The Evolution, Immunopathogenesis and Biomarkers of Type 2 Inflammation in Common Allergic Disorders
Funding: This work was supported by the Start-up Research fund by The First Affiliated Hospital of Zhejiang University School of Medicine (BQD 2306), the National Natural Science Foundation of China (72204214), and the Zhejiang Provincial Natural Science Foundation of China (LTGY24H260001, LQN25H030006, and LQN25H020010).
Ya-dong Gao and Zi-jun Wang contributed equally to this work.
ABSTRACT
The prevalence of allergic diseases, including allergic rhinitis, chronic rhinosinusitis, asthma, eosinophilic esophagitis, food and drug allergies, and atopic dermatitis, has been increasing globally over the past few decades. Allergic diseases are closely linked to type 2 immunity, which is characterized by the coordinated interplay between innate and adaptive immune responses. Significant advancements have been achieved in elucidating the cellular and molecular mechanisms that govern type 2 immunity, chiefly mediated by type 2 cytokines, including IL-4, IL-5, IL-9, and IL-13, which are primarily secreted by T helper 2 cells and group 2 innate lymphoid cells. In addition, a diverse array of effector cells, including mast cells, basophils, eosinophils, regulatory T cells, B lymphocytes, dendritic cells, and natural killer cells, are critically involved in orchestrating and modulating type 2 inflammatory responses. The activation of epithelial cells, secretion of alarmins and multiple chemokines, impairment of epithelial barrier integrity, and disruption of microbial dysbiosis serve as crucial mechanisms underlying not only the pathogenesis of allergic disorders but also the development of various systemic conditions. Biologic therapies targeting type 2 pathways—specifically effector functions of IL-4, IL-13, IL-5, thymic stromal lymphopoietin, and immunoglobulin E have—demonstrated promising efficacy. However, a subset of patients with severe allergic diseases remains unresponsive to these treatments, underscoring the need for deeper mechanistic insights and personalized therapeutic approaches. This review addresses the definition, evolution, cellular and molecular basis, and regulation of type 2 immunity. It then examines the common allergic diseases associated with type 2 responses and concludes by exploring the associations between inborn errors of immunity and type 2 responses.
1 Introduction
Allergic diseases have emerged as a global health burden, affecting nearly 1 billion people and imposing significant healthcare costs. There exists a broad spectrum of allergic diseases, encompassing allergic asthma (AS), allergic rhinitis (AR), atopic dermatitis (AD), eosinophilic esophagitis (EoE), as well as food and drug allergy [1-4]. These diverse disorders share inflammatory responses, although they manifest in different organ systems and present with distinct clinical features.
The immune system responds to various threats through distinct pathways, categorized as type 1 and type 2 immunity. Type 1 immunity, primarily regulated by T helper 1 (Th1) cells, involves phagocytic activity driven by cytokines, such as interleukin (IL)-2, interferon-γ (IFN-γ), and lymphotoxin-α [5]. Type 2 immunity, an evolutionarily conserved defense mechanism primarily directed against helminth infections, is marked by prominent tissue eosinophilia and heightened allergen sensitization. This immune response engages a wide array of innate immune cell populations, including mast cells (MCs), eosinophils, basophils, alternatively activated (M2) macrophages, and innate lymphoid cells 2 (ILC2s), along with components of adaptive immune cells. Th2 cells, a specialized subset of CD4+ helper T lymphocytes, play a pivotal role in type 2 immunity by secreting signature cytokines, including IL-4, IL-5, IL-9, and IL-13, which initiate immune responses by acting on immune cells, such as B cells and dendritic cells (DCs) [6].
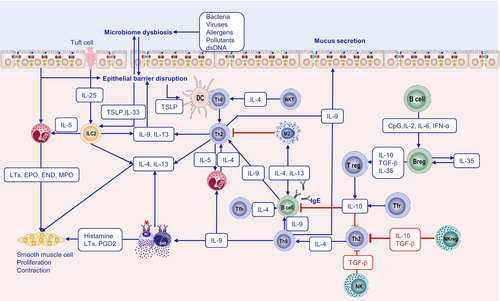
The epithelial barrier theory proposes that exposure to hazardous environmental agents associated with industrialization, urbanization, and modern lifestyle leads to the impairment of epithelial barrier integrity, thereby compromising its protective functions [1]. External exposomes, including allergens, pathogens, and environmental pollutants, disrupt epithelial barriers. This triggers epithelial cells to release alarmins, which activate ILC2s to produce IL-5 and IL-13, and thus initiates type 2 immunity [7, 8]. In addition, disruption of the epithelial barrier drives or coexists with microbial dysbiosis, characterized by a reduction in commensals and an overgrowth of opportunistic pathogens. This imbalance facilitates the translocation of bacteria into the inter- and subepithelial regions, leading to both tissue and systemic inflammation, along with immune dysregulation. The epithelial barrier dysfunction and bacterial translocation have been mechanistically linked to the rising prevalence and exacerbation of allergies [1] (Figure 1).
This review will introduce the definition of type 2 immunity and its associations with allergic diseases. It will further examine the central players in type 2 immunity, including Th2 cells, ILC2s, and effector cells like MCs, eosinophils, and basophils. Following this, the review will also explore the pathophysiological underpinnings of type 2 immunity, particularly in the context of epithelial barrier disruption, microbiome dysbiosis, and the roles of various regulatory cells in type 2 inflammation. Finally, we will summarize the most prevalent allergic diseases associated with type 2 immunity and discuss the relationship between inborn errors in immunity and type 2 immune responses.
2 Underlying Mechanisms of Type 2 Immunity
2.1 The Evolutionary Basis of Type 2 Immunity
Type 2 immunity plays a crucial role in providing protection against helminth infections and contributing significantly to the reduction of helminth burden within infected tissues. It effectively eliminates or expels helminths while minimizing tissue damage, preserving tissue homeostasis, and promoting regeneration and fibrosis [9-12]. The expulsion response against helminth larvae exhibits characteristic hallmarks of a robust type 2 immune response, which involves a cascade of molecular interactions that facilitate the co-survival of the worm and its host. The earliest scientific documentation of this phenomenon dates back to 1932 when Willem Löffler reported his observation of eosinophilic pneumonia—a pulmonary inflammation characterized by eosinophil infiltration—in response to infections of Ascaris, hookworms, Toxocara, and Schistosoma [13, 14]. Infection by helminths typically occurs when their fertilized eggs are inadvertently ingested by the host during their life cycle. These eggs hatch in the intestine, and the larvae then travel through the portal veins, passing through the inferior vena cava, right heart, and pulmonary artery, ultimately reaching the lungs. The larvae, ranging in size from 0.5 to 1 mm, grow and induce eosinophilic pneumonia, leading to symptoms like coughing, as Löffler originally described. Mechanistically, chitin expressed by helminths can stimulate epithelial cells to release alarmins, which can induce ILC2s differentiation and initiate type 2 immunity [15]. The larvae need to be completely expelled from the lungs before reaching adulthood to accommodate their size of 15–20 cm. The lungs offer no space for the growth of adult worms, becoming a major threat to the survival of both the host and the parasite. As a result, the larvae are expelled from the lungs while they are still small in size and then transition into adults in the larger, more accommodating space in the guts. Likewise, a similar expulsion-like immune response occurs against skin parasites, such as those causing scabies [16]. The type 2 immune response can eradicate the danger away from deeper tissues, leading to intense pruritus, persistent scratching, peripheral eosinophilia, and transepidermal elimination of the inflammation out of the skin, with cutaneous manifestations akin to AD [16, 17].
2.2 Cytokines and Chemokines Involved in Type 2 Immunity
2.2.1 IL-4, IL-5, IL-13, IL-9, and IL-31
Type 2 immunity is characterized by the production of cytokines, such as IL-4, IL-5, IL-9, IL-13, and IL-31 [3], which play critical roles in host defense and allergic disease pathogenesis [18]. Additionally, epithelial cell-derived alarmins, such as thymic stromal lymphopoietin (TSLP), IL-25, and IL-33 are important initiators of type 2 immunity [18]. IL-4 and IL-13 are structurally similar cytokines that affect the type 2 inflammatory response. IL-4, unique to mammals, is mainly produced by activated CD4+ T cells, but can also be secreted by other immune cells, including natural killer T (NKT) cells, ILC2s, macrophages, DCs, eosinophils, basophils, and MCs [19]. Both Th2 cells and ILC2s are major contributors to the cytokine production of type 2 immunity. Th2 cells secrete IL-4, IL-5, IL-9, IL-13, and IL-31, while ILC2s mainly produce IL-5, IL-9, and IL-13 [3].
The differentiation of naive CD4+ T cells into Th2 cells is driven by IL-4, which activates the signal transducer and activator of transcription 6 (STAT6) pathway. This pathway is crucial for the expression of IL-4-responsive genes and the upregulation of GATA-binding protein 3 (GATA3), a master transcription factor for Th2 cells [20]. GATA3 orchestrates the expression of IL-4, IL-13, and IL-5 in Th2 cells [21, 22]. IL-4 and IL-13 can also be released as pre-formed mediators from basophils, MCs, and eosinophils. IL-4 exerts its biological effects by signaling via two distinct receptor complexes, the type I and type II receptor complexes, whereas IL-13 primarily utilizes the type II receptor complex [23]. These cytokines activate intracellular signaling via receptor-associated kinases, such as the Janus family tyrosine kinases (JAKs)—STAT pathway. Additionally, IL-4 induces isotype switching in B cells and promotes the production of IgE, a key factor in allergic responses [3].
IL-5 is another crucial cytokine in type 2 immunity, produced by various cell types, including Th2 cells, MCs, invariant NKT cells, non-B/non-T cells, CD34+ progenitor cells, eosinophils, and basophils [24]. It plays a vital role in eosinophil biology, driving their differentiation and maturation in the bone marrow, facilitating their migration to tissues, and preventing apoptosis, particularly in allergic conditions. IL-5 also supports the proliferation and function of eosinophil precursors in tissues, such as the lungs [24]. Additionally, IL-5 enhances TSLP-dependent eosinophilopoiesis [25] and upregulates C-C motif chemokine receptor 3 (CCR3) expression, which is essential for eosinophil chemotaxis [26]. Beyond its role in eosinophils, IL-5 contributes to mucosal immunity by stimulating antigen-specific IgA production and supporting the function of ILC2s.
IL-9, primarily produced by Th9 cells under the influence of transforming growth factor-β (TGF-β) and IL-4, exerts its effects on various cell types, including Th cells, ILC2s, MCs, B cells, eosinophils, and epithelial cells, thereby amplifying allergic responses [27, 28]. IL-9 enhances the recruitment and activation of MCs and eosinophils, stimulates mucus production by epithelial cells, and promotes IgE production in B cells. IL-9 levels increased in respiratory and food-allergic patients, as well as in skin allergies, underscoring its prominent role in the development of allergic diseases [29].
