Risk of colorectal neoplasia according to histologic disease activity in patients with inflammatory bowel disease and colonic post-inflammatory polyps
Thomas Wolf and Ayanna Lewis are co-authorship.
The members of the Saint-Antoine IBD Network are listed in Appendix A.
The Handling Editor for this article was Dr Sreedhar Subramanian, and it was accepted for publication after full peer-review.
[Correction added on 14 November 2023, after first online publication: The copyright line was changed.]
Summary
Background and Aims
While post-inflammatory polyps (PIPs) have historically been a risk factor for colorectal neoplasia (CRN), histologic activity may explain this association. We aimed to assess the impact of histologic activity on CRN occurrence in IBD patients with colonic PIPs.
Methods
Patients with PIPs on surveillance colonoscopy at Saint-Antoine hospital between 1 January 1996 and 31 December 2020 were included and subsequent colonoscopies were assessed. Histologic IBD activity was assessed by the Nancy histologic index. Survival and Cox regression analysis were performed to assess the strength of the association of PIPs and other patient variables with progression to CRN.
Results
A total of 173 patients with at least two surveillance colonoscopies with PIPs at index colonoscopy were compared to a similar group of 252 patients without PIPs. In survival analysis, the presence or PIPs at index colonoscopy did not impact the risk of CRN in patients with histological inflammation (p = 0.83) and in patients without histological inflammation (p = 0.98). The risk of CRN was associated with increasing Nancy index score of 3 or 4 (HR: 4.16; 95% CI 1.50–11.52 and HR: 3.44; 95% CI 1.63–7.24), age (HR per 10-year increase: 1.37; 95% CI 1.13–1.66) and first-degree family history of colorectal cancer (HR: 5.87; v 1.31–26.26), but not PIPs (HR: 1.17; 95% CI 0.63–2.17).
Conclusions
After controlling for histologic activity, PIPs do not increase the risk of CRN in IBD patients. Histologic activity rather than PIPs should be considered in the risk assessment of CRN.
1 INTRODUCTION
Patients with colonic inflammatory bowel diseases (IBD) have an increased risk for colorectal neoplasia (CRN) after 7–10 years of disease evolution.1 This risk is stratified into high-, moderate- and low-risk categories by current guidelines, corresponding to endoscopic surveillance intervals of 1–5 years.2 The risk is mainly related to the occurrence of primary sclerosing cholangitis, personal history of CRN or first-degree familial history, IBD disease extent or histologic disease activity. As a feature of chronic inflammation, the presence of post-inflammatory polyps (PIPs) also known as ‘pseudopolyps’, was historically considered as a risk factor for neoplasia but these initial findings were followed by studies concluding to the absence of increased risk.3, 4 Recently, in a large retrospective cohort, PIPs were not associated with a higher risk of CRN.5 In this study, histologic disease activity was not assessed, while histologic disease activity assessed by the Nancy histologic index has been associated with an increased risk of CRN and should be considered in the neoplasia screening strategy.6 The first association observed between the presence of PIPs and the risk of colonic neoplasia may be explained by concomitant histologic disease activity, but the risk of colonic neoplasia in patients with PIPs according to histologic disease activity is unknown. Clarifying the impact of PIPs on colorectal neoplasia considering histological inflammation could help to guide the risk stratification in patients with PIPs. In this study, we aimed to assess the impact of histologic activity on CRN occurrence in IBD patients with colonic PIPs and to confirm the lack of influence of PIPs on CRN development.
2 METHODS
2.1 Patients
This retrospective study included patients from one French tertiary centre, Saint-Antoine Hospital. Eligible patients underwent at least one colonoscopy between 1 January 1996 and 31 December 2020 in Saint-Antoine Hospital. Included patients were men and women aged of 18 years old or more, with an IBD, and among whom PIPs were identified at surveillance colonoscopy. Patients with Crohn's disease (CD) and ulcerative colitis (UC) were pooled since the risk of CRN appears to be the same in CD and UC after adjustment for extent of colitis and disease duration.7-9 Patients with a past history of CRN or colectomy and patients with a follow-up shorter than 6 months or only one colonoscopy were excluded.
