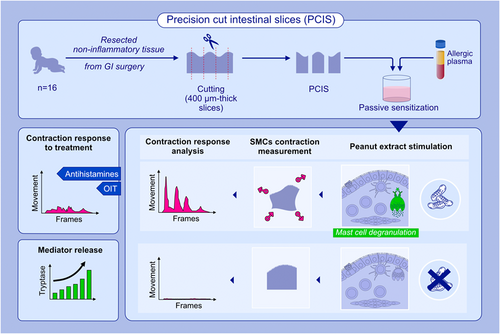
Precision cut intestinal slices, a novel model of acute food allergic reactions
Abstract
Background
Food allergy affects up to 10% of the pediatric population. Despite ongoing efforts, treatment options remain limited. Novel models of food allergy are needed to study response patterns downstream of IgE-crosslinking and evaluate drugs modifying acute events. Here, we report a novel human ex vivo model that displays acute, allergen-specific, IgE-mediated smooth muscle contractions using precision cut intestinal slices (PCIS).
Methods
PCIS were generated using gut tissue samples from children who underwent clinically indicated surgery. Viability and metabolic activity were assessed from 0 to 24 h. Distribution of relevant cell subsets was confirmed using single nucleus RNA sequencing. PCIS were passively sensitized using plasma from peanut allergic donors or peanut-sensitized non-allergic donors, and exposed to various stimuli including serotonin, histamine, FcɛRI-crosslinker, and food allergens. Smooth muscle contractions and mediator release functioned as readouts. A novel program designed to measure contractions was developed to quantify responses. The ability to demonstrate the impact of antihistamines and immunomodulation from peanut oral immunotherapy (OIT) was assessed.
Results
PCIS viability was maintained for 24 h. Cellular distribution confirmed the presence of key cell subsets including mast cells. The video analysis tool reliably quantified responses to different stimulatory conditions. Smooth muscle contractions were allergen-specific and reflected the clinical phenotype of the plasma donor. Tryptase measurement confirmed IgE-dependent mast cell-derived mediator release. Antihistamines suppressed histamine-induced contraction and plasma from successful peanut OIT suppressed peanut-specific PCIS contraction.
Conclusion
PCIS represent a novel human tissue-based model to study acute, IgE-mediated food allergy and pharmaceutical impacts on allergic responses in the gut.
Graphical Abstract
A novel human ex vivo GI tissue-based PCIS model was developed to study IgE-mediated food allergy. PCIS were generated using healthy, non-inflamed tissue from children. Passively sensitized PCIS displayed allergen-specific responses including mediator release and smooth muscle contractions which correlated with the clinical reactivity of the plasma donor. Antihistamines and plasma from patients who underwent successful oral immunotherapy suppressed contraction responses.Abbreviations: GI, gastrointestinal; OIT, oral immunotherapy; PCIS, precision cut intestinal slices; SMCs, smooth muscle cells
Abbreviations
-
- AUC
-
- area under the curve
-
- FA
-
- food allergy
-
- GI
-
- gastrointestinal
-
- LDH
-
- lactate dehydrogenase
-
- OFC
-
- oral food challenge
-
- OIT
-
- oral immunotherapy
-
- OVA
-
- ovalbumin
-
- PCIS
-
- precision cut intestinal slices
-
- PE
-
- peanut extract
-
- SMCs
-
- smooth muscle cells
-
- sNuc-Seq
-
- single nucleus RNA sequencing
-
- WST-1
-
- water-soluble tetrazolium salt
-
- WME
-
- Williams' medium E
1 INTRODUCTION
Food allergy (FA) is a growing public health concern.1 Current estimates report up to 10% of children are globally affected by FA.1 While many children outgrow their allergy over time, some FAs persist into adulthood resulting in a chronic disorder. The symptoms of an allergic reaction can be unpredictable and may result in potentially fatal anaphylaxis.2 The current treatment approaches for FA are limited to avoidance of the allergen and emergency interventions upon accidental exposure, although allergen-specific immunotherapies (e.g., oral, sublingual, and epicutaneous) are a promising potential treatment option for certain individuals.
