) (neodymium-doped yttrium aluminum garnet (Nd:YAG) laser system with a two-volume large format cell connected to an Agilent 7500 cs ICP-MS (Agilent Technologies Inc., Santa Clara, CA, USA) for isotopes 56Fe, 24Mg and 66Zn. This technique involves vaporising material with a UV laser that is transferred into an ICP-MS via argon gas.33, 34 Consecutive sections at 4 µm were also stained for Perls’ Prussian blue iron stain for iron distribution and structural identification.
Statistical analysis
Experimental data are presented as box plots or stacked bar charts. One-way analysis of variance (ANOVA) with Brown–Forsythe test (F*-test) was performed followed by Dunnett’s T3 post hoc tests against Vehicle group using GraphPad Prism Version 8.4.3 (GraphPad Software Inc., San Diego, CA, USA), unless indicated otherwise in the figure legend. If the assumption of normal distribution by Q–Q plot investigation was violated, the measured values were log2-(RT-qPCR assay) or log10-transformed before the analysis. Data were considered statistically significant, if adjusted P ≤ 0·05.
Results
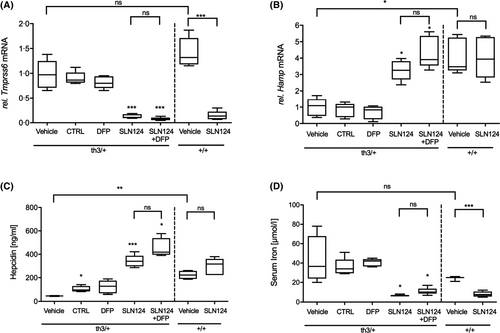
SLN124 induces hepcidin expression and reduces serum iron levels in Hbbth3/+ mice
The development of novel therapies for the management of anaemia and iron overload in haemoglobinopathies represents a major field of focus. It has been shown that Tmprss6 siRNA can be used to increase hepcidin synthesis and reduce ineffective erythropoiesis in β-thalassaemic mice.19, 20 Based on GalNAc-conjugate delivery technology, SLN124 was developed as an siRNA therapeutic to restore hepcidin expression and normalise iron homeostasis in β-thalassaemia. To evaluate the in vivo activity of SLN124, in silencing Tmprss6 and thereby reducing disease activity in β-thalassaemic mice, 6-month-old wild type (WT) and Hbbth3/+ mice, were treated with SLN124 (3 mg/kg on days 1 and 14) alone or in combination with the oral iron chelator DFP (1·25 mg/ml) in the drinking water and followed for 35 days. In this study, we used the same SLN124 dose, previously demonstrated to be effective in a murine model for hereditary haemochromatosis (HH) type 1.24 The SLN124-treated mice had significantly reduced liver Tmprss6 mRNA levels, dramatically increased liver Hamp mRNA (which encodes hepcidin) and increased serum hepcidin levels in Hbbth3/+ mice compared to controls (Fig 1A–C). SLN124 was also effective in lowering serum iron levels (Fig 1D), while treatment with the oral iron chelator DFP alone did not affect Tmprss6, Hamp or serum iron levels (Fig 1). Importantly, these results provide evidence that SLN124 represents a potent in vivo modulator of Hamp expression and serum iron availability by the transient inhibition of Tmprss6 expression.
SLN124 treatment improves RBC parameters in Hbbth3/+ mice
Treatment with SLN124 significantly reduced anaemia in Hbbth3/+ mice as measured by an increase in haemoglobin (Hb) levels, RBC counts and decreased red cell distribution width (RDW) when compared to Hbbth3/+ controls and DFP-treated mice (Fig 2A–C). In addition, SLN124 partially corrected reticulocytosis in Hbbth3/+ mice either alone or in combination with DFP (Fig 2D). Mice treated with SLN124 combined with DFP showed similar benefit as mice treated with SLN124 alone (mean Hb increase of 25 g/dl versus control) compared to vehicle control, whereas mice treated with DFP alone showed no improvement in RBC parameters. Moreover, the improvement in RBC parameters in SLN124 treated Hbbth3/+ mice was associated with a significant reduction in splenomegaly (Fig 2E).
