A single centre experience on the formation of double barrelled uro-colostomy in pelvic exenteration surgery: a cohort study
Abstract
Background
Double barrelled uro-colostomy (DBUC) is an alternative to traditional ileal conduit (IC) and separate colostomy in patients requiring simultaneous urinary and faecal diversion for reconstruction in pelvic exenteration surgery (PES).
Methods
This cohort study evaluated short- and long-term morbidity and mortality associated with DBUC formation in 20 consecutive adult patients undergoing PES in an Australian Complex Pelvic Surgical Unit. Data were obtained from a prospective database.
Results
Mean age 59 years (range 27–76 years). PES was performed for malignant disease in 18 patients (curative intent in 17). Mean operative duration 11.8 h (range 7–17 h). Mean follow-up duration 29.1 months (range 2.6–90.1 months). Early DBUC-related complications occurred in four patients (20.0%): urinary tract infection (UTI)/urosepsis (n = 4) and early ureteric stenosis requiring intervention (n = 1). Late DBUC-related complications occurred in five patients (25.0%): recurrent UTI/urosepsis (n = 4), chronic kidney disease (n = 4), ureteric stenosis (n = 2) and parastomal hernia (n = 4). No mortality occurred secondary to a DBUC complication.
Conclusion
DBUC is a safe reconstructive option with acceptable morbidity profile in patients requiring simultaneous urinary and faecal diversion.
Introduction
Pelvic exenteration is a radical surgical procedure that involves removal of two or more contiguous pelvic organs.1 The procedure was first described by Alexander Brunschwig in 1948 in New York as a palliative procedure for recurrent carcinoma of the cervix.
In addition to PES, Brunschwig described the first ureterocolostomy (‘wet’ colostomy). This approach involved uretero-colonic anastomoses proximal to a sigmoid end colostomy, resulting in the formation of a single stoma.2 The technique, however, was associated with high rates of electrolyte disturbance and pyelonephritis secondary to faecal reflux, and was superseded by the double barrelled uro-colostomy (DBUC; also formerly termed the ‘double barrelled “wet” colostomy’). The DBUC, first described by Carter et al. in 1989, involves ureteric anastomoses to a second ‘urine limb’ fashioned distal to the colostomy. Urine flows in an antiperistaltic direction to exit the stoma, without making contact with the faecal stream.2 In Carter's original series of 11 patients DBUC was not associated with any cases of pyelonephritis.3 Separation of the urinary and faecal streams is also thought to reduce the risk of malignant transformation within the colonic mucosa at the uretero-colonic anastomoses.2, 4, 5
Unlike the watery output of standard ‘wet’ colostomies, the DBUC has continuous urine output with intermittent semi-formed faecal output2; this is associated with a lower incidence of peristomal skin complications.6-8
Compared to the traditional colostomy and ileal conduit, the DBUC offers patients the advantage of a single stoma. This reduces patient costs related to stomal appliances and is associated with improved quality of life (QOL) scores in small case series.7 DBUC avoids the need for an additional small bowel anastomosis (particularly advantageous in patients who have undergone prior radiotherapy or who are malnourished).6, 9 The DBUC has comparable rates of pyelonephritis, renal failure and urine/enteric fistulas to traditional ileal conduit.10, 11
There are few local and international published series investigating outcomes in DBUC. An Australian series recently reviewed outcomes of 16 patients with DBUC formed through the Royal Adelaide Hospital (RAH) Pelvic Exenteration Service. This demonstrated that DBUC is a safe alternative to ileal conduit with potentially fewer complications.12 This study presents another Australian single centre experience reviewing short- and long-term morbidity and mortality associated with DBUC formation in adult patients undergoing PES.
Methods
This study included all consecutive adult patients (>18 years) undergoing PES who received a DBUC for benign or malignant disease at an Australian Complex Pelvic Surgical Unit between 1 September 2015 and 31 March 2023. Data from eligible patients were recorded in a prospectively collected database designed for teaching and research purposes. Ethics approval was obtained from the South Western Sydney Local Health District Human Research Ethics Committee, approval number 2022/ETH00137.