The gastrointestinal (GI) tract plays a central role in FA as the primary site of exposure and immune response to food allergens when oral or mucosal tolerance are not achieved.3 The GI mucosa is enriched with mast cells that are one of the primary effector cells of the allergic response.4-6 They express the high affinity IgE receptor (FcɛRI) that binds allergen-specific IgE antibodies. Upon exposure to a relevant allergen, the receptor-bound IgE recognizes the allergen and crosslinks the receptors, activating the cell.7 This results in the release of pre-formed (e.g., histamine, tryptase, and serotonin) and de novo synthesized (e.g., cytokines and leukotrienes) inflammatory mediators influencing both local and systemic allergic responses.8 The release of histamine causes increased intestinal smooth muscle contraction by binding to histamine receptors in the GI tract, which may manifest as abdominal pain during an allergic reaction.9-12 Similarly, other symptoms of an acute allergic reaction are often GI based (e.g., cramping, emesis, and diarrhea) and reflect the direct and in situ immune response.13
Due to the inaccessibility of the gut, allergic exposure and the resulting immune cascade within human GI tissues remain understudied. Models of FA that reflect the complexity of human intestinal tissue, including the immune system and the functionality of the enteric nervous system, are rare.14
Precision cut intestinal slices (PCIS) are viable gut explants of a fixed thickness that maintain the structure and cellular diversity of intestinal tissue.15 They contain all relevant cell populations of interest and preserve the spatial distribution of the distinct cell subsets.16 PCIS can potentially represent any region of the intestine based on the surgical tissue harvested. To date, the PCIS system has been used to study the metabolism, toxicity, and interaction of pharmaceuticals as well as models for viral infection and intestinal fibrosis.17-22 However, the use of PCIS has not yet been reported in the context of FA.
Here, we report that human PCIS can be used as a model of FA to study acute, IgE-mediated allergic reactions via measurement of smooth muscle contraction as a readout for allergic response.
2 MATERIALS AND METHODS
2.1 Source of human intestinal tissue
Human intestinal tissue was obtained from 16 children (median age: 4 months) undergoing clinically indicated resection of the small or large intestine (tissue donor information in Table S1). Patients with systemic inflammatory disorders, metabolic disorders, or systemic immunosuppression were excluded. Samples from patients undergoing surgery due to non-inflammatory disorders (e.g., stoma closure after an initial surgical correction of Hirschsprung's disease) were eligible. The use of human tissue for research was approved by the SickKids Research Ethics Board (REB #1000059282). All experiments were carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving human subjects. Privacy rights of all human subjects were observed, and informed consent was obtained from the parents/guardians.
2.2 Preparation of PCIS
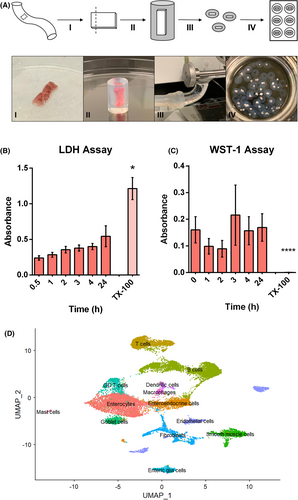
PCIS were prepared as previously published in detail by de Graaf et al. with modifications.21, 23 In short, a piece of full-thickness human intestinal tissue (approximately 1.0 × 0.5 × 0.2 cm) was cut from the healthy region of a surgically resected specimen by a trained pathologist directly following surgical removal. The sample was immediately placed into oxygenated, chilled (4°C, 95% O2, 5% CO2) Krebs Henseleit Buffer (KHB), and transported to the laboratory within one hour. The sample was then dissected into strips approximately 0.5 × 0.2 cm and embedded in low melting temperature agarose (37°C, 3% w/v, 0.9% NaCl; MilliporeSigma, Darmstadt, Germany). Once the agarose solidified, the tissue was cut into 400 μm thick slices using a Krumdieck Tissue Slicer (Alabama Research and Development, Munford, AL, USA), or a VT1200S Vibratome (Leica Microsystems, Nussloch, Germany). PCIS were then incubated in 12-well culture plates in 1.2 ml Williams' medium E (WME) containing L-glutamine and supplements (D-glucose 14 mM, gentamycin 50 μg/ml; Gibco, MA, USA) in an oxygenated incubator (37°C, 90% O2, 5% CO2; Thermo Fisher Scientific, MA, USA) with gentle shaking (Figure 1A).
2.3 Viability assays
The viability of PCIS were assessed using lactate dehydrogenase (LDH) release (cytotoxicity) assays and water-soluble tetrazolium salt (WST-1) (metabolic) assays (Roche, Mannheim, Germany) from 0 to 24 h in culture. Treatment with the detergent Triton X-100 (MilliporeSigma, Darmstadt, Germany) was included to show maximum LDH release and as a dead-control.