2.2.2 TSLP, IL-25, and IL-33
Thymic stromal lymphopoietin, IL-25, and IL-33 play critical roles in the regulation of type 2 immunity [19]. These alarmins are secreted by epithelial cells in response to damage caused by allergens, pathogens, or hazardous substances affecting the airway, skin, or gut.
IL-25, alternatively referred to as IL-17E, is a cytokine that belongs to the IL-17 family. It promotes the production of IL-4, IL-5, and IL-13, thereby mitigating IL-17-dependent autoimmune responses [30]. Additionally, IL-25 bolsters Th9 cell responses, aiding in the defense against parasitic helminth infections [31]. It is secreted by epithelial cells as well as various immune cells, including Th2 cells, ILC2s, macrophages, MCs, basophils, and eosinophils [32]. Elevated levels of IL-25 have been observed in inflammatory skin conditions, such as AD, where it stimulates ILC2s to secrete IL-13. This, in turn, promotes keratinocyte proliferation and the production of chemokines that recruit immune cells to the skin [33]. In asthma, IL-25 plays a pivotal role in pathogenesis. Mouse models have demonstrated that intratracheal administration of IL-25 induced lung eosinophilia, increased IgE production, and exacerbated airway hyperreactivity [19].
IL-33, which belongs to the IL-1 cytokine family, is essential for driving Th2-associated cytokine responses, particularly during helminth infections and allergic inflammations [34]. It is primarily secreted by non-immune cells, such as epithelial cells, endothelial cells, and fibroblasts, and by immune cells, including macrophages and MCs, particularly at barrier sites. At these sites, IL-33 functions as an alarmin, released in response to tissue damage caused by environmental allergens or mechanical stress [35, 36]. IL-33 interacts with its receptor, a suppressor of tumorigenicity 2 (ST2), which is highly expressed in Th2 cells, ILC2s, and MCs. This interaction drives Th2-skewed immune responses critical for eliminating pathogens and helminths [37]. However, chronic IL-33 expression, often resulting from repeated environmental exposure, can lead to allergic inflammation, impaired wound healing, and tissue remodeling [38]. Interestingly, IL-33 also contributes to tissue homeostasis and repair by interacting with ST2-expressing regulatory T cells (Tregs), particularly in non-lymphoid tissues [39]. This dual role highlights the complex functions of IL-33 in both promoting immune responses and maintaining tissue integrity.
IL-33 is crucial to the development of allergic diseases, such as AD, where excessive IL-33 produced by keratinocytes can activate ILC2s, which then produce IL-5 and IL-13, leading to the accumulation of eosinophils in the dermis [40]. IL-33-induced activation of basophils further enhances the activity of ILC2 via IL-4 signaling. Additionally, IL-33 promotes histamine production in MCs through the activation of the p38 signaling pathway [41, 42]. Furthermore, IL-33 mediates skin-gut crosstalk, driving intestinal MC expansion and contributing to food anaphylaxis, thus highlighting its role in systemic allergic inflammation [43]. The IL-33/ST2 signaling pathway also activates sensory neurons, which play a role in itch and pain responses, further exacerbating symptoms in allergic conditions [44].
Thymic stromal lymphopoietin is mainly secreted by epithelial cells but can also be produced by DCs, basophils, and MCs [45]. The TSLP receptor (TSLPR) is expressed on hematopoietic cells and sensory neurons. Upon binding to TSLPR, TSLP recruits IL-7Rα, forming a complex that activates the JAK/STAT and Phosphoinositide 3-kinases (PI3K) pathways, both of which are essential for immune cell activation and differentiation [46]. TSLP induces the expression of major histocompatibility complex (MHC) and co-stimulatory molecules on DCs, promoting Th2 differentiation through the secretion of chemokines, such as CC-motif ligand 17 (CCL17), CCL22, and OX40 ligand [47-49]. In addition, TSLP can activate naive CD4+ T cells, driving Th2 functions and facilitating Th9 differentiation in airway inflammation [50]. Moreover, TSLP stimulates basophils and ILC2s to promote Th2 immune responses [51, 52] and promotes the differentiation of CD4+ T cells into Th2 and T follicular helper (Tfh) cells [53]. Chronic TSLP release, observed in conditions like AD, leads to persistent barrier disruption, increased TSLP expression, and epigenetic changes that exacerbate the disease [47, 54].
2.2.3 Chemokines of the Type 2 Response
Chemokines are crucial in orchestrating immune cell trafficking and activation, particularly within type 2 immunity that involves the activation of Th2 cells, eosinophils, basophils, and MCs. Although the role of alamins has been repeatedly emphasized, chemokines released early together with them are decisive for the development of an allergic inflammation. Recent research has elucidated the roles of specific chemokines in modulating type 2 immunity, with implications for allergic diseases and parasitic infections. CCL17 and CCL22 are chemokines that bind to the CCR4 receptor, which is primarily expressed on Th2 cells, cutaneous lymphocyte antigen-positive skin-homing T cells, and Treg cells [55]. These chemokines are critical for recruiting Th2 cells to inflammation sites, thereby amplifying type 2 immune responses. Elevated levels of CCL17 have been observed in AD, where it may act as a biomarker for disease severity and response to therapy [56]. Therapeutic strategies targeting the CCL17-CCR4 axis, including monoclonal antibodies against CCR4 [57], are being investigated to mitigate Th2-mediated diseases. Similarly, CCL22 plays a crucial role in inflammatory conditions like AD and AS, making its inhibition an attractive therapeutic approach [58].
CCL18 is primarily produced by antigen-presenting cells and is regulated by Th2 cytokines, such as IL-4 and IL-13, making it a key player in sustaining chronic Th2 responses. Elevated levels of CCL18 have been implicated in hypersensitivity diseases [59], including AD [60], underscoring its role in immune activation and immunosuppression. CCL18 also contributes to immune regulation by attracting naive T cells, immature DCs, and B cells [61], further influencing the inflammatory environment in type 2 responses. Another important chemokine, CCL2, is induced by Th2 cytokines and produced by macrophages and endothelial cells [62]. CCL2 facilitates the recruitment of monocytes and other immune cells to inflamed tissues. Elevated CCL2 levels are closely linked to type 2 inflammation-driven diseases, such as AS [63], where it significantly contributes to tissue remodeling and inflammation. Therapeutic strategies targeting the CCL2-CCR2 axis have been proposed to reduce monocyte and macrophage recruitment in allergic conditions [64].
CCL11 (eotaxin-1) and CXCL8 also play distinct roles in type 2 immune responses. CCL11 acts as a potent chemoattractant for eosinophils, with elevated levels correlating with disease severity in allergic diseases, particularly AS. Targeting CCL11 or its receptor CCR3 has emerged as a promising therapeutic strategy to control eosinophil-mediated inflammation. In contrast, CXCL8 (interleukin-8), while primarily recognized for its role in neutrophil recruitment, has also been observed at elevated levels in certain type 2 conditions [65]. This suggests a more complex and less well-defined role in modulating immune responses within type 2 immunity. Further research is needed to clarify CXCL8's specific contributions to type 2 immunity.
2.2.4 Regulation and Functions of IgE and IgG4 Antibodies in Type 2 Response
IgE is a critical mediator of type 2 immune responses, particularly in allergic initiation and inflammatory responses mediated by MCs and basophils. The secretion of IgE by B cells is tightly regulated by IL-4 and IL-13 [66], which promote class switching to IgE. Low-affinity IgE is produced through direct class switching from μ to ε, with minimal SHM. High-affinity IgE arises from sequential class switching from μ to γ to ε, involving an intermediate IgG phase and extensive SHM [67]. The IgE memory is primarily maintained by a small population of IgE memory B cells, with a more substantial contribution from IgG1+ memory B cells that undergo class switching to produce IgE upon re-encounter with antigen [68]. Antigen-specific IgE antibodies bind FcεRI on MCs and basophils to recognize allergens [69]. Upon antigen-binding and the spleen tyrosine kinase (Syk)-mediated signaling, degranulation occurs, releasing stored mediators, such as histamine, leukotrienes, prostaglandin D2, proteases, and proteoglycans [70]. With the activation of MCs, cytokine synthesis (such as IL-4, IL-5, IL-13, TSLP, TNF-α, and TGF-β1) [70, 71] is induced, contributing to vasodilation, increased vascular endothelial permeability, and systemic effects, such as hypovolemic shock and anaphylaxis [70]. Targeting IgE-mediated pathways, including anti-IgE monoclonal antibodies (e.g., omalizumab), FcεRI inhibitors, Syk inhibitors, glucocorticosteroids, and antihistaminic treatment [72-75], has shown efficacy in treating allergic diseases [75].
In addition to its classical role in allergic responses, IgE also participates in the development of allergic diseases by autoimmune mechanisms [76]. In non-classical allergic conditions, such as chronic spontaneous urticaria (CSU) [77, 78], patients display elevated serum levels of autoreactive IgE antibodies against autoallergens or immune complexes involving IgG-anti-IgE and IgG-anti-FcεRI, implicating type I and type II autoimmunity [2, 77].
Antigen-specific IgG4 serves as a natural counterbalance to IgE-mediated inflammation in type 2 immune responses. IgG4 competes with IgE for allergen binding sites, preventing the cross-linking of IgE on FcεRI expressed by MCs and basophils. This inhibitory effect is facilitated by IgG4's unique ability for fragment antigen-binding (Fab)-arm exchange, which confers bispecificity and monovalence [79-81]. IgG4 also exhibits low affinity for complement component C1q and Fcγ receptors, reducing its pro-inflammatory potential [82]. These properties are particularly important in allergen-specific immunotherapy (AIT), where IgG4 levels increase in response to allergen exposure, mediated by regulatory T and B cells. AIT induces immune tolerance by reducing type 2 cytokines and promoting the production of protective antibodies, particularly IgG4 [83, 84].
Interestingly, in natural tolerance models, such as helminth infections or occupational allergen exposure (e.g., beekeepers), elevated IgG4 levels correlate with reduced inflammatory responses and improved immune regulation [85, 86]. Regulatory B cells expressing IL-10 and IgG4 suppress inflammation and expand following allergen exposure, further mitigating IgE-driven immune responses [87, 88]. The anti-inflammatory properties of IgG4 make it a key therapeutic target in managing IgE-mediated diseases, complementing the efficacy of anti-IgE therapies like omalizumab. While IgG4 is widely acknowledged as the primary antibody exhibiting IgE-blocking activity, alternative isotypes, including IgD and IgG2 have also shown comparable functional properties, though their clinical significance remains less substantiated [84, 89, 90]. Together, these findings underscore the interplay between IgE and IgG4 in type 2 immunity and highlight their therapeutic implications in allergic and autoimmune diseases.