Clinical parameters analysed included patient demographics (age, sex, body mass index (BMI), American Society of Anaesthesiologists (ASA) physical status classification, pre-operative renal function, and presence of pre-existing stoma), disease characteristics (indication for PES, TNM stage, neoadjuvant chemotherapy or radiotherapy), procedure characteristics (visceral resection, lymph node dissection, bone resection, method of pelvic reconstruction, operative time) and postoperative outcomes (length of stay, postoperative renal function, histopathology, short- and long-term morbidity and mortality data).
Surgical technique
A standard oral bowel preparation is used preoperatively. Following removal of the pathology and pelvic organs, attention turns to reconstruction, including formation of a DBUC. If not already performed, lateral to medial splenic flexure and left colic mobilization occurs. Usually, the left colic vessels are preserved during resection ensuring a satisfactory conduit blood supply. The sigmoid colonic urinary conduit is formed by transecting the sigmoid colon distally with a TLC75™ linear stapler (Ethicon™, USA). The sigmoid apex, 15–20 cm proximally, is similarly transected. Complete division of the bowel wall both reduces stomal retraction (in a manner analogous to the Abcarian construction) and results in better separation of the faecal from the urinary stream.
The ureters are transected by the urologist to a well vascularised, non-irradiated distal segment. Mobilization of the proximal ureter is minimized to preserve periureteric blood supply. After spatulation of the ureteric ends, a Bricker anastomosis to the taenia of the distal colonic conduit in an antiperistaltic fashion is performed with interrupted 4–0 Vicryl over Bander™ (Cook® Medical, Bloomington, Indiana, USA) ureteric stents. The aim is to achieve tension-free, mucosal to mucosal, watertight anastomosis to the taenia coli. An on-table leak test is performed on completion with saline distension of the colonic conduit. Any obvious leaks are reinforced.
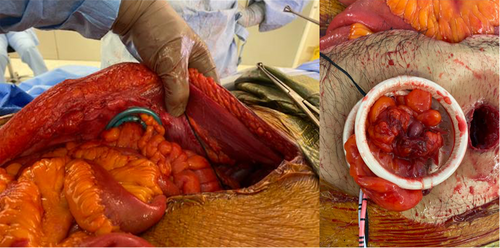
The DBUC is matured with 3–0 Vicryl at a pre-operatively marked site. When a SMART (Stapled Mesh stomA Reinforcement Technique) procedure is performed, a 28 mm circular stapled trephine is made using a Chex CS COMPACT™ stapler (Frankenman International, China) through the centre of a circular piece of 70 mm onlay polypropylene mesh, secured to the anterior sheath with either AbsorbaTack™ (Covidien-Medtronic, Minneapolis, Minnesota, USA) or sutures. The colostomy and urinary conduit are delivered through the trephine with the aid of two XXS Alexis™ wound protector-retractors (Applied Medical, Santa Margarita, California, USA) and matured (Fig. 1).13 An initial Alexis retractor is placed to deliver the urinary conduit (inferiorly). A second Alexis retractor is passed through the first (beside the urinary conduit) to facilitate delivery of the faecal stoma (superiorly) alongside the urinary conduit. The Alexis retractors are then removed in the reverse order during closure, and the DBUC matured.
All patients are admitted to the intensive care unit and receive 48 h of further prophylactic antibiotics. Drain creatinine is assessed on post-operative day three and seven to ensure no delayed breakdown of the ureteric anastomoses. Ureteric stents are removed on day 14. Timing of stent removal is a balance between anastomotic healing and infection risk.
Endpoints
The primary endpoint evaluated was post-operative morbidity, defined as any Clavien–Dindo grade II–IV complication.14 Morbidity was sub-classified as short-term (occurring within 30 days) or long-term (occurring after 30 days); DBUC-related or non DBUC-related. Secondary endpoints were length of stay (LOS) and all-cause mortality.
Kidney Disease Improving Global Outcomes (KDIGO) guideline definitions were used for Acute Kidney Disease (AKI: a rise in serum creatinine to ≥1.5 times baseline) and Chronic Kidney Disease (CKD: glomerular filtration rate < 60 mL/min/1.73m2 for >3 months).15 Ureteric stenosis was defined as radiological evidence of hydroureter with obstruction demonstrated on combination nuclear medicine scan or nephrostogram requiring nephrostomy or ureteric stent insertion.12 Mean follow-up was calculated from date of surgery to date of most recent follow-up or date of death, whichever occurred first.