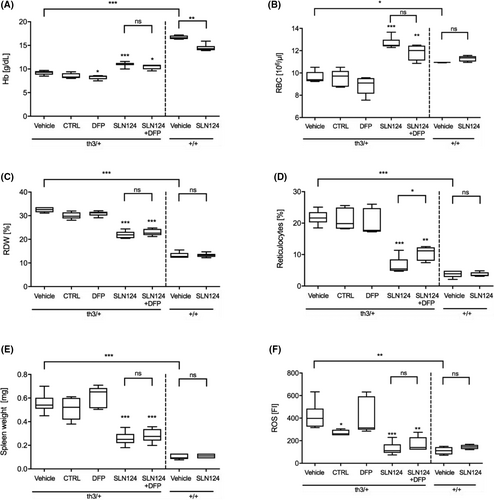
In β-thalassaemia, the unpaired α-globin chains precipitate, forming large, insoluble aggregates in RBCs, which increases ROS production resulting in haemolysis and greatly exacerbating the anaemic phenotype in β-thalassaemia.2, 35 We next assessed the ROS levels in RBCs obtained from SLN124-treated mice. FACS analysis of RBCs was used to measure the oxidative conversion of DCFH-DA in RBCs. Remarkably, mice treated with SLN124 alone or in combination with DFP significantly reduced ROS to near normal levels, whereas DFP-treated mice showed no change in ROS levels (Fig 2F). Overall, these results demonstrate that SLN124 alone or in combination with DFP can improve RBCs and reticulocyte cellular indices as well as disease activity.
SLN124 treatment reduces tissue iron levels and restores splenic architecture
To assess the impact by SLN124 and DFP on iron loading, liver and spleen tissue sections from Hbbth3/+ mice and WT mice were Perls’ Prussian blue stained for iron distribution and organ structure. As expected, liver and spleen sections from Hbbth3/+ mice treated with vehicle or control siRNA displayed dense blue punctate staining, representing abnormally high levels of insoluble iron stores termed haemosiderin. In addition, morphological examination of the Hbbth3/+ spleen revealed significant structural disorganisation, with absent white pulp and red pulp regions. Notably, Perls’ Prussian blue-staining indicated DFP or SLN124 alone or in combination decreased liver iron levels when compared with vehicle or control siRNA (Fig 3A). Morphological examination of the spleen indicated, that SLN124 alone or in combination with DFP decreased splenomegaly and also improved splenic architecture, showing a normalisation of the proportion of white pulp and red pulp regions (Fig 3A; Figure S3). This is in contrast to the results obtained with DFP alone, which did not improve splenic architecture, splenomegaly or anaemia in Hbbth3/+ mice.
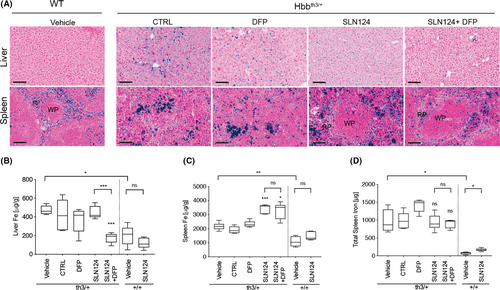
Next, the tissue iron levels in liver and spleen were quantified by LA-ICP-MS, which allows the detection of major and minor elements with a high level of precision and accuracy (Fig 3B; Figure S2). Tissue sections were prepared from formalin-fixed, paraffin-embedded liver and spleen tissue samples. As expected, quantitative analysis revealed that liver and spleen iron levels were about twofold higher in Hbbth3/+ mice when compared to WT mice. Notably, following treatment, the strongest reduction of liver iron levels in Hbbth3/+ mice was achieved in mice treated with SLN124 and DFP, and attained normal physiological iron levels, while SLN124 or DFP alone did not reduce liver iron levels significantly (Fig 3B). Intriguingly, iron levels in the spleen appeared to increase following treatment with SLN124 alone or in combination with DFP. These results appear to be the consequence of a significant reduction in spleen size due to improved erythropoiesis, and concentration of insoluble haemosiderin within the confines of the spleen. When the total weight of the spleen was taken into account, the total iron content in the spleen did not significantly differ between treated and control groups (Fig 3D). These results suggest that the efficiency of iron removal by SLN124 in combination with DFP appears to be higher for the liver than for the spleen. We hypothesise that insoluble haemosiderin particles in the spleen may not be readily cleared by SLN124 and DFP within the 35 days of treatment, and thus remain within the confines of the spleen. Importantly, these results demonstrate that treatment with SLN124 in combination with DFP can achieve physiological concentrations of iron in the liver; however, further optimisation of treatment will likely be required to restore spleen iron to normal levels.