2.3 Role of Innate Immunity in Type 2 Inflammation
2.3.1 Dendritic Cells and Macrophages
Type 2 immunity has traditionally been understood as an adaptive immune response, with differentiated cells taking center stage. Th cells drive eosinophil recruitment and immunoglobulin production by the production of type 2 cytokines, resulting in the development of conditions, such as asthma, AR, AD, and anaphylaxis [3]. However, recent findings reveal that ILC2s can secrete large amounts of type 2 cytokines before the initiation of the adaptive response [91], indicating the crucial role of innate immunity in type 2 inflammation.
Mucosal and skin epithelial cells constitute the frontline defense system against harmful pathogens. Disruption of epithelial barriers triggers a pro-inflammatory response by activating myeloid cells and DCs, which are central to innate immune defenses [92, 93]. DCs, macrophages, and B cells are the most well-studied and specialized antigen-presenting cells (APCs). DCs are specialized tissue-resident and circulating leukocytes that sense microbial invaders and initiate innate and adaptive immune responses. They are primarily located in lymphoid tissues, mucosal epithelium, and organ parenchyma, where they initiate rapid immune responses [94]. In human circulation, DCs are categorized into plasmacytoid dendritic cells (pDCs) and myeloid dendritic cells (mDCs), based on their surface markers. pDCs can negatively regulate allergic airway responses by secreting IFN-α to inhibit the function of ILC2s and reduce the secretion of IL-33 from epithelial cells [95]. Notably, studies have demonstrated an increase in peripheral blood pDC levels following 1 year of AIT [96]. In peripheral tissues, classical DCs (cDCs), also known as myeloid/conventional DCs, capture protein antigens that breach the epithelial barrier and subsequently present them to T cells. These cDCs can be divided into two distinct subsets: cDC1, which is primarily involved in cross-presentation, and cDC2, the predominant subset associated with Th2 cell differentiation, as demonstrated in both murine models and human in vitro studies. When DCs are exposed to epithelial alarmins, such as TSLP, IL-25, and IL-33, they exhibit reduced IL-12 production, while upregulating co-stimulatory molecules like OX40L and ligands for the Notch receptor. This creates a positive feedback loop that biases CD4+ T-cell differentiation toward a Th2 phenotype, driving Th2 polarization [97, 98]. Moreover, the secretion of IL-5 and IL-13 by ILC2, along with TNF-α by MC, enhances the migration of DCs toward draining lymph nodes [99]. In addition, there is another special type of DC, the monocyte-derived dendritic cell (mo-DC), which also participates in antigen presentation and has been proven to be locally recruited in the inflammatory tissues of AD and AR [100] (Figure 2).
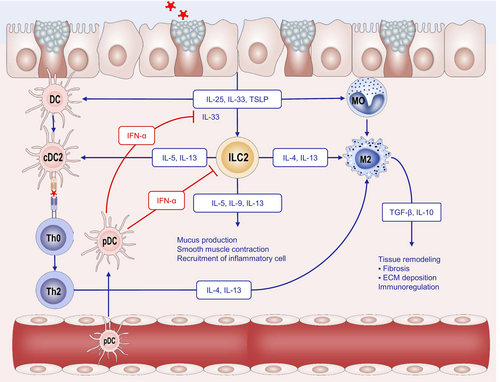
Macrophages constitute another category of APCs and are critical players in orchestrating the immune response. They can recruit other immune cells, such as eosinophils, neutrophils, and monocytes, and promote airway remodeling by secreting cytokines IL-4 and IL-13, as well as profibrotic growth factors, such as TGF-β and PDGF [101]. Research indicates that macrophages can be broadly categorized into two functional subsets: pro-inflammatory M1 and anti-inflammatory M2. M0 macrophages, which represent the unpolarized state, can undergo classical activation via TNF-α and IFN-γ stimulation, leading to polarization toward the pro-inflammatory M1 phenotype. Conversely, M0 macrophages can be alternatively activated upon stimulation with IL-13 and IL-4, leading to polarization into the M2 phenotype. M1 macrophages appear in an inflammatory environment dominated by Toll-like receptor (TLR) and interferon signaling and are typically associated with immunity against bacteria and intracellular pathogens. M2 macrophages are present in environments dominated by the Th2 response [102]. M2 is categorized into M2a, M2b, M2c, and M2d on the basis of activation markers and transcriptional changes [103]. In recent years, the role of M2a macrophages in AA has gained significant attention. The cytokines IL-4 and IL-13 bind to the IL-4 receptor, activating the STAT6 signaling pathway and inducing M2a macrophage activation [104-107] (Figure 2).
2.3.2 Type 2 Innate Lymphoid Cells
Based on the immune response they mediate, innate lymphoid cells can be categorized into three distinct ILC subsets: ILC1s, ILC2s, and ILC3s, which parallel Th1, Th2, and Th17 cells in their primary cytokine production. Specifically, ILC1s produce the typical type 1 cytokine IFN-γ, ILC2s generate type 2 featured cytokines IL-5 and IL-13, and ILC3s secrete type 3 cytokines IL-17 and IL-22. ILC2s, along with other type 2 innate immune cells, such as basophils, MCs, and eosinophils, could act as antigen-presenting cells and cooperate with DCs to perpetuate type 2 inflammtion [99]. Upon exposure to appropriate exogenous stimuli, epithelial cells release alarmins that activate ILC2s, thereby boosting the production of type 2 cytokines like IL-4, IL-5, IL-9, and IL-13, even before the initiation of the adaptive immune response [108, 109]. TSLP plays a critical role in ILC2 survival and activation, especially in skin [110]. In asthma, ILC2s serve as effector cells driving airway inflammation. ILC2s activated by IL-33 are implicated in virus-induced bronchial hyperreactivity and asthma, contributing to hallmark symptoms, such as mucus production, smooth muscle contraction, and recruitment of inflammatory cells through type 2 cytokine secretion. Notably, ILC2s are prevalent in nasal polyps from individuals with chronic rhinosinusitis (CRS) and in skin biopsies from patients with AD. Additionally, they are primary sources of IL-5 and IL-13 in the lung tissue of patients with asthma [111]. Interestingly, a subpopulation of ILC2 in mucosal tissues demonstrates autonomous production of IL-10, a cytokine that correlates with disease severity [112]. These IL-10+ILC2s have a regulatory function, downregulating cytokine production and reducing eosinophil recruitment. In addition, IL-10+ ILC2s maintain epithelial barrier integrity and are clinically beneficial to suppress inflammatory and allergic conditions, such as grass pollen allergy [112]. This demonstrates the functional plasticity within major ILC subpopulations, which generate variable cytokine profiles in response to local microenvironmental signals. These cytokine profiles, in turn, influence immune regulation or dysregulation [113]. However, the phenotypical and functional similarity of regulatory ILC2s to Tregs or ILC1s to exhausted CD8+ T cells remains to be elucidated [114].
Natural killer T cells are also major players in innate immunity and can directly regulate type 2 immunity. They influence immune responses by the rapid production of abundant cytokines (mainly IFN-γ, IL-4, and IL-13) [115-117]. NK cells, a subset of large granular lymphocytes, play a similar role in innate immunity. Recent studies demonstrated that NK cells can inhibit ILC2s' activity both in vitro and in vivo during allergic lung inflammation by the production of IFN-γ. This suggests a regulatory mechanism by which NK cells help control type 2 inflammation [118, 119].
2.4 Adaptive Immunity
2.4.1 Cell Immunity: Th2 Cells, Tfh Cells, B Cells
In type 2 immunity, Th2 and Tfh cells play essential roles in initiating, regulating, and executing germinal center (GC) responses, which significantly influence antibody production and the development of allergic reactions [120]. GCs are specialized microenvironments within secondary lymphoid organs, where activated B cells undergo somatic hypermutation (SHM) and class-switch recombination (CSR) to produce high-affinity antibodies [121-124]. Upon encountering their cognate antigen, B cells upregulate the chemokine receptor CCR7, enabling them to migrate to the T- and B-cell border, where they present antigens to T helper cells and receive activation signals primarily through CD40-CD40L interactions and cytokines. These signals activate B cells, which then migrate into the B-cell follicle to form GCs and further maturation.
Tfh cells, essential for GC formation, express Bcl-6, CXCR5, and PD-1 [125], and provide critical signals, such as IL-21 and IL-4 to GC B cells, promoting their proliferation, survival, and differentiation [126]. IL-21 supports CSR to IgG1 and inhibits IgE production, while IL-4 enhances IgE production, thereby contributing to allergic responses [126]. Interestingly, early IL-21 expression by Tfh cells precedes IL-4 and plays a dominant role in initiating GC formation [126]. Th2 cells, primarily localized outside GCs, also produce IL-4, supporting GC seeding and IgE class switching [127]. Recent research has identified a subset of Tfh cells, known as Tfh13 cells, which express high levels of IL-13 in addition to IL-4 and IL-5 [128]. These cells, which emerge after repeated allergen exposure, drive the production of high-affinity IgE antibodies and can trigger severe allergic reactions. This underscores the complex interplay between IL-4 and IL-13 in IgE production and presents potential therapeutic targets for managing allergies. Follicular regulatory T (Tfr) cells, a distinct subset of Tregs, also play a crucial role in modulating GC responses [129]. Tfr cells regulate the GC reaction by directly inhibiting B cells or by influencing Tfh–B-cell interactions [130]. They maintain GC size and function, which is essential for preventing autoimmunity and ensuring proper affinity maturation. Furthermore, Tfr cells can also regulate allergen-specific immune responses by modulating Tfh activity and directly inhibiting B cells through IL-10 signaling [131].
2.4.2 Humoral Immunity
The transcription factor STAT6 is pivotal for B-cell responses in type 2 immunity. STAT6 directly promotes CSR to IgE and IgG1 by binding specific DNA elements in the germline ε and γ1 promoters [132-134]. It also induces the expression of nuclear factor interleukin 3-regulated transcription factor, which is required for IgE switching [135]. B cells can undergo CSR from IgM to IgE either directly or via an intermediate IgG1 step, with sequential IgE CSR being crucial for producing affinity-matured IgE and establishing durable immune memory [67]. STAT6 also regulates multiple genes involved in GC responses, including those related to MHC-II and CD86 expression, activation-induced cytidine deaminase induction, and the low-affinity IgE receptor CD23 [133]. In contrast, STAT3 promotes CSR to IgG1 but inhibits IgE CSR, although strong CD40 signaling can overcome this inhibition [136, 137]. Additionally, the transcription factor Bcl-6, which is essential for GC formation, inhibits IgE CSR, potentially explaining the lower frequency of IgE+ GC B cells [138] (Figure 3).