Statistical analysis
Data were analysed using Jamovi statistical software [The jamovi project (2022). Jamovi(Version 2.3), Sydney, Australia]. Values expressed as means (ranges) for continuous variables; frequencies (percentages) for categorical variables.
Results
DBUC was formed in 20 patients, including 14 males (70%); mean age 59 years (range: 27–76). Patient and disease characteristics are detailed in Table 1. Indication for PES was malignant disease in 18 patients (17 with curative intent) and benign disease in two. Eighteen patients (90%) had prior pelvic radiotherapy. Exenteration details are summarized in Table 2. A SMART procedure was performed in 17 patients (85.0%). Mean operative duration was 11.8 h (range 7–17 h).
| n (%) | |
|---|---|
| Male | 14 (70.0) |
| Age (years) | 59.2 (27–76)† |
| BMI overweight or obese (>25 kg/m2) | 10 (50.0) |
| ASA | |
| II | 5 (25.0) |
| III | 14 (70.0) |
| IV | 1 (5.0) |
| Indication for PES | |
| Malignant disease | 18 (90.0) |
| Colorectal | 11 (50.0) |
| Rectal adenocarcinoma | 9 (45.0) |
| Mixed adeno-neuroendocrine carcinoma of rectum | 1 (5.0) |
| Urological | 3 (15.0) |
| Urothelial carcinoma of bladder | 2 (10.0) |
| Urethral carcinosarcoma | 1 (5.0) |
| Gynaecological | 4 (20.0) |
| Cervical cancer | 2 (10.0) |
| Endometrial cancer | 1 (5.0) |
| Serous ovarian cancer | 1 (5.0) |
| Benign disease | 2 (10.0) |
| Severe radiation cystitis and faecal incontinence | 1 (5.0) |
| Benign urethral fistula (to rectum) and pubic bone osteomyelitis | 1 (5.0) |
| Prior pelvic radiotherapy | 18 (90.0) |
| Pre-existing stoma | 4 (20.0) |
- † Expressed as mean (range).
| n (%) | |
|---|---|
| Pelvic exenteration type | |
| Total pelvic exenteration | 19 (95.0) |
| Posterior pelvic exenteration | 1 (5.0) |
| Visceral resection | |
| Abdominoperineal resection | 20 (100.0) |
| Cystectomy/cystoprostatectomy | 18 (90.0) |
| Vaginectomy | 5 (25.0) |
| Hysterectomy | 1 (5.0) |
| Bone resection | 8 (40.0) |
| Pubic bone | 5 (25.0) |
| Sacrum/coccyx | 5 (25.0) |
| Lateral pelvic lymph node dissection | 14 (77.8)† |
| Aortocaval lymph node dissection | 5 (27.8)† |
| SMART procedure | 17 (85.0) |
| Pelvic reconstruction | |
| Omental graft | 16 (80.0) |
| VRAM flap | 4 (20.0) |
| Mesh to pelvis | 8 (40.0) |
| BioA | 5 (17.6) |
| Vicryl | 1 (5.0) |
| Parietex | 1 (5.0) |
| Strattice | 1 (5.0) |
| Operative time (h) | 11.8 (7–17)‡ |
- † Lymph node dissection percentages based on the 18 patients who underwent PES for malignancy. All other percentages based on total cohort of 20 patients.
- ‡ Expressed as mean (range).
Short-term postoperative outcomes
Mean length of stay was 22.0 days (range: 10–49 days). DBUC-related short term complications in the form of UTI/urosepsis occurred in four patients (20%). One of these patients developed urosepsis secondary to early ureteric obstruction at the uretero-colic anastomosis, requiring nephrostomy on post-operative day 20 and antegrade ureteric stent insertion. Non-DBUC-related Clavien Dindo II–IV short term complications occurred in 15 patients (75%) and are outlined in Table 3. No patients had a leak from the ureteric anastomosis.
