Chimeric antigen receptor T cell immunotherapy in colorectal cancer: primetime yet?
Immunotherapy has revolutionized treatment in cancers such as melanoma, non-small cell lung cancers (NSCLC) and renal cell cancers.1 Immune checkpoint inhibitors are being used as first line therapy in metastatic NSCLC, as consolidation therapy for locally advanced disease and in the adjuvant setting for patients with resectable disease.2 Chimeric Antigen Receptor (CAR) T cell therapy is no exception, with significant developments in treatment for haematological malignancies which brings promise and hope in its use in solid tumours such as colorectal cancer. While oncological resection remains the mainstay of colorectal cancer treatment, the majority of patients with Stage IV disease are unresectable, and current chemotherapeutics has reached its ceiling with no appreciable durable response to prolong survival.3
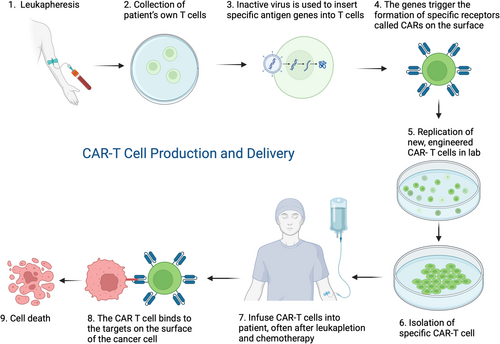
Solid tumours including colorectal cancers are traditionally treated with a combination of surgery, chemotherapy and/or radiotherapy. For refractory and recurrent disease, alternate therapies have been explored. CAR T cell therapy is a novel, cell-based immunotherapy treatment. T lymphocytes are genetically modified to recognize specific antigens on cancer cells. Lymphocytes have the capacity to migrate and infiltrate tissues through both vascular connections and directly through the interstitium. By modifying the T lymphocyte, the CAR T cells migrate towards the tumour which expresses the specific antigen and eliminates them by causing significant tumour regression or destruction (Fig. 1).4
There are significant hurdles when applying the CAR T cell therapy to solid tumours. The major limitations of its use in colorectal cancer are due to the heterogeneity of tumour specific antigens, poor migration and infiltration into the tumour and the active suppression of the tumour microenvironment, both physical and immunological.5, 6 Not to mention the toxicity that can potentially trigger an overwhelming immune response due to the pro-inflammatory cytokine release, a condition named cytokine release syndrome (CRS).
However, despite these limitations with solid tumours, a potential exciting prospect in the application of CAR T cell therapy is in colorectal peritoneal metastasis (CPM). Peritoneal carcinomatosis from colorectal cancer is an exceedingly difficult condition to treat but one that affects 10%–15% of all colorectal cancer patients.8 If left untreated, patients often progress to fatal malignant bowel obstruction.
Conventional treatment in highly selected patients, is cytoreductive surgery with instillation of hyperthermic intraperitoneal chemotherapy (CRS-HIPEC), after a randomized control trial showed survival benefit over palliative 5-fluorouracil systemic chemotherapy in 2004.9 The HIPEC component of this treatment regimen has recently been under scrutiny after the results of the French PRODIGE 7 trial. This trial published in the Lancet Oncology, is a randomized-control trial demonstrating that when comparing those who underwent oxaliplatin-based HIPEC after completion of CRS showed an absence in survival benefit and had a higher 60-day morbidity rate than those who underwent CRS alone.10 This trial only included a specific HIPEC chemotherapy regimen (oxaliplatin) in a select group of patients. Opponents to the technique question the rationale for the principles of HIPEC in its entirety.10 The data from this study cannot be extrapolated to other HIPEC regimens. It does however, bring to light the concept of other intraperitoneal therapeutics such as CAR T cell therapy.
Katz et al. demonstrated local peritoneal infusion of CAR-T cell therapy targeting CEA+ tumours was superior to systemic delivery in the murine model.11 They showed a significant reduction in intraperitoneal tumour burden as well as a synchronous site distant to the peritoneum. Another notable finding in this study was the demonstration of improved efficacy when intraperitoneal CAR T cells were combined with depleting antibodies against myeloid-derived suppressor cells (MDSC) and regulatory T cells, both components of the stubbornly impermeable tumour microenvironment. This pre-clinical trial shows promise in addressing the clinical need of targeting peritoneal metastasis in colorectal cancer and advances in local therapeutic approaches.
There are significant physical and biological barriers that make CAR T cell applicability in solid tumours more difficult than in haematological diseases. Despite these challenges, the hurdles are being overcome by exploring and exploiting the full benefits of CAR T cell therapy, co-stimulation with chemotherapeutics and exploration into different therapeutic delivery methods. CAR T cell therapy in solid tumours is an exciting cross over between immunology and surgery and while immunotherapy has changed the landscape in haematological malignancies, its efficacy and translation to solid tumours such as colorectal cancer is not quite there yet, but it's close.
Acknowledgement
Open access publishing facilitated by Monash University, as part of the Wiley - Monash University agreement via the Council of Australian University Librarians.
Author contributions
Maria Kristina Vanguardia: Conceptualization; writing – original draft. Hannah Hassum: Writing – review and editing. Sara Roth: Supervision. Joseph Cherng Huei Kong: Conceptualization; supervision; writing – review and editing.