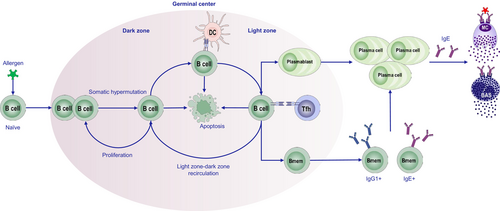
Plasma cells (PCs) are the primary antibody-producing cells, originating from B cells during specific stages of the immune response [139]. The differentiation of B cells into PCs is tightly regulated by Tfh and Tfr cells. Interaction between ICOS and CD40L on Tfh cells and ICOS-L and CD40 on GC B cells promotes PC differentiation, while Tfr cells help prevent the generation of autoreactive or nonspecific antibody-producing PCs [139]. IgE+ PCs have a shorter half-life compared with IgG1+ PCs, but chronic allergen exposure can lead to their accumulation [140]. IgE memory [141] is maintained by CD23+IL4R+IgG+ memory B cells that transcribe immunoglobulin heavy constant epsilon and are potential precursors of IgE-producing PCs upon reactivation. These memory B cells preserve the clonal imprint of IgE responses and are considered central to long-term allergic sensitization and pathogenic IgE production [142].
2.5 Regulatory Immune Cells
Regulatory immune cells are a diverse group of cells with immunomodulatory functions that play a crucial role in controlling allergic diseases. These cells include Tregs, regulatory B cells (Bregs), regulatory NK (NKreg) cells, regulatory DCs (DCregs), Tfr, and regulatory ILCs (Figure 4). Each subset contributes to immune tolerance and prevents excessive immune responses that drive allergy and inflammation.
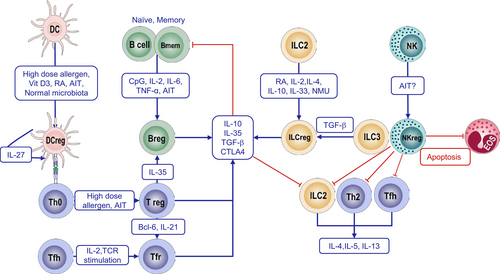
2.5.1 Regulatory T Cells
Tregs are a subset of CD4+ T cells characterized by the expression of the transcription factor forkhead box P3 (FoxP3) [143, 144]. The primary Treg population, known as natural regulatory T (nTreg) cells, mostly emerges in the thymus and mediates tolerance to self-antigens. Another subset, induced Tregs (iTregs), originates from conventional T cells in peripheral lymphoid tissues, induced by antigen exposure in the presence of TGF-β [145]. T-cell receptors (TCRs) on nTregs primarily recognize self-antigens, which are crucial for maintaining self-tolerance and preventing autoimmunity. In contrast, iTreg cells are believed to regulate immune responses against foreign antigens and microorganisms [146].
Tregs play a key role in maintaining peripheral tolerance and immune homeostasis [143]. In asthma, Tregs are rapidly recruited to the airways, coinciding with the onset of type 2 inflammation [147], and increased circulating Tregs are associated with asthma control and exacerbation [148]. Tregs constitutively express cytotoxic T lymphocyte-associated protein 4 (CTLA-4), a negative co-stimulatory molecule essential for their suppressive function [149, 150]. Moreover, Tregs are significant producers of suppressive cytokines, such as IL-10 and TGF-β [151, 152]. Emerging evidence suggests that Tregs can directly influence MCs to suppress immediate hypersensitivity by blocking their degranulation [151]. In secondary lymphoid organs, Tregs upregulate Bcl-6 and interact with B cells to modulate antibody production. iTreg cells also mediate suppression by selectively depleting peptide–MHC-II complexes from DCs in an antigen-specific manner [153].
Tregs exhibit plasticity and can adopt a pathogenic and pro-inflammatory phenotype in allergic diseases. In these settings, Tregs may lose their ability to maintain tolerance and adopt a Th2 cell-like phenotype, producing IL-4 while retaining Foxp3 expression [154]. Additionally, IL-33 can drive Tregs to shift toward a Th17-like phenotype through an antigen-pulsed mature DC-mediated pathway [155, 156]. These mechanisms contribute to the breakdown of Treg-mediated immune tolerance observed in patients with allergic diseases.
2.5.2 Regulatory B Cells
Accumulating evidence underscores the importance of Bregs in regulating allergic diseases. Various stimuli, including CpG, IL-2, IL-6, or IFN-α, can promote different B-cell types—including naïve B cells, memory B cells, and plasma cells—to differentiate into Bregs [157]. Bregs undergo development and maturation in the bone marrow before entering systemic circulation, where they constitute approximately 0.5% of peripheral B cells. Several subsets of B cells with immunoregulatory functions have been identified [157]. The regulatory function of Bregs is mediated by suppressive molecules, including the cytokines IL-10, IL-35, and TGF-β. In addition, Bregs utilize membrane-bound proteins, such as programmed death-ligand 1, CD39, CD73, aryl hydrocarbon receptor, BCR, CD80, CD86, CD40, Fas ligand, inducible co-stimulatory ligand, MHC, and TLR4 [157], to engage with various immune cells and suppress inflammatory responses. Bregs that produce IL-10 and TGF-β are amplified when exposed to high doses of allergens [158]. In experimental models, transferring IL-10-producing Bregs significantly attenuated allergic reactions induced by helminth infection [159]. Mechanically, Bregs suppress immune responses by inducing apoptosis in effector cells through FasL and by killing effector T cells via granzyme B [160]. Bregs are critical to induce allergen tolerance during AIT via suppression of Th2 and Tfh responses, induction of Tregs and inhibitory IgG4 antibodies, and IL-10-mediated inhibition of DC maturation [161, 162].
2.5.3 Regulatory NK Cells
NKregs are a subset of IL-10-secreting NK cells capable of suppressing antigen-specific T-cell responses and IgE production [163]. Various mechanisms have been proposed to explain the regulatory effect of NKregs on allergic diseases. Firstly, NKregs produce regulatory cytokines IL-10 and TGF-β to help dampen inflammatory responses [164]. Additionally, NKregs can modulate the Th1/Th2 balance partly by reducing the activity of Th2 cells, thereby addressing the imbalance often seen in allergic diseases. Moreover, NKregs may mitigate the severity and persistence of allergic reactions by inhibiting pro-inflammatory cell activity, blocking the release of allergic mediators, and inducing apoptosis of eosinophils [165]. Although these hypotheses highlight the potential role of NKregs, the precise mechanisms and functions of NKregs in allergic diseases remain under investigation. This is particularly important for exploring their therapeutic potential.
The impairment of NK cell function has been demonstrated in allergic disease [166, 167]. In human peripheral blood mononuclear cells, TGF-β+NK cells are dramatically lower in patients with AD compared with healthy controls [168]. Experimental studies show that transplantation of TGF-β+NK cells into mice significantly inhibits Th2 immune responses, effectively attenuating the severity of AD [168]. These findings underscore the potential of TGF-β+NK cells in modulating type 2 inflammation and highlight their therapeutic promise for allergic conditions.
2.5.4 Regulatory ILCs
A subset of regulatory IL-10-producing ILC2s has been identified [169] and has been shown to inhibit airway hyperreactivity [170] and contribute to allergen tolerance induced by AIT [112]. Regulatory innate lymphoid cells (ILCregs) are a specialized subset of innate lymphoid cells that play a crucial role in maintaining immune homeostasis and regulating inflammatory responses by producing the anti-inflammatory cytokine IL-10. Retinoic acid (RA) induces IL-10 secretion by human ILC2s [169]. In addition, other cytokines have also been shown to be capable of inducing the production of IL-10 by ILC2, including IL-2, IL-4, IL-10, IL-33, and neuromedin U (NMU) [113]. Whereas TGF-β inhibits the production of IL-10 from ILC2 [113] but promotes the conversion of ILC3s into ILCregs [171]. These IL-10-producing ILCregs exhibit a regulatory profile akin to regulatory T cells, characterized by the expression of key markers, including IL-10, CTLA-4, and CD25. These cells demonstrate reduced expression of type 2 effector-associated markers, such as the chemoattractant receptor-homologous molecule expressed on TH2 cells and ST2. Functionally, IL-10+ ILCregs exert suppressive effects on the activation of both CD4+ T cells and ILC2s. ILCregs numbers were increased in nasal tissue from patients with CRS with nasal polyps and in lung tissue from house dust mite-sensitized mice [169]. Antibodies and chemical drugs promoting the conversion to ILCregs from different ILC subsets may be useful in controlling allergic diseases with type 2 inflammation.
2.5.5 Other Types of Regulatory Cells
In addition to Tregs, Bregs, and NKregs, regulatory dendritic cells (DCregs) play a pivotal role in modulating the magnitude and direction of immune responses by influencing antigen presentation and the activation state of immune cells [172, 173]. Additionally, IL-10-producing Th2 cells, which include two distinct subsets with immunoregulatory potential, have been identified [174, 175]. These cells may regulate type 2 inflammation and allergic diseases, though their precise role remains to be fully elucidated.
2.6 Effector Cells
Effector cells, including MCs, eosinophils, and basophils, are pivotal in allergic diseases. These cells aggregate and become activated at sites of allergic inflammation, significantly impacting disease progression and outcomes.
2.6.1 Mast Cells
Mast cells are essential immune effector cells located in the skin and mucosal tissues of the gut and airways, and their activation contributes significantly to the onset of the symptoms of allergic diseases [176]. MCs originate from the bone marrow and circulate as mast cell progenitors (MCp) before migrating to tissues. The migration of MCp to tissues is a regulated process that is stimulated by inflammation and leads to an increase in tissue MCp [177]. MCp matures into diverse phenotypic and functionally heterogeneous MCs in peripheral tissues, influenced by microenvironmental signals during their development [178].
The activation of MCs is primarily mediated by IgE-FcRI cross-linking with allergens, which triggers the release of biologically active mediators, such as histamine, leukotrienes, and inflammatory cytokines TNF-α, IL-4, IL-5, and IL-13 [179]. These substances promote immune cell aggregation, tissue remodeling, smooth muscle contraction, mucus secretion, vasodilation, and increased vascular permeability—hallmarks of allergic diseases [179].
In addition to their direct effector functions, MCs stimulate Th2 cell activation and differentiation through IL-4 and IL-13, which further amplify allergic responses [180]. They interact indirectly with other immune cells, such as DCs and B cells, influencing overall immune regulation. In chronic allergic diseases, such as AR and chronic urticaria, MCs may play a key role in the persistence of the inflammatory response [2]. Interestingly, recent studies revealed that MCs and IgE engage in food allergen avoidance behaviors [181].