| n (%) | ||
|---|---|---|
| Clavien–Dindo Grade | DBUC-related | 4 (20.0)† |
| IIIb | Ureteric obstruction (at uretero-colonic anastomosis) | 1 (5.0) |
| II | UTI/Urosepsis | 4 (20.0) |
| Non DBUC-related | ||
| Required intervention | 7 (35.0)† | |
| IIIb | Pelvic collection requiring operative drainage | 2 (10.0) |
| Perineal wound dehiscence requiring operative management | 1 (5.0) | |
| IIIa | Pelvic collection requiring percutaneous drainage | 4 (20.0) |
| ST-elevation myocardial infarction requiring PCI | 1 (5.0) | |
| Managed nonoperatively | 14 (70.0)† | |
| II | Abdominal wound dehiscence | 1 (5.0) |
| Atrial fibrillation | 2 (10.0) | |
| Bacteraemia | 1 (5.0) | |
| Delirium | 3 (15.0) | |
| Fungaemia | 2 (10.0) | |
| Heparin-induced thrombotic thrombocytopenia syndrome (HITTS) | 1 (5.0) | |
| Hospital acquired pneumonia | 1 (5.0) | |
| Ileus | 2 (10.0) | |
| Intra-abdominal collections | 1 (5.0) | |
| Internal jugular vein thrombosis | 1 (5.0) | |
| Non-ST elevation myocardial infarction | 1 (5.0) | |
| Type 1 respiratory failure | 1 (5.0) |
- † Some patients had multiple complications.
Postoperative AKI occurred in nine patients (45.0%) however serum creatinine returned to baseline prior to discharge in all patients. There was no mortality at post-operative day 30.
R0 resection was achieved in 16 patients (88.9%) treated for malignancy. Two patients had an R1 resection (11.1%). Lymph node metastases were present in two patients (11.1%), including one PES performed with palliative intent.
Long-term outcomes
Mean follow-up duration was 29.1 months (range 2.6–90.1 months). Five patients (25.0%) had long-term DBUC-related complications (Table 4) including recurrent UTI/urosepsis with associated CKD in four patients (20.0%) and ureteric stenosis in two patients (10%). One of these patients had bilateral ureteric stenosis at the anastomoses due to fibrosis (radiation-related endarteritis obliterans) and required bilateral nephrostomies and antegrade ureteric stents. This patient had prior external beam radiotherapy and brachytherapy for cervical cancer. The second patient with ureteric stenosis also required nephrostomies and stents, and subsequently underwent surgical separation of the urinary conduit from the colostomy, with maturation of a neo-colonic conduit at a separate site, at 12 months post PES; the same patient progressed to stage 5 CKD requiring long-term haemodialysis.
| n (%) | Clavien–Dindo grade/s | |
|---|---|---|
| DBUC-related | 5 (25.0)† | |
| Ureteric stenosis | 2 (10.0) | IIIb, IIIb |
| Parastomal hernia | 4 (20.0) | I, I, I, IIIb |
| Recurrent UTI/urosepsis | 4 (20.0) | II, II, IIIb, IVa |
| Chronic kidney disease | 4 (20.0) | II, II, IIIb, IVa |
| Subacute small bowel obstruction between the limbs of the stoma | 1 (5.0) | II |
| Non DBUC-related | 2 (10.0)† | |
| Chronic pelvic collection | 2 (10.0) | IIIa, IIIa |
| Benign enterocutaneous fistula | 1 (5.0) | IIIb |
- † Some patients had multiple complications.
Four patients had a clinically evident parastomal hernia; one symptomatic. All of these patients had a SMART procedure. The symptomatic patient underwent elective laparoscopic mesh repair 13 months following PES. Another patient developed subacute small bowel obstruction between the two limbs of the stoma and was successfully managed nonoperatively. No patient had malignancy within the conduit at surveillance colonoscopy/conduitoscopy (performed in three patients, range 7–42 months post-operatively). No patient developed a urine leak, documented peristomal dermatitis or lithiasis within the conduit.
Other complications (Table 4) included chronic pelvic collection in two patients (10.0%) requiring repeated drainage procedures. One patient developed a benign enterocutaneous fistula in the right gluteal region, treated with laparotomy, excision of fistula tract and small bowel resection with Vicryl mesh reconstruction of the pelvic floor 65 months post PES.
Malignant disease recurrence occurred in eight patients (44.4%); including seven treated with curative intent. Local recurrence occurred in one patient (5.6%) and distant metastatic disease in seven patients (38.9%). Sites of metastasis included pulmonary (n = 4), spinal (n = 2), non-regional lymph nodes (n = 2) and liver (n = 1). Mean time to recurrence was 7.0 months (range 1.0–27.2 months).
