Mast cell chymase suppresses functional parameters in primary human airway smooth muscle cells
Increased airway smooth muscle cell (SMC) mass is a common feature in asthma development.1, 2 Mast cells (MCs) have an important role in asthma, by releasing various bioactive compounds, including exceptionally large quantities of MC-restricted proteases (e.g., tryptase and chymase). However, the possible functional impact of these proteases on airway SMCs is only partially understood.3 To investigate this, primary airway SMCs were treated with either chymase or β-tryptase, followed by assessment of SMC morphology, viability, proliferation, contraction, migration, and cytoskeletal organization.
Neither tryptase nor chymase was cytotoxic for airway SMCs (Figure 1A, Figure S1A). However, chymase markedly suppressed the metabolic activity of the SMCs and caused reduced SMC proliferation (Figure 1B,C), whereas tryptase had little or no effects (Figure S1B,C). SMC contraction is a hallmark feature of asthma and we thus assessed whether MC proteases can affect this process. Indeed, chymase caused a partial suppression of airway SMC contractility (Figure 1D,E). As SMC contractility is linked to Ca2+ fluxes, effects of the MC proteases on intracellular Ca2+ levels were assessed. This analysis showed that the MC proteases cause a reduction of intracellular Ca2+ levels (Figure S2).
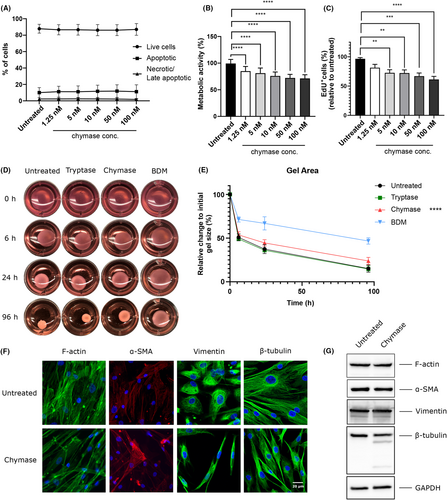
Next, we explored whether MC proteases can influence SMC morphology. As seen in Figure S3 , and in line with previous findings,4 chymase caused a marked separation of the SMCs, both from each other and from the underlying surface, the latter suggesting effects on the extracellular matrix (ECM). Based on these observations, we investigated if the MC proteases can affect cellular migration. Indeed, chymase caused significantly increased SMC migration whereas modest effects of tryptase were seen (Figure S4).
Cell morphology, migration and interaction with neighboring cells, and ECM is intimately linked to cytoskeletal organization. As our findings reveal that chymase can affect all of these parameters, we next investigated for effects of chymase on key cytoskeletal components. These analyses revealed that chymase caused extensive F-actin remodeling, without affecting the total levels of intracellular F-actin (Figure 1F,G). Chymase also caused reorganization of vimentin, an intermediate filament, without affecting the total vimentin levels (Figure 1F,G). Marked remodeling of β-tubulin was also observed in response to chymase, and it was seen that chymase promoted β-tubulin degradation (Figure 1F,G). In contrast, α-smooth muscle actin was not affected by chymase (Figure 1F,G).
Our observations above suggest that chymase may modify SMC–ECM interactions. In agreement with this, we noted that chymase degraded SMC-produced fibronectin, accompanied by potentiated fibronectin mRNA expression (Figure 2A,B). Further, chymase was shown to cause degradation of focal adhesion kinase (FAK), FAK being downstream linked to fibronectin, accompanied by reduced FAK phosphorylation (Figure 2C). In contrast, chymase did not influence paxillin, vinculin, or phospho-myosin light chain kinase (Figure S5).
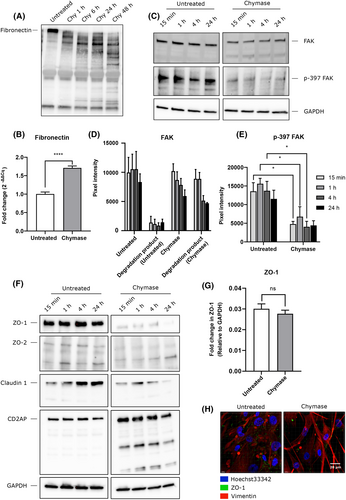
Next, we addressed the mechanisms by which chymase might cause SMC separation, hypothesizing that chymase might interfere with cell–cell contacts. We thereby focused on tight junction proteins, based on previous findings showing that chymase can promote the degradation of such proteins.3 Intriguingly, the airway SMCs were shown to express the tight junction proteins zona occludens-1 (ZO-1), ZO-2, and claudin-1 as well as CD2AP (Figure 2F–H), which is contrary to the general notion that tight junction proteins are epithelial cell markers. Further, chymase was shown to promote the degradation of all these proteins (Figure 2F–H).
Altogether, our findings suggest that chymase has an overall dampening impact on airway SMCs. Notably, previous studies have suggested that chymase can have protective functions in asthma,3, 5, 6 and the present findings thus introduce the possibility that this could be attributed to effects of chymase on airway SMC populations.
AUTHOR CONTRIBUTIONS
XOZ designed and performed most of the experimental work, interpreted data, and wrote the manuscript; FRM contributed to the design of the study and performed experimental work; CPS contributed with essential reagents and contributed to the writing of the manuscript; AP contributed to the design of the study; GP conceived of the study, contributed to the design of the study, interpreted data, and wrote the manuscript.
ACKNOWLEDGMENTS
This study was supported by grants from The Swedish Heart and Lung Foundation, The Swedish Research Council, The Erling-Persson Foundation, The Swedish Cancer Foundation, and The Knut & Alice Wallenberg Foundation.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest in relation to this work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