2.6.2 Eosinophils
Eosinophils originate from bone marrow leukocytes and are pivotal cells in allergic diseases [182]. While constituting < 5% of blood leukocytes, they are more prevalent in tissues like bone marrow and gastrointestinal tract. Eosinophil migration from blood to tissues is orchestrated by a complex network involving cytokines, adhesion molecules, chemotactic agents, and receptors [182]. Key growth factors promoting eosinophils include IL-5, GM-CSF, IL-3, and IL-33 [183, 184].
Peripheral eosinophil counts have been used as a biomarker of type 2 immunity and are correlated with the severity of allergic diseases [185]. Eosinophils exert their effects through various mechanisms. First, they release inflammatory mediators, such as histamine, IL-4, IL-5, IL-13, IL-8, and IL-1β, which amplify allergic reactions by increasing vascular permeability and recruiting and activating other immune cells [186]. Second, eosinophils exacerbate allergic responses by releasing intracellular mediators and protease-like substances, such as major basic protein, eosinophil cationic protein (ECP), eosinophil-derived neurotoxin, eosinophil peroxidase, and eosinophil-derived tumor necrosis factor-α, which directly cause tissue damage and apoptosis [187, 188]. Additionally, eosinophils interact with macrophages, DCs, and T cells, activating and causing the accumulation of these immune cells. Eosinophils may induce epithelial barrier damage, tissue remodeling, fibrosis, mucus plugging, airway hyperreactivity, skin blistering, pruritus, edema, and neural dysfunction in asthma, AD, etc. [189]. Eosinophils also play a role in tissue repair and regeneration by promoting angiogenesis and increasing stromal cell activity [190, 191]. Recent advances in understanding the functions of eosinophils have demonstrated the heterogeneity and plasticity of eosinophils, and two major subsets of murine eosinophils have been identified: tissue-resident eosinophils (rEos) and inducible eosinophils (iEos). In humans, the parenchymal rEos found in nonasthmatic lungs (Siglec-8+CD62L+IL-3Rlo cells) were phenotypically distinct from the iEos isolated from the sputa of patients with eosinophilic asthma (Siglec-8+CD62LloIL-3Rhi cells) [192]. The rEos contribute to the resolution of inflammation, whereas the iEos display a pro-inflammatory phenotype and contribute to the development and exacerbation of inflammation [193]. Similarly, rEOS- and iEOS-like eosinophils exhibit functional differences in patients with AS and severe non-allergic eosinophilic asthma [194]. Despite the absence of human evidence in certain research areas, therapeutic strategies targeting eosinophils and IL-5 have been applied to allergic diseases characterized by eosinophilia because of their significant role in allergic inflammation [195].
2.6.3 Basophils
Basophils are key effector cells involved in both acute and chronic allergic reactions, despite comprising < 1% of peripheral blood leukocytes [196]. After their release from the bone marrow, basophils primarily circulate in peripheral blood and the spleen and migrate to lymph nodes and sites of tissue inflammation. Basophils contribute to various immune processes, including protective immunity against parasites, the promotion of humoral memory responses, and the initiation of Th2 responses [196]. In response to allergen stimulation, basophils produce effector molecules, such as histamine and leukotrienes [197, 198], as well as cytokines like IL-4, IL-13, and IL-8 [199]. These molecules contribute to the allergic response by enhancing the production and action of IgE, promoting T-cell polarization and activation, and exacerbating the inflammatory response [200]. Basophils also express FcεRI, the high-affinity receptor for IgE, which mediates pro-inflammatory secretion upon stimulation with allergens [201]. In addition to their effector functions, basophils participate in antigen presentation through MHC class II and co-stimulatory molecules [202]. This results in the activation and differentiation of Th2 cells [197]. Moreover, basophils modulate the function of other immune cells, such as macrophages and DCs, through secreting type 2 cytokines like IL-4 and IL-13. This interaction enhances antigen presentation and pro-inflammatory responses, thereby exacerbating allergic reaction severity [203].
2.7 Biomarkers of Type 2 Immunity
Type 2 (T2) immunity is characterized by various biomarkers, including specific immune cells and cytokines (such as IL-4, IL-5, IL-9, IL-10, IL-13, and IL-33), which vary across allergic diseases. T2-high asthma is associated with alterations in sputum and blood eosinophils, total and allergen-specific IgE, fractional exhaled nitric oxide (FeNO), periostin, TSLP, IL-25, and the receptor for advanced glycation end products [204, 205]. Clinically, the most established biomarkers for T2 airway inflammation are blood eosinophil counts, FeNO, and serum IgE. Correspondingly, these biomarkers are widely used in the diagnosis of T2-high asthma and guide the application of biologicals targeting T2 inflammation in asthma. Additional T2 inflammatory biomarkers include serum ECP and tryptase, which correlate with the onset and deterioration of AS [206]. Furthermore, circulating CCR10-expressing ILC2s demonstrate diagnostic and prognostic value in asthma [59], while CCL27 acts as a shared biomarker for both asthma and AD [207]. Other chemokines, such as CCL17, CCL18, CCL22, CCL26, CCL37, IL-13, and CCL1 levels, may also be used as biomarkers for AD [207, 208]. IL-25, periostin [209], TSLP, cysteinyl leukotriene receptor 1 (CysLTR1), ST2, CD23, and specific microRNAs (miR-155, miR-206, miR-338-3p, and miR-26a) could be used as biomarkers for AR [59, 210, 211]. The potentially applicable biomarkers for type 2 inflammation are listed in Table 1.
| Allergic asthma | AD | AR |
|---|---|---|
| sIgE | sIgE | sIgE |
| IL-13 | IL-13 | IL-13 |
| Periostin | Periostin | |
| TSLP | TSLP | |
| IL-4 | IL-4 | |
| IL-5 | IL-5 | |
| IL-25 | IL-25 | |
| IL-33 | IL-33 | |
| Sputum and blood eosinophils | CCL17 | CysLTR1 |
| FeNO | CCL18 | ST2 |
| RAGE | CCL22 | IL-9 |
| CCR10 expressing ILC2s | CCL26 | CD23 |
| ECP | CCL37 | miR-155 |
| Tryptase | IL-13 | miR-206 |
| CCL 1 | miR-338-3p | |
| miR-26a |
- Abbreviations: CCL, C-C Motif Chemokine Ligand; CCR, Circulating chemokine receptor; CysLTR1, Cysteinyl Leukotriene Receptor 1; ECP, eosinophil cationic protein; FeNO, fractional exhaled nitric oxide; IL, interleukin; ILC2s, innate lymphoid cells; RAGE, the receptor for advanced glycation end products; sIgE, serum-specific Immunoglobulin E; SNPs, single nucleotide polymorphisms; ST2, suppression of tumorigenicity 2; TSLP, thymic stromal lymphopoietin.
3 Epithelial Barrier Disruption and Microbiome Dysbiosis
3.1 Epithelial Barrier Disruption
The epithelial barrier hypothesis suggests that dysfunction of epithelial barriers is central to the pathogenesis of type 2 inflammatory diseases. This hypothesis posits that environmental exposures and lifestyle changes due to industrialization and urbanization result in the disruption of epithelial barriers in the skin, respiratory, and gastrointestinal tracts [1, 3], which protect the body from external attack. In addition to functioning as a physical barrier, the epithelial barrier responds to environmental and immunological stimuli by secreting cytokines, thereby triggering and amplifying type 2 immune responses [24]. The compromised barriers lead to microbial dysregulation and increased permeability, allowing allergens and pathogens to penetrate subepithelial spaces, triggering immune responses characterized by type 2 immunity [1, 3] (Figure 5).
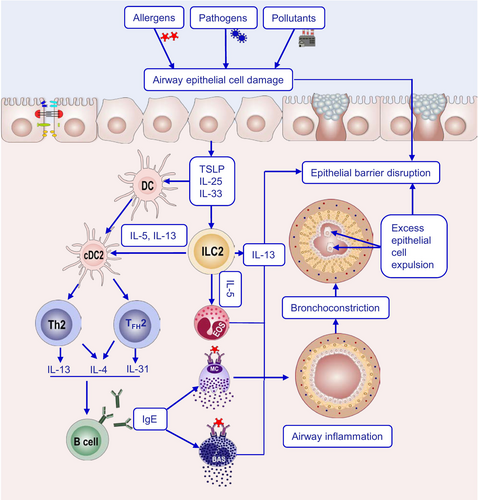
The human exposome—representing the totality of environmental exposures (including dietary patterns, microbial communities, and environmental contaminants) accumulated over an individual's lifespan—has undergone profound transformations due to the processes of industrialization and modernization [7, 212, 213]. In the past six decades, more than 350,000 new chemicals have been incorporated into our daily lives, often without sufficient assessment of their potential impacts on human and animal health, with more than 110,000 lacking proper reporting. The pervasive utilization of these substances is leading to their escalating accumulation in the human body, thereby intensifying their long-term health impacts.
Toxic substances, such as sodium lauryl sulfate (SLS) and polysorbates (P20 and P80), have significant detrimental effects on epithelial barriers and may cause various health issues, for example, SLS rapidly and strongly damages epithelial barriers, even in highly diluted doses, as shown in mouse models [214, 215]; polysorbates (P20 and P80) cause epithelial barrier disruption at concentrations as low as 0.05%, which is 20 times lower than the currently allowed 1% [216]. They can induce cell death, molecular toxicity, and trigger inflammatory responses in epithelial cells [216] and damage gut barriers by disrupting tight junctions, increasing paracellular flux, and reducing transepithelial electrical resistance [217].