2.4 Single nucleus RNA sequencing of human colon tissue
Colon tissue samples (n = 4), also used to generate PCIS, were snap-frozen, and stored at -80°C prior to single nucleus RNA sequencing (sNuc-Seq). After library generation and quality control, the samples were deep sequenced using a NovaSeq (target 10,000 nuclei, 80,000 reads/nuclei). FASTQ files were aligned to a reference human transcriptome using 10X Genomics Cell Ranger (CA, USA). The resulting gene expression data were merged using the Seurat R package.24, 25 Batch effects were removed by “regressing out” variables, and clustering was performed using the Leiden algorithm implemented in Seurat. Cell identities were assigned and confirmed using a list of pre-defined marker genes in reference to single cell gene expression databases and the top distinct genes expressed by each cluster.26, 27
2.5 Passive sensitization
PCIS were passively sensitized in a 1:10 dilution of human plasma from clinically confirmed peanut allergic donors or peanut-sensitized non-allergic donors (Table S2) in WME for 90–120 min (37°C, 90% O2, 5% CO2) with gentle shaking.28, 29 Human plasma was derived from ongoing and completed trials including the Markers of Nut Allergy Study (MONAS) cohort at the Hospital for Sick Children.30 The use of human plasma for research was approved by the SickKids REB for MONAS (#1000053791) and the Low Dose Multi-OIT (LoMo) study (#1000060633).
2.6 Video microscopy
PCIS muscle contraction was filmed using a stereomicroscope with a camera attachment (Walter Products, ON, Canada; TCapture Software, Tucsen Photonics, Fuzhou, China) for 10 min without the addition of stimuli to establish baseline movement of the individual sample. The slices were then filmed for an additional 10 min upon addition of either peanut extract (PE) (1 μg/ml, ALK-Abelló, Hørsholm, Denmark), serotonin (10 μg/ml, MilliporeSigma, Darmstadt, Germany), FcɛRI-crosslinker (10 μl, Bühlmann, Amherst, NH, USA), histamine (10 μg/ml, ALK-Abelló, Hørsholm, Denmark), or ovalbumin (OVA; 1 μg/ml, InvivoGen, San Diego, CA, USA) as an irrelevant allergen. Any samples not passing quality control criteria (Table S3) were excluded from analysis.
2.7 Analysis of PCIS smooth muscle contractions
Statistical analysis was conducted using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). Responses between different stimulations were compared using the Mann–Whitney U test with a p value < 0.05 considered statistically significant.
A novel video analysis software was developed internally (available at: https://github.com/celalp/video_parser). This software measures muscle contraction using individual pixel movement per frame (Figure 2). Videos were analyzed using a semi-supervised approach with variables kept consistent between the unstimulated control and stimulated sample. Videos were processed using OpenCV (Open Source Computer Vision Library) and scikit-image Python packages.31, 32 Pixels that changed from frame x-1 to frame x were calculated using a Gaussian mixture-based background/foreground segmentation algorithm.33, 34 A background subtraction algorithm was chosen for this analysis as videos consisted of one large central object with a mostly static background, meaning that any observed movement was captured as foreground. The selection of the specific algorithm (MOG2 in OpenCV) was influenced by the large variability of exposure levels (e.g., glare) between different slices. A dynamic Gaussian Mixture Method allowed for the same background removal method for all experiments. Measurements were validated and confirmed by an independent assessor. Overall pixel movement per frame of the video was plotted for each stimulation and total Area Under the Curve (AUC) was calculated and compared for each contraction response using GraphPad Prism 6.
2.8 Tryptase quantification in cell free supernatant
Culture supernatant from PCIS passively sensitized and stimulated with PE (1 μg/ml) or controls was collected 10 min post-stimulation and stored at −80°C. Tryptase levels in supernatant were measured using a tryptase beta-2 ELISA (MilliporeSigma, Burlington MA, USA) after concentration using centrifugal filter devices (Amicon Ultra-0.5 10 K device, MilliporeSigma, Burlington, MA, USA).
2.9 Treatment with antihistamines
Passively sensitized PCIS were incubated with diphenhydramine hydrochloride (10 μg/ml), cetirizine dihydrochloride (1 μg/ml), and rupatadine (1 μg/ml) (MilliporeSigma, Darmstadt, Germany) for 10 min prior to stimulation with histamine (10 μg/ml) and filmed for 10 min post-stimulation. Concentrations were chosen based on published reports and dose–response experiments.35-38
2.10 Effect of immunomodulation from successful OIT patients
PCIS were passively sensitized with plasma (1:10 in WME) and stimulated with PE and controls to assess differences in pre- (oral food challenge [OFC] positive) and post-successful peanut OIT (ingestion of 5× the baseline eliciting dose).
3 RESULTS
3.1 PCIS maintain viability for at least 24 h
This model was generated to study acute, IgE-mediated allergic reactions, which are known to occur within seconds of exposure to a relevant allergen. To confirm that PCIS remained viable during the incubation process, they were monitored from 0–24 h post-slicing. Cell death (LDH) and metabolism (WST-1) were assessed in each sample and demonstrated consistent viability of PCIS during the investigated 24 h in culture (Figure 1B,C).