Every patient with an IBD for 8–10 years is eligible for CRN screening in our centre. Two biopsies at least are performed for each segment associated with targeted biopsies. Patients were enrolled from the date of the first colonoscopy at St-Antoine's Hospital in the period between 1 January 1996 and 31 December 2020. Entry dates were defined as the date of the first colonoscopy in St-Antoine without CRN. They were followed up until occurrence of low-grade or high-grade dysplasia or colorectal cancer, colectomy or death.
2.2 Data collection
Variables were collected from the SUiVi Intégré soins-recherche des Maladies Inflammatoires Chroniques intestinales registry (a prospective clinical database of all patients with IBD evaluated by Saint-Antoine Hospital digestive disease medical staff), endoscopic and medical records, and the Saint-Antoine Hospital's Pathology Department database. For each patient, following data were recorded: date of birth, sex, IBD type (UC or CD, considering indeterminate colitis in the UC group), disease duration, colonic extent (pancolitis, extensive colitis involving ≥50% of the colon, non-extensive colitis involving <50% of the colon), presence of PSC, familial history of colorectal cancer at first-degree. Current treatment within 6 months was also assessed between methotrexate, mesalamine, thiopurines and biologics. At each follow-up colonoscopy, the presence of PIPs, including numbers and location, and endoscopic disease activity based on the presence of ulcerations were assessed. Histologic disease activity was also assessed according to the Nancy histologic index: ulceration, mild to severe acute inflammation and mild to severe chronic inflammation.10
2.3 Outcome
Primary outcome was the occurrence of any colonic neoplasia (low-grade dysplasia, high-grade dysplasia or colorectal cancer, or lesions indefinite for dysplasia). CRN was defined according to Vienna classification (no dysplasia, indefinite for dysplasia, low-grade dysplasia, high-grade dysplasia or adenocarcinoma).11 If several neoplastic lesions were identified during the same procedure, the most severe lesion was included for the analysis. Lesions were classified as endoscopically visible and nonvisible lesions12 and localisation was assessed. Diagnosis and classification of dysplasia were confirmed by a second expert gastrointestinal pathologist.13 In cases of lesion indefinite for dysplasia, p53 immunohistochemistry was performed to discriminate between regenerative changes and intraepithelial dysplasia.14
2.4 Statistical analysis
Continuous data are expressed as means (SD), and differences between groups were tested for significance by the Student t-test or the Wilcoxon test if appropriate. Discrete data are provided as percentages, and comparisons were made with the Pearson chi-squared test or the Fisher test if appropriate. We considered differences to be statistically significant when the p value was less than 0.05 (all tests were two sided).
2.4.1 Cohort of patients without PIPs
In order to assess the impact of histologic IBD activity according to the presence of PIPs, we assembled a cohort of patients without PIPs based on a previous study.6 Briefly, this study assessed the impact of histologic disease activity on the risk of CRN in patients with IBD who underwent at least two colonoscopies at Saint-Antoine Hospital between 1 January 1996, and 1 March 2015, and whose first procedure was a surveillance colonoscopy. The Nancy histologic index was assessed for each colonoscopy, as well as the presence of ulcerations.
2.4.2 Impact of PIPs on the risk of neoplasia
Survival without CRN was assessed in the patients with and without PIPs, according to the presence of histologic activity based on Nancy index score greater than one and the presence of endoscopic disease activity based on the presence of ulcerations.
Cox regression analysis was used to assess the relationship of clinical, endoscopic and histologic variables to the risk of CRN. Variables significant at a p < 0.20 were entered into a multivariate Cox regression analysis to assess the strength of the associations while controlling for possible confounding variables. The occurrence of PIPs was a priori included in the multivariate Cox model. Additionally, a secondary analysis stratified according to the IBD subtype was performed. We also performed several sensitivity analyses. First, we excluded patients with lesion indefinite for dysplasia. Second, we restricted the analysis to patients included after 2007, as the time scale of the inclusion period was long and includes changes in endoscopy techniques that could impact lesion visualisation. Lastly, we adjusted for the mean Nancy histologic index during follow-up to assess how the inclusion of chronic histological inflammation could impact the findings.