These hazardous substances cause structural damage to tight junctions and disruption of epithelial barrier integrity, leading to increased permeability [216, 217]. The biological effects of epithelial barrier disruption included the following: (1) Cellular responses: upregulate the expression of genes and proteins related to apoptosis, inflammatory responses, and stress responses [216, 217], and trigger various cellular processes, including tissue damage and alterations in cell signaling [218]; (2) pro-inflammatory responses: induce the secretion of cytokines and chemokines [217], and activate PI3K-Akt and mitogen-activated protein kinase signaling pathways, further promoting inflammation [217]; (3) microbiome dysbiosis: epithelial barrier damage leads to microbial dysbiosis and bacterial translocation to subepithelial areas [1, 216]; and (4) systemic effects: the disruption of epithelial barriers can facilitate allergen entry and induce inflammatory responses, potentially initiating or exacerbating chronic inflammatory disease [218, 219]. The disrupting factors of the epithelial barrier are summarized in Table 2. These findings highlight the urgent need for reevaluating the safety of these substances and developing safer alternatives to protect epithelial barrier integrity and overall health.
| Disrupting factors | Restoring factors |
|---|---|
| Cigarette smoke | Corticosteroids: dexamethasone, fluticasone, budesonide? |
| Air pollutants: particulate matters, microplastics, etc. | β2 agonists |
| Oxidants | 1,25-(OH)2-Vit D3 |
| Ozone | Growth factors: epithelial growth factor, Heregulin |
| Respiratory viruses: human rhinovirus, respiratory syncytial virus, SARS-CoV-2 | Metabolites of fatty acid: polyunsaturated fatty acids, lipoxin A4 |
| Pseudomonas aeruginosa | Toll-like receptor ligands: CpG-DNA |
| Allergens containing protease activity | β-eudesmol |
| Pro-inflammatory cytokines: TNF-α, IFN-γ | Antioxidants: N-acetyl cysteine |
| Th2 cytokines: IL-4, IL-5, IL-13 | Cystatin SN |
| Th17-associated cytokines: IL-17, IL-22, IL-26 | Histone deacetylase 6 inhibitor |
| Toll-like receptor ligands: Lipopolysaccharide, double-stranded RNA | cAMP derivates |
| Bronchoconstriction damages airway epithelium by excessive cell extrusion in asthma | Allergen immunotherapy |
| Biologicals: anti-IgE, IL-4Rα, IL-5/R, anti-TSLP monoantibodies |
Recently, we reported 68 important diseases linked to the epithelial barrier theory [218]. The worldwide prevalence of all these diseases has increased in recent years, which is an important criterion. Common features among these diseases include epithelial barrier disorders, loss of commensal bacteria and microbial dysbiosis with colonization by opportunistic pathogens, and circulating inflammatory cells and cytokines. In addition, athletes and physically active individuals are at higher risk of exposure to agents that damage epithelial barriers and the microbiome, and their excessive physical exercise causes tissue damage and inflammation in multiple organs [220].
Pets, especially cats and dogs, share the same living spaces as humans and are exposed to household cleaning products, personal care products, air pollutants, and microplastics [221]. The use of cosmetic products and food additives for pets is increasing, but unfortunately, these products are subject to less stringent safety regulations than those applied to human products. In addition, future research areas address the interconnections between human, animal, and environmental health in line with the “One Health” concept.
3.2 Epigenetic Regulation of Defective Epithelial Barriers in Chronic Type 2 Inflammation
Environmental exposures, such as air pollutants and diets, can modify epigenetic mechanisms, influencing gene expression and resultant phenotypes [222]. The integrity of the epithelial barrier is epigenetically regulated by histone acetylation and deacetylation mechanisms, which precisely control the expression of genes coding for essential tight junction proteins that are fundamental to barrier function [223, 224]. Pro-inflammatory cytokines, such as IL-6R, IL-1R1, IL-1R2, IL-36B, IL-17B, IL-17RE, and type 2 gene IL-4I1, exhibit reduced methylation levels, whereas regulatory cytokines (IL-10 RA and TGF-βR2) show increased methylation. In addition, higher methylation was identified in several genes involved in regulating epithelial barrier integrity, such as genes encoding zonula occludens and claudins, the structural proteins of tight junction [224]. Epigenetic changes also impair the healing capacity of epithelial barriers. Notably, in defective areas, epithelial stem cells display diminished tight junction formation capability; however, this functional impairment can be successfully reversed by applying histone deacetylase inhibitors [223]. Furthermore, persistent epigenetic alterations exacerbate chronicity and barrier dysfunction, perpetuating localized or systemic inflammatory responses [223, 224]. For example, type 2 cytokine IL-13 directly damages the epithelial barrier, fueling a vicious cycle of peri-epithelial inflammation and leakiness [225] (Figure 5).
3.3 Role of Epithelial Barrier Disruption in the Development of Type 2 Allergic Diseases
Epithelial barrier disruption triggers the release of alarmins [226], such as TSLP, IL-33, and IL-25, leading to a pro-allergic microenvironment through the activation of Th2 and ILC2 cells [227]. Epithelial barrier impairment is an important mechanism in type 2 immunity, with allergic diseases often resulting from environmental factors that dysregulate the epithelial barrier function [228]. For instance, patients with AD, AR, FA, and AS show significantly higher epithelial cell permeability than healthy individuals [213]. Thus, epithelial barrier dysfunction has now been implicated in the pathogenesis of these allergic diseases.
The skin serves as a physical barrier against allergens and pathogens [229]. The impaired skin barrier is a risk factor for FA and EoE [230, 231], correlating with the progression of FA [232]. This finding explains why the initial manifestation of allergic disease in childhood is usually in the skin, known as AD, followed by interlocking developments of FA, EoE, AR, and AS [233].
Similarly, the respiratory epithelium protects airways against inhaled allergens, microorganisms, and airborne irritants [234]. Genetic factors, chronic exposure to environmental factors, such as cigarette smoke, air pollution, and respiratory infections, disrupt the integrity and function of the airway epithelium, increasing permeability and inflammation [213]. Beyond acting as a physical barrier, the airway epithelium plays an active role in modulating the innate immune system, and these functions are strongly linked to asthma pathogenesis [235].
The intestinal barrier is a physiological functional unit that separates the host from harmful substances, such as toxins and pathogens, exhibiting protective, nutritive, and immunizing functions [236]. However, mechanical damage, infection, microbiota dysbiosis, and dietary triggers can lead to intestinal barrier disruption [237]. Studies have shown a link between the intestinal barrier and food allergies [238]. There is a negative cycle in which increased intestinal permeability in FA sufferers makes it easier for allergens to get through the intestinal barrier, inducing worse immunity. This process induces the secretion of allergen-specific IgE and the activation of MCs and basophils. The resulting immunological cascade further compromises the function of the intestinal mucosal barrier [239].
Genetic mutations affecting epithelial barrier function are also linked to allergic diseases. For example, mutations in the epidermal barrier gene filaggrin increase the risk not only of AD but also of FA and EoE [240, 241]. Additionally, asthma susceptibility is associated with specific genes, especially the locus on chromosome 17q12-21, a region rich in epithelium-related genes [242]. Additionally, type 2 cytokines promote epithelial differentiation toward a goblet cell phenotype via 15-lipoxygenase activity, exacerbating asthma symptoms [243]. Meanwhile, dectin-1 signaling, an epithelial-derived mediator, can inhibit IL-33 expression, thereby dampening innate immunity and type 2 responses [244].
In summary, epithelial barrier dysfunction not only facilitates allergens to enter the body but also influences immune responses and allergic sensitization. When the epithelial barrier is compromised, pro-inflammatory cytokines are released, DCs are activated, and Th cells are polarized toward a Th2 phenotype. These immune responses collectively promote the production of allergen-specific IgE antibodies and drive the development of allergic diseases.
3.4 Restoration of the Integrity of the Epithelial Barrier
Recently, interest has grown in barrier-restoring therapies for allergic diseases. Strengthening skin and mucosal barriers prevents allergen penetration and immune activation. Protective factors that enhance epithelial barrier integrity reduce the possibility of allergens crossing the epithelial barrier and entering the systemic circulation.
Barrier-restoring therapies, such as emollients, moisturizers, and barrier creams, are designed to repair and strengthen the skin and mucosal barriers, thereby reducing allergen penetration and inflammation [245, 246]. Ceramides are one promising barrier-restoring treatment, as these lipid molecules play a crucial role in maintaining skin barrier integrity. Studies indicate that ceramide-based moisturizers improve skin barrier function, reduce inflammation, and alleviate symptoms of AD [247]. Maintaining the level of vitamin D is also critical for the generation of the skin barrier [248].
In addition to ceramides, other barrier-restoring therapies include probiotics, prebiotics, and dietary supplements, such as omega-3 fatty acids [249, 250]. These therapies support a healthy gut microbiome, strengthen the intestinal barrier, and modulate the immune response to allergens [251].
Overall, barrier-restoring therapies offer a novel and promising approach to managing allergic diseases by targeting the underlying pathophysiological mechanisms and enhancing the body's intrinsic defenses. Current studies suggest that barrier-restoring therapies could be an effective and safe treatment option for individuals with allergic diseases.
3.5 Microbiome Dysbiosis
The microbiota refers to a vast array of microorganisms that colonize and thrive in distinct parts of the body, including the gut, skin, and respiratory tract. These microorganisms, ranging from bacteria and viruses to fungi and other microorganisms, form an indispensable biological foundation for preserving health and homeostasis [252]. The microbiota in various parts of the body, such as the gut, skin, and respiratory tract, have different compositions and functions. Dysbiosis, or an imbalance in the microbiota, can lead to immune system dysregulation and contribute to disease development [253]. Multiple factors, including diet, antibody overuse, environmental pollution, and disease, can cause microbiome dysbiosis (Figure 6) [254]. In addition, the “hygiene hypothesis” suggests that reduced exposure to the diverse microbiota in early life can increase the Th2/Th1 response ratio, thereby increasing the risk of allergic diseases [255], emphasizing the importance of acquiring more diverse gut flora early in life. Decreased biodiversity has been proposed as the “biodeiversity hypothesis” to explain the increasing prevalence of allergic diseases [256].
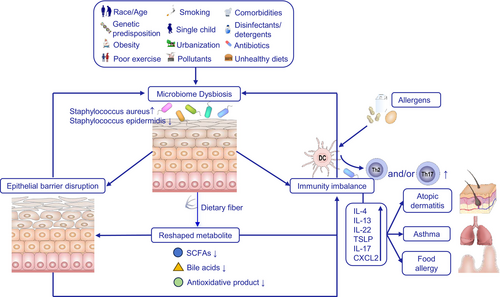
The skin microbiota has been implicated in the development of allergic diseases [257]. Major phyla on the skin include Firmicutes, Actinobacteria, Proteobacteria, and Bacteroidetes, with Staphylococcus epidermidis being the most common species [258, 259]. In patients with AD, microbiome diversity is often reduced [260], while the abundance of Staphylococcus aureus, S. epidermidis, Propionibacteria, Corynebacteria, and Streptococcus increases in lesions [261]. Moreover, AD severity has been associated with increased levels of Corynebacterium and Proteobacteria [262], and studies also link fungal species, particularly Malassezia, with AD [263].
Respiratory microbiota, which consists of the bacteria residing in the respiratory tract, is similarly linked to allergic diseases. Studies have shown that changes in the respiratory microbiota are associated with an increased risk of asthma and AR [264]. For example, a study by Markus Hilty et al. found that adults with asthma had a higher abundance of pathogenic Proteobacteria, particularly Haemophilus spp., in their respiratory tract compared with healthy adults, a trend also observed in children with asthma [265].