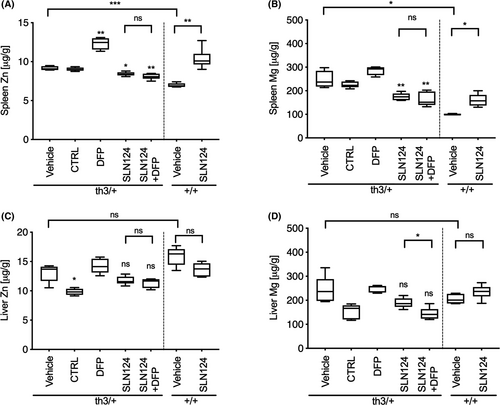
SLN124 improves splenic zinc and magnesium homeostasis in Hbbth3/+ mice
We also measured the concentration of two elements, Zn and Mg from the same tissue sections using LA-ICP-MS (Fig 4). Firstly, we compared the level of metal ions in the liver and spleen of WT and Hbbth3/+ mice under normal conditions. Minimal differences were observed in the concentration of Zn and Mg in the liver of Hbbth3/+ and WT mice. Interestingly, significantly higher levels of Zn and Mg were observed in the spleen of Hbbth3/+ mice, thus confirming a general dysregulation of Zn and Mg homeostasis in Hbbth3/+ mice. Importantly, following treatment of Hbbth3/+ mice with SLN124 alone or in combination with DFP, Zn and Mg levels were significantly reduced to near normal levels in the spleen (Fig 4A,B). In the liver, Zn and Mg levels were largely unaffected following similar treatment (Fig 4C,D). Unexpectedly, DFP treatment alone markedly increased Zn and to lesser extend Mg levels in the spleen of Hbbth3/+ mice. Interestingly, SLN124 treatment of WT mice significantly increased Zn and Mg levels in the spleen. This observation is consistent with a recent study, which reported elevated Zn levels in the spleen of 12-week-old Tmprss6−/− knockout mice. These results demonstrate that Tmprss6 may also regulate the transport of other divalent metal ions, which is thought to be under hepcidin-mediated ferroportin control.36, 37 Taken together, these data suggest that the restoration of splenic Zn and Mg homeostasis in Hbbth3/+ mice may be an important additional factor in the activity of SLN124.
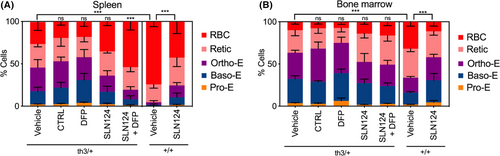
SLN124 treatment ameliorates ineffective erythropoiesis in Hbbth3/+ mice
FACS analysis was used to identify different stages of erythroid differentiation in the spleen and BM using Ter119, CD71 and CD44 cell surface markers (Figure S1). The additional parameter, FSC, was used to discriminate between five distinct erythroid populations: (i) pro-erythroblasts, (ii) basophilic, (iii) polychromatic, (iv) reticulocytes and (v) RBCs. Importantly, SLN124 ameliorated ineffective erythropoiesis in Hbbth3/+ mice as indicated by the reduced proportion of immature erythroid progenitor cells in the spleen and to a lesser extent in the BM of Hbbth3/+ mice, compared with mice treated with vehicle or control siRNA. Treatment with DFP alone did not ameliorate the ineffective erythropoiesis in the spleen or in the BM. However, DFP appeared to further enhance the positive impact of SLN124 on erythroid maturation (Fig 5). This was evidenced by the small but significant increase in the percentage of reticulocytes and enucleated erythroid cells in the spleen of Hbbth3/+ mice treated with SLN124 and DFP, compared to mice treated with SLN124 alone. While positive effects of DFP were noted with SLN124 therapy, DFP alone appeared to mitigate erythroid maturation in the spleen and BM of Hbbth3/+ mice. This is shown by a small but notable decrease in reticulocytosis and a concomitant increase in the proportion of erythroblasts. Collectively, these results demonstrate that SLN124 alone or in combination with DFP significantly enhanced in vivo maturation and differentiation of erythroid cells in Hbbth3/+ mice.
Discussion
Considering the central role of the TMPRSS6/HJV/hepcidin axis in iron homeostasis, the transient inhibition of TMPRSS6 expression represents a promising treatment modality to address iron-loading disorders. Several studies targeting TMPRSS6 protein through enhanced mRNA degradation in models of iron overload, such as HH and β-thalassaemia intermedia, have reported increased hepcidin expression and reduced serum and liver iron concentrations using ASO or RNA interference therapy.22-24 As the liver plays a major role in the regulation of iron metabolism through the modulation of hepcidin expression, GalNAc-siRNA is uniquely suited for liver-specific delivery with rapid, reversible and titratable gene silencing activity.22-24
The results presented in the present study show that the GalNAc-siRNA, SLN124, produces a robust knockdown of liver Tmprss6 mRNA expression, which was associated with a two–threefold increase in hepcidin expression. Notably, two doses of SLN124 led to a 70% reduction in serum iron levels, an increase in haemoglobin concentration and improvement in red cell parameters in Hbbth3/+ mice. In addition, SLN124 mitigated ineffective erythropoiesis and splenomegaly in Hbbth3/+ mice, which represent major clinical parameters in β-thalassaemia patients. Furthermore, SLN124 diminished iron overload in the liver when administered in combination with the clinically approved oral iron chelator DFP, while the total spleen iron levels remained unchanged.