Statistical analyses were performed using SAS software V.9.4 (SAS). The use of the Saint-Antoine hospital database for the purpose of observational studies has been authorised by the French data protection agency (Commission Nationale Informatique et Liberte´) decision DR-2016-373 on 5 September 2016.
3 RESULTS
A total of 200 patients were identified who met the criteria of having at least two surveillance colonoscopies between 1 January 1996 and 31 December 2020 with PIPs at index colonoscopy. Of those 200 patients, 18 patients with a previous history of colonic neoplasia were excluded. An additional nine patients were excluded for having a follow-up of less than 6 months. In total, 173 patients with at least two surveillance colonoscopies and a history of PIPs at the index colonoscopy were identified. Overall, 89.5% (n = 154) of patients had more than one PIP on the index colonoscopy and 63% (n = 109) had many PIPs (more than 5 PIPs). PIPs were mainly located in the left colon and sigmoid (71.7% of patients, n = 124).
A comparison group of 252 patients without PIPs and at least two surveillance colonoscopies was also identified. Patient characteristics at cohort entry are shown in Table 1. Patients with PIPs at index colonoscopy did not differ substantially from patients who did not have PIPs at index colonoscopy with regard to age at cohort entry, IBD diagnosis, IBD duration or disease extent. In the domain of gender, there appeared to be an increased proportion of males with PIPs compared to those without PIPs. Patients with and without PIPs were followed during a mean time of 4.8 (SD 2.9) and 5.5 (SD 3.3) years, respectively.
| No PIPs (n = 252) | PIPs (n = 173) | All (n = 425) | |
|---|---|---|---|
| Sex | |||
| Female | 105 (41.7) | 95 (54.9) | 200 (47.1) |
| Male | 147 (58.3) | 78 (45.1) | 225 (52.9) |
| Age at cohort entry (year) | 40.9 (13.6) | 40.1 (13.5) | 40.6 (13.5) |
| IBD type | |||
| Ulcerative colitis | 99 (39.3) | 78 (45.1) | 177 (41.6) |
| Crohn's disease | 153 (60.7) | 95 (54.9) | 248 (58.4) |
| IBD duration (year) | 14.5 (8.4) | 12.0 (7.5) | 13.5 (8.1) |
| Disease extension | |||
| Non-extensive colitis | 35 (13.9) | 25 (14.5) | 60 (14.1) |
| Extensive colitis | 99 (39.3) | 58 (33.5) | 157 (37.0) |
| Pancolitis | 118 (46.8) | 90 (52.0) | 208 (48.9) |
| Primary sclerosing cholangitis | 39 (15.5) | 10 (5.8) | 49 (11.5) |
| Family history of CRC, first-degree relative | 4 (1.6) | 6 (3.5) | 10 (2.4) |
| Treatment exposure at cohort entry | |||
| Aminosalicylates | 123 (48.8) | 84 (48.6) | 207 (48.7) |
| Azathioprine | 100 (39.7) | 70 (40.5) | 170 (40.0) |
| Methotrexate | 16 (6.3) | 18 (10.4) | 34 (8.0) |
| Anti-TNF | 40 (15.9) | 64 (37.0) | 104 (24.5) |
| Nancy histological index | |||
| 0 | 116 (46.0) | 65 (37.6) | 181 (42.6) |
| 1 | 34 (13.5) | 16 (9.2) | 50 (11.8) |
| 2 | 14 (5.6) | 31 (17.9) | 45 (10.6) |
| 3 | 23 (9.1) | 10 (5.8) | 33 (7.7) |
| 4 | 65 (25.8) | 51 (29.5) | 116 (27.3) |
| Presence of endoscopic ulcerations | 122 (48.4) | 78 (45.1) | 200 (47.1) |
- Note: Results are expressed as mean (SD) or n (%).
Direct comparison of patients with and without CRN shows that patients who had CRN tended to have higher Nancy index scores at baseline colonoscopy with 44.6% of patients with Nancy score of 4 in the CRN group compared to 24.7% in the group without CRN (Table S1). Among patients with PIPs, 55% of neoplastic lesions were identified in a colonic area where PIPs were also identified (Figure 1).