The gut microbiota is the largest and most active component of the intestinal barrier and plays a critical role in regulating immune tolerance and response [266]. A strong correlation exists between gut microbiota and allergic diseases. Firmicutes and Bacteroidetes are the primary phyla of the gut microbiota [255], with studies showing that the gut microbiota in food-allergic patients differs from healthy individuals. In food-allergic individuals, the gut microbiota is characterized by a decreased abundance of Bacteroidetes and Proteobacteria, and an increased abundance of Firmicutes and Actinobacteria [267]. In children with food allergies (FA), the gut microbiota is altered, showing enriched Clostridia and Firmicutes [268]. In infants, the continuous low abundance of Bacteroidetes and elevated levels of Enterobacteriaceae/Bacteroidetes are associated with the sensitization of FA [269]. Additionally, germ-free mice show greater sensitivity to cow's milk allergy compared with normal mice [270], suggesting that FA is partly caused by the alteration of the gut microbiome. Similar gut microbiota alterations have also been detected in AR and atopic eczema [271].
Microbial dysbiosis contributes to allergic disease development through multiple mechanisms. First, microbial dysbiosis causes immune dysregulation, leading to an exaggerated response to harmless antigens by exacerbating Th2 responses. In addition to Th2 cells, microbiota dysbiosis also affects other T-cell subsets, such as Th9 and Th17 [272]. Second, microbial dysbiosis results in barrier disruption, as illustrated in the previous section [273, 274]. Finally, dysbiosis impairs the synthesis of protective metabolites, particularly short-chain fatty acids, which have protective effects against allergic diseases [275].
In summary, microbial dysbiosis plays a critical role in the initiation and progression of allergic disorders. Alterations in the microbiota, particularly in the gut, skin, and respiratory tract, are associated with an increased risk of allergic disease. Notably, dysbiosis can act as both a consequence and a cause of allergic diseases. Further investigation is essential to uncover the mechanisms linking microbial dysbiosis to allergic diseases and to develop targeted therapies to modulate microbiota.
4 Allergic Diseases With Type 2 Immunity
4.1 Allergic Asthma
Asthma, a heterogeneous inflammatory airway disorder, is characterized by recurrent episodes of cough, chest tightness, wheezing, and dyspnea. These symptoms can be provoked by various stimuli, including viral respiratory tract infection, exposure to aeroallergens and air pollutants, and exercise, and relieved by β2 receptor agonists [276-278]. The main pathophysiological characteristic of asthma includes chronic airway inflammation, airway hyperreactivity, and reversible airflow obstruction. In chronic asthma, persistent inflammation can lead to airway remodeling, contributing to irreversible airflow limitation. To better understand asthma's underlying immunological mechanisms, asthma endotypes have been defined and researched [5]. These include T2-high, T2-low, mixed Th1/Th2/Th17, and paucigranulocytic asthma [279]. In addition, cluster analyses have identified different phenotypes within each asthma endotype according to age, gender, atopy, lung function, health care utility, and body mass index, which denotes the heterogeneity of asthma [280].
T2-high asthma is defined by elevated levels of type 2 inflammatory biomarkers, such as increased blood eosinophils, FeNO, and, to a lesser degree, increased serum IgE levels [281]. This type of asthma is commonly initiated by aeroallergen sensitization and is most common in pediatric asthma. Airway epithelial barrier disruption contributes significantly to the initiation and exacerbation of type 2 inflammation of asthma [3]. Moreover, a recent study demonstrated that bronchoconstriction causes airway epithelial cell crowding and excess cell extrusion, which results in airway epithelial barrier disruption [282] (Figure 5). T2-high asthma typically responds well to the treatment of glucocorticoids and biologicals targeting type 2 inflammation, such as monoclonal antibodies of anti-IgE, anti-IL4Rα, anti-IL-5/IL-5R, and anti-TSLP [283]. Thus, the novel concept of remission as a treatment goal is now widely adopted in T2-high asthma [284].
4.2 Allergic Rhinitis
Allergic rhinitis is a common type 2 chronic inflammatory disorder affecting the upper airways. Allergens related to AR include pollens (trees, grasses, and weeds), molds, and indoor aeroallergens (dust mites and pet dander), with significant geographic variations among countries [285]. Traditionally, AR was categorized into perennial and seasonal types; however, as most individuals are sensitized to multiple allergens, it is now commonly classified into intermittent and persistent forms [286].
The diagnosis of AR is established through a comprehensive evaluation that includes medical history, physical examination, and nasal endoscopy. Clinically, typical AR symptoms include nasal congestion, rhinorrhea, postnasal drainage, sneezing, and pruritus involving the eyes, nose, and throat, with rhinorrhea and nasal congestion being the most common. The examination of AR includes observing facial characteristics and an internal examination by nasal endoscope. In some patients, allergic conjunctivitis may manifest on the face along with allergic shiner or double creases beneath the eyes (Dennie-Morgan lines). When examining the nasal cavity with nasal endoscope, the typical features of AR are swelling of the inferior turbinate and increased mucosal secretion [287].
AR occurs when the epithelial barrier of the nasal mucosa is compromised, allowing allergens to penetrate the subepithelial space. This leads to a Th2 inflammatory response and the production of allergen-specific IgE [286, 288]. AR treatment usually includes allergen avoidance and pharmacologic interventions, such as intranasal corticosteroids and second-generation antihistamines, to manage symptoms. If there are clear allergens, such as dust mites and pollen, allergen immunotherapy is a viable treatment option.
4.3 Atopic Dermatitis
AD is one of the most prevalent chronic inflammatory diseases affecting the skin [289], characterized by prominent heterogeneity [290]. This disorder affects approximately 10% of adults and 20% of children in developed countries, with an increasing prevalence in developing countries [289]. The featured symptoms of AD are intense itching and recurrent eczematous lesions [289]. The immune activation of the Th2 pathway is a hallmark of AD [290], and its pathogenesis is linked to a complex interplay between epidermal barrier dysfunction, type 2-predominant immune response, genetic predisposition, and microbiome dysbiosis [289, 291] (Figure 7).
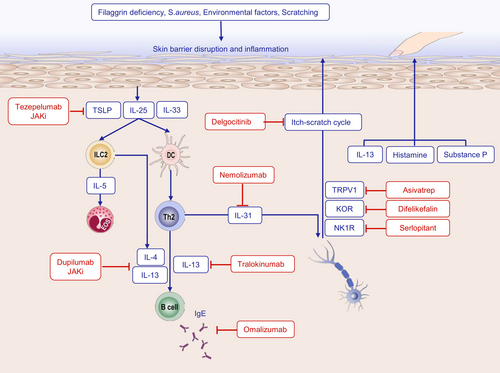
Epidermal barrier dysfunction can be caused by filaggrin deficiency, colonization of pathogens like Staphylococcus aureus, scratching, and type 2 inflammation [289, 292]. This dysfunction leads to the release of epidermal alarmins, which subsequently stimulate differentiation of Th2-predominant CD4+ cells [293]. Type 2 cytokines, such as IL-4 and IL-13, stimulate IgE production and eosinophil activation in both the skin and peripheral blood [294, 295]. IL-4 and IL-13 also mediate lipid abnormalities through STAT6 signaling, interfering with lipid synthesis and elongation [296, 297]. The IL-4/STAT6 axis also upregulates transmembrane 232 in AD, exacerbating the inflammation response [298]. Additionally, Th2 cytokines reduce the expression of proteins crucial for epidermal barrier formation, such as loricrin and involucrin [299, 300]. Itch is the predominant symptom of AD, often leading to psychological outcomes. Several factors contribute to this symptom, including Th2 cytokines like IL-4, IL-13, and IL-31, as well as IL-33, TSLP, histamines, neurotransmitters, neurotrophins, and substance p [301]. Among these cytokines, IL-31 represents a crucial role in itch signaling in AD, and is primarily secreted by Th2 cells and to a smaller extent by macrophages and MCs [302]. IL-31 interacts with TSLP, periostin, and basophils to generate the sensation of itching [303].
The management of AD aims to provide symptom relief and disease control by avoiding triggers, restoring the epidermal barrier, and reducing inflammation [289]. Biological therapies targeting type 2 inflammation, such as the anti-IL-4Rα monoclonal antibody dupilumab and JAK inhibitors, have shown effectiveness in symptom remission and improvement in quality of life [304, 305]. Moreover, dupilumab has been shown to improve skin barrier function by inhibiting IL-4 and IL-13 signaling, and it also reduces the colonization of Staphylococcus aureus [290, 306-308].
4.4 Food Allergy
Food allergy refers to hypersensitivity to normally innocuous food protein allergens [309]. The onset and progression of FA result from genetic predisposition, environmental factors, compromised intestinal barrier, and microbiome dysbiosis [310]. In recent years, the prevalence of FA has increased dramatically, especially in developed countries [311]. Common food allergens include eggs, cow's milk, peanuts, soy, wheat, fish, and shellfish [312].
FA develops when the immune system erroneously recognizes certain food proteins as harmful substances. FA is divided into IgE-mediated, non-IgE-mediated, and mixed types, with type 2 immune response being central to IgE-mediated FA [313]. The onset of “atopic march” begins with AD and FA, which may be risk factors for AA and AR in children [314]. In children, the development of FA is often associated with skin barrier dysfunction in AD, which increases the risk of sensitization to food allergens by the age of two. Recent studies have demonstrated that the early oral introduction of solid foods like peanuts, eggs, and dairy has been shown to reduce the incidence of allergies to these food [315], with early peanut introduction decreasing allergy risk, early egg introduction reducing egg allergy incidence by age 3, and early dairy introduction lowering milk allergy risk by 6 months [316].
Symptoms of FA vary from mild to life-threatening and may include hives, itching, swelling, gastrointestinal symptoms, respiratory symptoms, and in severe cases, anaphylaxis. Diagnosis involves a detailed medical history, followed by elimination diets [317], skin prick tests, and blood tests for specific IgE. Advanced tools, such as basophil and MC activation tests, and bead-based epitope assays, can provide further insights. Oral food challenges remain the gold standard [316]. Recently, oral immunotherapy has been approved for the treatment of peanut allergy [318, 319]. Biologicals targeting type 2 inflammation, such as omalizumab and dupilumab, are currently evaluating the efficacy of FA treatment [316].