In β-thalassaemia, unlike in HH, excessive amounts of splenic haemosiderin are seen when the RBCs are removed at a faster rate, leading to enhanced erythrophagocytosis by macrophages and excess iron deposition in splenic macrophages.38 Over time, large deposits of insoluble and poorly accessible iron-loaded haemosiderin are formed, consisting predominantly of denatured H-ferritin subunits.39 These findings suggest the composition of insoluble haemosiderin particles in the spleen differ from composition in the liver and therefore may not be readily cleared by SLN124 and DFP from the confines of the spleen. In addition, elemental iron may be locked in the reticuloendothelial macrophages, as a consequence of hepcidin elevation and the inhibition of ferroportin-mediated iron export. A similar retention of iron in splenic macrophages by treatment with SLN124 was also observed in a murine model for HH type 1.24 These observations indicate that further optimisation of treatment will likely be necessary to restore spleen iron to normal levels. Interestingly, SLN124 alone or in combination with DFP normalised the splenic architecture, with re-establishment of red pulp and white pulp compartments, suggesting a restoration of splenic function. The main clinical consequence of defective spleen function is increased susceptibility to bacterial infection.40, 41 As infections represent one of the most common complications in β-haemoglobin disorders, SLN124 treatment could potentially provide benefits for spleen function and immune responses to infection. We suggest further research is needed to determine the utility of SLN124 on immune function in β-thalassaemia.40, 41
In the present study, we also provide evidence for dysregulated Zn and Mg homeostasis in the spleen of Hbbth3/+ mice. The reason for this is not immediately obvious; however, a range of ion transporters are likely to be involved. For example, the Zn importer Slc39a8 is differentially regulated during erythroid differentiation. Slc39a8 is highly expressed during early stages of erythropoiesis leading to increased intracellular zinc.42 Similarly, the Mg receptor (MagT1) is expressed in high levels by erythroblasts and may also contribute to higher Mg content in erythrocytes during anaemia.43-45 Considering the Hbbth3/+ spleen represents a major site of ineffective erythropoiesis, with increased number of immature erythroblast at the polychromatic and orthochromatic phase, we propose that immature erythroblasts contribute to elevated Zn and Mg levels in the spleen. Interestingly, following erythroid differentiation with SLN124 alone or in combination with DFP, Zn and Mg levels decreased suggesting the splenic Zn and Mg content was largely dependent on erythroid differentiation. Further investigations into the contributions of known or suspected metal transporters are required to clarify the mechanisms underlying the physiological distribution of these elements. Due to the importance of these elements in haematopoiesis, it is highly relevant to expand research focussed directly on Zn and Mg homeostasis and understand how these changes affect terminal erythroid differentiation in β-thalassaemia.45
In conclusion, the present study demonstrates the efficacy of SLN124 as a potent drug candidate for the treatment of β-thalassaemia. SLN124 can be used as monotherapy or in combination with DFP treatment. SLN124 alone, reduced systemic iron levels and normalised erythropoiesis in Hbbth3/+ mice. When used in combination with the DFP, SLN124 reduced hepatic iron stores and ameliorated several major clinical signs of β-thalassaemia.19, 20, 46 Taken together, our results support the clinical development of SLN124 in β-thalassaemia either as a monotherapy or in combination with iron chelators. First-in human clinical studies with SLN124 have commenced for treatment of patients with β-thalassaemia (NCT04718844).
Acknowledgments
Monash Pathology for granting access to their facilities and equipment. Jim Vadolas acknowledges funding from Silence Therapeutics plc, London, UK". The DFP was kindly provided by ApoPharma Inc., Toronto, ON, Canada.
Author contributions
Jim Vadolas, Garrett Z. Ng, Kai Kysenius, Peter J. Crouch, Shahla Vilcassim, George Grigoriadis and Ute Schaeper designed the research project. Jim Vadolas, Garrett Z. Ng, Kai Kysenius, Tiwaporn Nualkaew and Mona Eisermann performed the experiments. Sibylle Dames designed the siRNA and performed the statistical analysis. Jim Vadolas, George Grigoriadis and Ute Schaeper wrote the manuscript. All authors reviewed and approved the manuscript. Kai Kysenius is currently employed by Medaffcon Oy.
Conflict of interest
Ute Schaeper, Sibylle Dames, and Mona Eisermann are employed by Silence Therapeutics GmbH.