Six patients (30.0%) died within 5 years of undergoing PES; four who underwent PES for colorectal malignancy (one with palliative intent), one for gynaecological malignancy and one for urological malignancy. Causes of death were distant metastatic disease (n = 4), local disease recurrence (n = 1) and sepsis from right calcaneal osteomyelitis, treated palliatively (n = 1).
Detailed outcomes in supplemental table (Table S1).
Discussion
This study demonstrates that reconstruction using DBUC during PES is safe, and associated with satisfactory short and long-term morbidity. The majority of postoperative complications observed in our cohort occurred secondary to the complex nature of the pelvic surgery rather than the DBUC.
Rates of infective complications following DBUC vary considerably in the literature, and may be as high as 50% for early UTI and 60% for recurrent UTI.16 In our series, 20% of patients developed early UTI/urosepsis (n = 4) and 20% late UTI/recurrent urosepsis (n = 4), which is lower than rates reported in the literature.16, 17
When comparing our cohort (n = 20) to Nguyen et al.'s prospective series from the Royal Adelaide Hospital Pelvic Exenteration Service (n = 16), our cohort experienced lower rate of ureteric stricture (10.0 versus 25.0%), urinary leak (0.0 versus 6.3%) and urosepsis (20.0 versus 43.8%), slightly higher incidence of postoperative acute kidney injury (40.0 versus 31.3%) and similar rate of parastomal hernia requiring repair (5.0 versus 6.3%).12 Our cohort had longer mean follow up duration (29.1 versus 18.0 months), higher rates of lateral pelvic lymph node dissection (77.8 versus 25%) and bone resection (40.0 versus 18.8%), and similar rates of prior radiotherapy (90.0 versus 93.8%).12
In the largest international published series of 169 patients with DBUC (115 in the context of PES) with median follow-up of 17 months, Guimaraes et al. (Brazil) reported a 5.2% incidence of late postoperative UTI, 3.5% urinary leakage, 8.2% ureteric stenosis, and 1.7% DBUC complication-related mortality.18 In a second large international series (41 patients, Spain) Golda et al. reported 9.8% pyelonephritis, 7.3% lithiasis within the conduit, 4.9% ureteric anastomotic dehiscence, 2.4% CKD and 2.4% stenosis at the ureterocolonic anastomosis.6 In comparison, no patients in our cohort experienced ureteric lithiasis or anastomotic dehiscence. Two patients developed stenosis of the ureterocolonic anastomosis in the context of prior radiotherapy (10%).
Golda et al. utilized anti-refluxing ureteric anastomoses and a 20–25 cm colonic conduit.6 Subsequent series, including this cohort, have favoured shorter (15–20 cm) conduits in an effort to reduce urinary stasis that may predispose to lithiasis within the conduit. A shorter distal urinary reservoir is thought to reduce the incidence of metabolic complications, including hyperchloraemic hypocalcaemic metabolic acidosis secondary to absorption of urinary ammonia, hydrogen and chloride through the colonic mucosa.8, 19 There were no cases of clinically significant metabolic disturbances using a 15–20 cm colonic conduit in our cohort.
Anti-refluxing anastomoses are advocated by some authors to reduce rates of pyelonephritis, however are associated with significantly higher rates of ureteric stenosis compared to refluxing anastomoses (such as the Bricker and Wallace techniques).20 This has been demonstrated in several cohorts including Pantuck et al. (13.5% versus 1.7% respectively, P < 0.05) and Roth et al. (20.4% versus 3.6% respectively).21, 22 Ureteric stenosis can lead to significant septic complications and requirement for nephrostomy, as observed in two patients in our series.