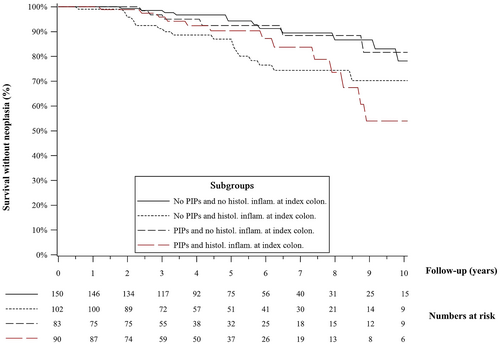
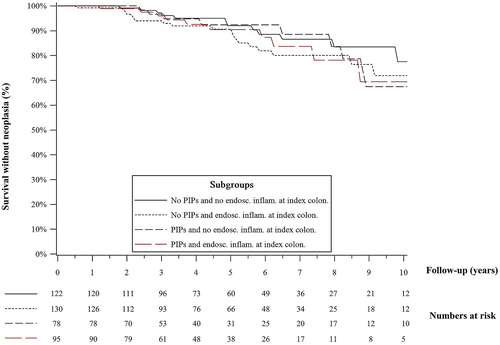
In survival analysis, the presence or PIPs at index colonoscopy did not impact the risk of CRN in patients with histological inflammation (p = 0.83) and in patients without histological inflammation (p = 0.98). The risk of CRN in patients with PIPs and no histological inflammation closely mirrored those of patients without PIPs and without histological inflammation at the index colonoscopy (Figure 2). However, patients without PIPs who had histological inflammation at the index colonoscopy were more likely to develop CRN on subsequent surveillance colonoscopies compared to patients without PIPs and without histological inflammation (p = 0.01), and a similar trend was observed in patients with PIPs although not statistically significant (p = 0.08). A similar stratification of patients with and without PIPs using the presence or absence of endoscopic ulceration did not show a statistical difference in terms of survival without CRN (Figure 3).
By univariate analysis, elevation of Nancy score into 3 (HR: 3.82; 95% CI 1.43–10.24; p = 0.02) or 4 (HR: 3.13; 95% CI 1.57–6.24; p = 0.02), and age at cohort entry (HR: 1.31; 95% CI 1.09–1.58, p < 0.01, per 10-year increase) were associated with occurrence of colorectal cancer. The analysis of other variables showed trends but no statistically significant difference (Table 2).
| Hazard ratio (95% CI) | p value | |
|---|---|---|
| Agea | 1.31 (1.09–1.58) | <0.01 |
| Male sex | 0.61 (0.36–1.04) | 0.07 |
| Family history of CRC, first-degree relative | 2.71 (0.65–11.22) | 0.17 |
| IBD phenotype, Crohn's disease | 0.83 (0.49–1.40) | 0.48 |
| IBD durationa | 1.12 (0.82–1.53) | 0.48 |
| Disease extension | ||
| Non-extensive colitis | Ref. | 0.28 |
| Extensive colitis | 0.63 (0.29–1.35) | |
| Pancolitis | 0.55 (0.26–1.14) | |
| Primary sclerosing cholangitis | 1.86 (0.94–3.69) | 0.08 |
| Post-inflammatory polyps | 1.04 (0.60–1.80) | 0.89 |
| Treatment exposure at cohort entry | ||
| Aminosalicylates | 0.66 (0.39–1.13) | 0.13 |
| Azathioprine | 0.94 (0.55–1.63) | 0.84 |
| Methotrexate | 1.24 (0.49–3.13) | 0.65 |
| Anti-TNFs | 0.50 (0.20–1.27) | 0.15 |
| Nancy histological index | ||
| 0 | Ref | 0.02 |
| 1 | 2.16 (0.88–5.33) | |
| 2 | 2.24 (0.78–6.38) | |
| 3 | 3.82 (1.43–10.24) | |
| 4 | 3.13 (1.57–6.24) | |
| Endoscopic ulcerations | 1.41 (0.83–2.40) | 0.21 |
- a Per 10 years increase.