4.5 Eosinophilic Gastrointestinal Disorders
Eosinophilic gastrointestinal disorders (EGID) are a spectrum of disorders caused by eosinophilic infiltration in the intestinal mucosa, resulting in organ dysfunction and symptoms [320]. These groups of disorders include EoE, eosinophilic gastritis, eosinophilic gastroenteritis, eosinophilic enteritis, and eosinophilic colitis. The symptoms of EGID depend on the location and the gastrointestinal layers affected by eosinophils infiltration. In EoE, common symptoms include heartburn/regurgitation, vomiting, dysphagia, food impactions, and even abdominal pain [321], while other EGID symptoms include abdominal pain, nausea, vomiting, early satiety, diarrhea, and weight loss [320]. EGID is now recognized as a group of type 2 inflammatory diseases, often triggered by food allergen ingestion and eosinophil-predominant inflammation [320, 322]. The development of EGID is influenced by an interplay between environmental and genetic factors that predispose individuals to allergy, along with impaired epithelial barrier function, enhanced Th2 activity, food allergy (both IgE- and non-IgE-mediated), and microbiome dysbiosis [320, 321]. Diagnosis of EGID can be supported by mucosal abnormalities observed during endoscopy and increased eosinophil levels on tissue biopsy [323]. Treatment approaches for EGID include control of environmental concurrent allergic disease, proton pump inhibitors, diet therapy, such as elemental diet or elimination diet, topical corticosteroids, and esophagus dilation in those with stricture [321, 322]. Biological therapies targeting type 2 inflammation have been explored for EGID treatment, including anti-IgE, anti-IL-5, anti-IL-4/IL-13, anti-IL-13, anti-TNF-α, and anti-siglec-8. Currently, dupilumab is the only FDA-approved treatment for EoE [324].
4.6 Alopecia Areata
In recent years, data are accumulating linking alopecia areata (AA) with atopy. There is emerging epidemiological data that highlight that atopy in general and AD in particular as comorbid conditions of AA, and with each atopic condition, the risk to develop AA increases [325, 326]. Also, IL-13 has been associated with the strongest genetic association in patients with AA in a large GWAS study [327]. Furthermore, AA is linked to seasonality and high IgE, and antihistamines are used as adjuvants in AA treatment [328-330]. New pathogenic insights from AA scalp and blood samples showed significant increases in Th2 cytokines in AA, with increases in eosinophils and MCs found in AA tissues [331-335]. Last, dupilumab treatment in patients with AA showed significant hair regrowth, particularly in those patients with either high IgE or associated atopy [336-339]. Collectively, these data link AA with the atopic march, particularly in those with personal or familial atopy.
5 Inborn Errors of Immunity and Type 2 Response
Inborn errors of immunity (IEI) play critical roles in inflammation modulation, oncological surveillance, autoimmune maintenance, and allergy response. Monogenic IEI with immune deficiency and dysregulation could also skew toward type 2 immunity-related allergic inflammation, often in conjunction with severe atopic diseases, driving the conceptualization of “primary atopic disorders” (PAD) [340]. Current classification stratifies PAD into mechanistically defined subgroups (Figure 8), acknowledging potential inter-categorical overlap in underlying molecular pathways. Additionally, monogenic disorders manifesting allergic phenotypes without immunodeficiency had also been documented, which was invaluable as they could provide important insights into the pathogenesis of atopic diseases [340].

5.1 Immune Deficiencies Associated With Altered T-Cell Signaling
Dysregulation of TCR signaling cascades, cytokine-mediated communication networks, or actin cytoskeletal dynamics may compromise immune homeostasis and predispose to pathological type 2 inflammatory polarization. The CARD11–BCL10–MALT1 complex is essential for transducing signals from T- and B-cell receptors to nuclear factor-kappa B (NF-kB) and mTOR signaling pathways [341]. Alterations in this complex have been associated with an atopic predisposition, characterized by conditions, such as asthma, eczema, and AR. Two primary mechanisms may account for this association: disruption of NF-kB signaling, as observed in patients with mutations in NF-kB essential modulator (NEMO) and NFKB2, leads to atopic manifestations. Impaired mTOR1 regulation undermines Treg activity and promotes an allergic phenotype [341, 342]. Evidence also shows that individuals with CBM complex deficiencies have both reduced Treg populations and diminished Treg function, reinforcing the importance of Treg dysfunction in the development of atopy [343].
Autosomal dominant hyper-IgE syndrome (Job's syndrome), defined by pathogenic variants in the STAT3 transcription factor, was a prototypical IEI with type 2 immune dysregulation [344]. STAT3 plays a critical role in the signaling of a wide range of type 2 and other cytokines, including IL-4, IL-6, and IL-11 [345, 346]. The clinical features of Job's syndrome often present early, including severe atopic manifestations, such as elevated IgE levels, eosinophilia, anaphylaxis, and severe asthma. Targeted biologics (e.g., dupilumab) and JAK inhibitors have shown significant efficacy in reducing allergic conditions [347]. Other non-allergic clinical manifestations include recurrent pulmonary infections complicated by the formation of pneumatoceles, absence of acute phase reactants, and dental, skeletal, and vascular abnormalities.
Wiskott–Aldrich syndrome (WAS), arising from mutations in the WASp gene, disrupts cytoskeletal dynamics through impaired coordination of the Dedicator of Cytokinesis 8 (DOCK8)-Actin-Related Protein 2/3 regulatory axis [348]. The actin cytoskeleton deficiencies in WAS result in impaired T-cell function, notably affecting immunological synapse assembly, chemotactic mobility, clonal expansion, and cellular specialization. These pathophysiological alterations predispose affected individuals to recurrent infectious episodes alongside type 2 inflammatory pathologies (AD, asthma, food allergy) and immune dysregulation. A diagnostic hallmark of this disorder is persistent thrombocytopenia, which provides critical differentiation from immunodeficiencies involving DOCK8 or ARBC1B gene defects [348] (Figure 8).
5.2 Immune Deficiencies and Reduced T-Cell Repertoire Diversity
Severe combined immune deficiency (SCID) represents the most critical phenotype among IEI, characterized by extremely low or absent T and B cells, resulting in the absence of functional adaptive immunity. Consequently, conventional type 2 immunity is generally absent in classical SCID presentations. While classic SCID presents in infancy with life-threatening infections, atypical presentations emerging later in life—termed “leaky SCID” variants, such as Omenn syndrome—demonstrate partial immune reconstitution [349]. In contrast to typical SCID, Omenn syndrome exhibits pathological Th2-skewed immunity driven by oligoclonal autoreactive T lymphocytes with limited TCR repertoire, inducing multisystem infiltration presenting as dermatitis, lymphadenopathy, and hepatosplenomegaly [350, 351]. Laboratory findings are reflective of type 2 dysregulation, with peripheral eosinophilia and IgE dysregulation being common. While immunosuppressive treatment is effective in suppressing T-cell expansion and tissue infiltration, its therapeutic benefits are counterbalanced by increased vulnerability to opportunistic infections resulting from systemic immune suppression. Hematopoietic stem cell transplantation remains the definitive therapeutic intervention for long-term immune reconstitution [350].
5.3 Immune Deficiencies With Defects in the Development or Function of Regulatory T Cells
IPEX syndrome (immune dysregulation-polyendocrinopathy-enteropathy x-linked) arises from pathogenic variants in the FOXP3 gene, a key transcriptional regulator indispensable for Treg differentiation and functional maintenance. While the number of immune cell subsets in IPEX syndrome may remain within normal ranges, FOXP3-mutated Tregs fail to properly suppress autoreactive T cells, leading to severe symptoms and immune dysregulation [352]. Furthermore, multiple genetic variants disrupting Treg maturation or functional integrity have been identified, including pathogenic alterations in IL-2RA, IL-2RB, IL-10, IKAROS, LRBA, CTLA4, BACH2, DEF6, and FERMT1. In contrast to IPEX syndrome, these Treg-opathies exhibit a clinical predilection for multiorgan autoimmunity and chronic inflammatory states, while demonstrating a relative paucity of classical atopic manifestations [353, 354].
5.4 Monogenic Disorders Manifesting Allergic Phenotypes Without Immunodeficiency
Several gain-of-function STAT6 germline variants (e.g., NM_001178079.2 and NM_003153.4) were identified in patients with severe early-onset allergic disease and Th2-skewed transcriptional profiles. Although there was evidence of systemic inflammation with elevations in WBC, platelets, serum immunoglobulin levels, adaptive immune markers (T, B, and NK cell counts), and other aspects of the immune system were all within the normal range [355, 356]. Similarly, JAK gain-of-function could drive severe allergic inflammation, as evidenced by IL-4/IL-13 and Th2 gene expression, and surprisingly upregulate innate immune and IFN-stimulated genes in the absence of immunodeficiency [357].
6 Conclusion and Outlook
Type 2 inflammation plays a vital role in the pathogenesis of allergic diseases, providing a foundational target for therapeutic strategies. The increased prevalence of type 2 inflammatory diseases from very low numbers to almost 1 billion patients with AR and 350 million patients with asthma, particularly over the last six decades, has been taking a huge attention. Inflammation starting from the epithelium in response to environmental toxic agents and microbial dysbiosis, namely the epithelial barrier theory, has been proposed to cause this increase. The immune response to many toxic substances, such as SLS and polysorbates, is typical type 2, as demonstrated in asthma, colitis, and EoE models [4, 215, 358].
The burden of epithelial barrier-related diseases currently consumes a significant portion of global health resources and poses a major threat to public health and sustainability. However, epithelial barrier theory also offers a significant opportunity: by controlling environmental exposures, promoting healthier diets and lifestyles, and prioritizing the reduction of systemic inflammation through the preservation of epithelial barriers, the prevalence and severity of these diseases can be significantly reduced. Recent advances in therapeutic therapies targeting type 2 inflammation have demonstrated promising efficacy and safety in clinical settings, significantly deepening our understanding of the underlying immunological mechanisms. Leveraging advanced methodologies and multi-omics approaches can shed light on the complexities of these mechanisms and explain the rising prevalence of allergic diseases, potentially linked to factors, such as epithelial barrier dysfunction and microbiome dysbiosis driven by exposomes. Furthermore, identifying reliable biomarkers of type 2 inflammation across a broader range of allergic diseases beyond T2-high asthma is essential for improving diagnosis and treatment precision. Additionally, the role of IEI in the development of allergic diseases warrants further investigation, as these genetic defects may provide new insights into the pathophysiology and treatment of allergic conditions.
Author Contributions
Conception and design of the study: Cezmi A. Akdis and Ya-dong Gao. Manuscript drafting: Ya-dong Gao, Zi-jun Wang, Ismail Ogulur, Sheng-jie Li, Duygu Yazici, Xue-hui Li, Yagiz Pat, Yang Zheng, Huseyn Babayev, Can Zeyneloglu, Ya-chun Li, Sena Ardicli, Wen-qu Tian, Ozge Ardicli, Shi-wei Chen, Xiang-ting Bu, Gan Lu, Li-hong Chang, and Ying He. Manuscript review and revision: Ya-dong Gao, Emma Guttman-Yassky, Beatriz Cabanillas, Cevdet Ozdemir, Ayca Kiykim, Mohamed Shamji, Kari Nadeau, Maria J. Torres, Mubeccel Akdis, and Cezmi A. Akdis.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.