A 2023 Spanish study (Lago et al.) of 49 patients undergoing PES for gynaecological malignancy found no significant difference between Bricker ileal conduit (n = 29) and DBUC (n = 20) in terms of urinary leakage (3% versus 0%), AKI (10% versus 20%), long-term pyelonephritis (69% versus 65%), ureteral stricture (10% versus 6%), CKD (38% versus 29%) and electrolyte disturbance (24% versus 18%).11 In comparison, Nguyen et al. (Adelaide, 2023) reported a higher rate of ureteric stricture in their DBUC group compared to ileal conduit (25.0 versus, 8.7%) but lower rates of urine leak (6.3 versus 8.7%), urosepsis (43.8 versus 60.9%) and parastomal hernia requiring repair (6.3 versus 13.0%).12
Pavlov et al. (Serbia, 2013) also found that when compared to ileal conduit and end colostomy (n = 77), DBUC (n = 104) was associated with lower 30-day morbidity (23.4% versus 11.5%) and reduced median LOS (18.1 versus 13.1 days).9 Our cohort had 30-day DBUC-related morbidity of 20% and mean LOS of 22.0 days. Pavlov's comparatively shorter LOS is likely due to the lower proportion of exenteration procedures in their cohort (77% pelvic exenteration, 12% palliative diversion and 11% post radiation damage diversion).9
Arguably one of the key advantages of DBUC over separate colostomy and ileal conduit is the psychological impact and potential QOL improvement associated with managing one stoma rather than two. A single stoma is more convenient and acceptable to many patients and is associated with reduced costs related to stoma appliances.7 A QOL assessment of five patients with DBUC (using the QLQ-C30 Version 3 questionnaire) found that all patients reported ease in caring for a single stoma bag and conducting personal hygiene.8 Pavlov et al. also found that no patient chose two stomas if they were offered the alternative of one.9
DBUC offers further benefit in cases where there is a significant perineal defect following APR. Placement of a single abdominal stoma, rather than two, provides the option of reconstruction with a VRAM (vertical rectus abdominis myocutaneous) flap; as was utilized in four patients in our study (20%).
We advocate the use of a DBUC in patients with history of prior pelvic irradiation. The use of a non-irradiated sigmoid colonic conduit rather than an ileal conduit (often with irradiated small bowel) removes the risk of anastomotic leak and enterocutaneous fistula from the enteroenteric anastomosis.8 DBUC avoids the requirement for an entero-enteric anastomosis, potentially involving irradiated small bowel, and associated risk of anastomotic leak. Another advantage of DBUC is the ease of re-entry into the abdominal cavity for early or later laparotomy. After conventional adhesiolysis the mesentery can be mobilized in the normal manner without the tethering of an ileal conduit.
To date, malignancy has not been described within a DBUC14. In contrast, the original uro-colostomy was associated with a 6%–40% rate of malignancy at the uretero-colonic anastomosis, as a result of Gram negative faecal bacterial conversion of urinary nitrites and secondary amines to carcinogenic nitrosamines.19, 23 In Osorio Gullon et al.'s series, conduit biopsies obtained from five patients at three and 16 months post-operatively demonstrated reduced mucous-secreting caliciform cells and slightly increased fibrosis in the lamina propria without dysplasia or malignancy.4 Multiple subsequent studies, including our series, have not reported evidence of malignancy in the conduit during the follow-up period.4, 6, 24 However, endoscopic surveillance is still recommended due to the latency of carcinogenesis and usual history of rectal cancer.16
We acknowledge that our study is limited by relatively small patient numbers, heterogenous cohort, lack of a comparison group (with separate colonic and urinary stomas) and variable follow-up. Despite this, to our knowledge, this is the largest single Australian study reporting short- and long-term outcomes of DBUC and adds to the current body of evidence supporting the use of DBUC in PES. Comparison of outcomes to prior studies is limited by the heterogeneity of patients undergoing PES with DBUC. For instance, our patient population included more advanced malignant pathology, with higher rates of lateral pelvic lymph node dissection and bone resection compared to other series.1, 8, 16
The data from this study shows that DBUC is a safe reconstructive option for patients requiring concurrent urinary and faecal diversion as a result of PES. We advocate for patients to be offered DBUC where technically feasible, rather than separate stomas, due to its acceptable short- and long-term morbidity profile, advantages in the context of prior pelvic irradiation, and potential QOL benefit to patients.
Author contributions
Alexandra M. Limmer: Conceptualization; data curation; writing – original draft; writing – review and editing. Rebecca J. Lendzion: Writing – review and editing. Christopher Leung: Conceptualization; data curation. Eddy Wong: Supervision; writing – review and editing. Andrew J. Gilmore: Conceptualization; data curation; supervision; writing – review and editing.
Acknowledgement
Open access publishing facilitated by Macquarie University, as part of the Wiley - Macquarie University agreement via the Council of Australian University Librarians.
Conflict of interest
None declared.