The Cox multivariate analysis did not show any statistically significant difference in terms of risk for progression to CRN for patients who had PIPs compared to patients who did not (HR: 1.17; 95% CI 0.63–2.17; p = 0.61; Table 3). However, there did appear to be a statistically significant difference in the multivariate analysis for risk of development of CRN based on increasing Nancy index score into Nancy score 3 (HR: 4.16; 95% CI 1.50–11.52; p < 0.01) or 4 (HR: 3.44; 95% CI 1.63–7.24; p < 0.01), age at entry (HR: 1.37; 95% CI 1.13–1.66; p < 0.01) and presence of colorectal cancer in a first-degree relative (HR: 5.87; 95% CI 1.31–26.26; p = 0.02). Use of anti-TNF therapy at cohort entry had a trend towards reduction in CRN but did not reach statistical significance (HR: 0.55; 95% CI 0.21–1.45; p = 0.22). Secondary and sensitivity analyses were consistent with the main analysis (Table S2).
| Hazard ratio (95% CI) | p value | |
|---|---|---|
| Agea | 1.37 (1.13–1.66) | <0.01 |
| Male sex | 0.66 (0.38–1.16) | 0.15 |
| Family history of CRC, first-degree relative | 5.87 (1.31–26.26) | 0.02 |
| Primary sclerosing cholangitis | 2.15 (0.99–4.62) | 0.05 |
| Post-inflammatory polyps | 1.17 (0.63–2.17) | 0.63 |
| Treatment exposure at cohort entry | ||
| Aminosalicylates | 0.71 (0.40–1.26) | 0.24 |
| Anti-TNF | 0.55 (0.21–1.45) | 0.22 |
| Nancy histological index | ||
| 0 | Ref | <0.01 |
| 1 | 1.83 (0.69–4.86) | |
| 2 | 2.49 (0.84–7.45) | |
| 3 | 4.16 (1.50–11.52) | |
| 4 | 3.44 (1.63–7.24) | |
- a Per 10 years increase.
4 DISCUSSION
Our study shows that the presence of PIPs was not associated with an increased risk of CRN in patients with IBD undergoing surveillance colonoscopy when adjusting for the presence of histological inflammation. This study used a validated histologic activity index in UC to assess histologic disease activity.
Historically, PIPs were thought to be an important risk factor for CRN. A 2006 case control study listed a history of PIPs among one of the most important factors associated with colorectal cancer (OR, 2.5; 95% CI: 1.4–4.6).15 Later, another case control study showed that compared to controls, the risk of IBD-related colorectal cancer in patients with PIPs was elevated (RR, 1.92; 95% CI 1.28–2.88).16 A recent meta-analysis reported that PIPs were considered as a risk factor of advanced CRN with moderate evidence.4 However, a 2019 multicentric study showed that during a median follow-up period of 4.8 years, the time until development of advanced CRN did not differ significantly between patients with PIPs and those without PIPs. Further, PIPs did not independently increase the risk of advanced CRN (adjusted hazard ratio 1.17; 95% CI 0.59–2.31).5
After careful analysis of our data, it appears that PIPs may not significantly increase the risk of progression to CRN. After stratifying patients with and without PIPs, what appeared to differentiate which patients would go on to develop CRN was the amount of histological inflammation present as scored by the Nancy index. PIPs may be rather a surrogate marker of previous disease severity. Interestingly, a recent study with 504 patients with IBD did show that a high PIPs burden was associated with treatment escalation, hospitalisation and need for surgery.17
This study also serves to underscore the importance of histological inflammation when it comes to the risk of development of CRN over endoscopic appearance. Stratification by the presence or absence of endoscopic ulceration did not appear to show any statistically significant difference in risk of progression. However, increasing histological features of inflammation as scored using the Nancy index, showed a clear association with subsequent development of CRN. In addition, the data appear to have good external validity as the other variables that were assessed in the multivariate analysis appear to match current known risk factors for CRN such as the presence of PSC and the presence of colorectal cancer in a first-degree relative.1 We also observed a trend for a protective effect associated with anti-TNFs exposure, as it has been recently reported.18
However, our study is not without limitations. Despite the advantage of our extended follow-up time, the data were limited to a single centre in Paris, France, which raises questions about generalisability to more diverse populations. Further, this was a retrospective analysis of a prospectively collected cohort with the inherent limitations with regard to data collection. Data collection was based on the same database but inclusion period and follow-up were extended until 2020 in patients with PIPs in order to increase the sample size of the study. However, the duration of follow-up was very close between groups. Endoscopic disease activity was only based on ulcerations. The presence of ulcerations may not detect mild disease activity, but ulcerations items are widely included in endoscopic disease activity scores.19 Additionally, histologic disease activity may be a more precise and accurate measure of inflammation compared to endoscopic disease activity for the assessment of the risk of colorectal neoplasia, as 20 to 30% of patients with UC have histologic disease activity on colonic biopsies while considered in endoscopic remission based on the Mayo score.20 Lastly, family history of CRC (first-degree relative) was assessed but age of the diagnosis of cancer was not collected.
Large field of PIPs may prevent assessment of neoplasia but the growing use of artificial intelligence may also be an opportunity to further differentiate neoplasia in the colon even in the presence of dense PIPs. New technology that incorporates artificial intelligence appears to demonstrate an independent increase in adenoma detection rate for routine colonoscopy.21 There has also been attempts to develop novel deep learning-based scoring systems and other artificial intelligence tools to evaluate endoscopic images from patients with lBD which can also accurately describe the severity and distribution of inflammatory activity as well as presence of polypoid lesions.
We observed that PIPs alone do not independently increase risk for CRN. Although previous studies did show an association with the presence of PIPs with progression to CRN, this association may have been due to a limited ability to distinguish between PIPs with underlying histological inflammation and PIPs without it. While PIPs may make it more difficult and time-consuming to survey the colon in patients with inflammatory bowel disease, thus necessitating careful colonoscopy, our findings suggest that PIPs are not independent risk factors for CRN. We believe that it is the presence or absence of histological inflammation in the colon, with or without PIPs, that ultimately matters when it comes to risk of progression to CRN. Therefore, routine utilisation of the Nancy index in pathological assessment of IBD patients may give more information about patients' risk for development of dysplasia. It is for these reasons that we believe our study adds support to the need for a change in the paradigm with regard to PIPs in IBD surveillance.
AUTHOR CONTRIBUTIONS
Thomas Wolf: Conceptualization (supporting); data curation (equal); formal analysis (supporting); writing – original draft (equal). Ayanna Lewis: Conceptualization (supporting); data curation (equal); formal analysis (supporting); writing – original draft (equal). Laurent Beaugerie: Conceptualization (equal); formal analysis (supporting); methodology (supporting); writing – review and editing (supporting). Magali Svrcek: Conceptualization (equal); formal analysis (equal); supervision (equal); writing – review and editing (equal). Julien Kirchgesner: Conceptualization (equal); formal analysis (lead); methodology (lead); supervision (equal); writing – review and editing (supporting).
ACKNOWLEDGEMENTS
Declaration of personal interests: Laurent Beaugerie has received research support from Abbvie, Celltrion, Ferring Pharmaceuticals, Hospira-Pfizer, Janssen, MSD, Mylan, Takeda and Tillots. The remaining authors disclose no conflicts.
FUNDING INFORMATION
This work was not supported by dedicated funding.
CONFLICT OF INTEREST
Laurent Beaugerie has received consulting fees from BMS, Janssen, Nordic Pharma and Mylan; lecture fees from Abbvie, BMS, Janssen, MSD, Ferring, and Takeda. Julien Kirchgesner has received lecture fees from Janssen and consulting fees from Roche, Pfizer, and Gilead. The remaining authors disclose no conflicts.
AUTHORSHIP
Guarantor of the article: Julien Kirchgesner.
APPENDIX A: Members of the Saint Antoine IBD Network
Lionel Arrive, Laurent Beaugerie, Anne Bourrier, Marine Camus, Najim Chafai, Edouard Chambenois, Ulriikka Chaput, Clotilde Debove, Xavier Dray, Nadia Hoyeau, Pauline Ioro, Julien Kirchgesner, Cécilia Landman, Marie LAZARETH, Jérémie H. Lefevre, Romain Leenhardt, Paul McLellan, Laurence MonnieR-Cholley, Isabelle Nion-Larmurier, Violaine Ozenne, Yann PARC, Laurène Parrot, Philippe Seksik, Harry Sokol, Magali Svrcek.