3.2 sNuc-Seq of gut tissue confirms the presence of mast cells and other cell types
The cellular composition of PCIS was analyzed using sNuc-Seq. Single cell gene expression profiles from colon tissue donors (n = 4) were compiled to reveal the cellular composition of PCIS (Figure 1D). Expression profiles aligned with the cellular identities of each cluster and were confirmed using single cell gene expression databases.26, 27 All expected cell types in colon tissue were observed (Figure 1D, Table S4) including epithelial cells (enterocytes, goblet cells, enteroendocrine cells, and endothelial cells), stromal cells (fibroblasts, smooth muscle cells), and immune cells (T cells, γδT cells, B cells, mast cells, dendritic cells, and macrophages).
3.3 PCIS contract in response to antigen-independent stimuli
To validate the model and determine the sensitivity of PCIS to non-specific antigen stimulations, FcɛRI-crosslinker, histamine, and serotonin were selected as positive controls. All conditions elicited a strong, reproducible smooth muscle contraction response, elevated in comparison with the unstimulated control (Figure 3A; Videos S1–S6).
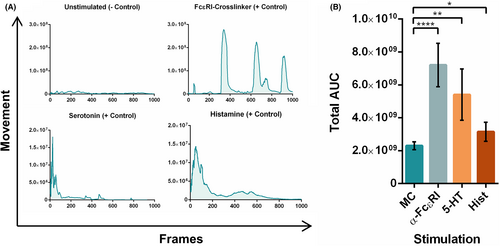
Based on original video data, a novel software was designed to quantify smooth muscle contractions using object and movement detection of irregular shapes. This was achieved by tracking the overall movement of individual pixels within each frame (Figure 2) which were then quantified and graphed resulting in a curve of the contraction response. Total AUCs of each response per stimuli were compared, showing a significant increase in movement between the unstimulated slices and PCIS stimulated with the positive controls (Figure 3B).
3.4 Allergen-specific induction of PCIS contractions reflects allergic status of the donor
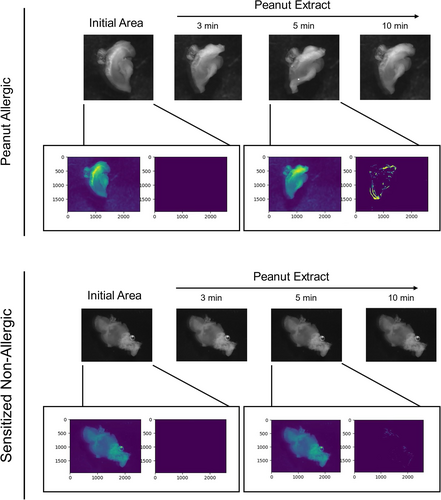
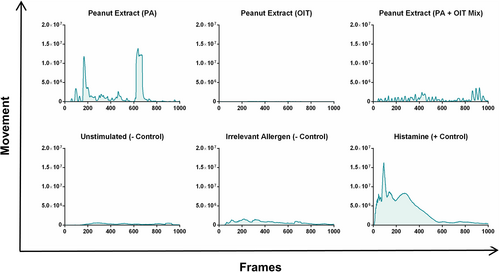
In order to establish PCIS as a model of FA, responses must be reproducible, allergen-specific, and clinically relevant. PCIS passively sensitized with plasma from clinically confirmed peanut allergic children displayed a strong contractile response after stimulation with PE (Figure 4A; Videos S7 and S8). This response was strictly allergen-specific, as stimulation with a clinically irrelevant food allergen (OVA) did not result in an increased response (Figure 4A; Videos S9 and S10).
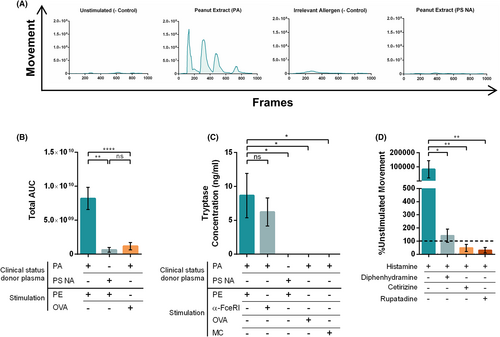
To evaluate whether response patterns were linked to allergen-specific IgE or match clinical reactivity at the individual level, PCIS were passively sensitized with plasma from peanut-sensitized non-allergic donors. These donors had measurable peanut-specific IgE but were asymptomatic upon peanut exposure. PCIS sensitized with this plasma did not show significant muscle contraction responses following stimulation with PE, while maintaining reactivity to positive controls (Figure 4A; Videos S11 and S12). The total AUCs of each contraction response revealed a significantly greater response following stimulation with a relevant allergen (PE), in comparison to an irrelevant allergen (OVA) in slices sensitized with peanut allergic plasma, as well as to PE stimulation in sensitized non-allergic tissue (Figure 4B).
Tryptase was measured in the culture supernatant following stimulation to confirm the release of mast cell derived mediators (Figure 4C). Tryptase concentrations in the supernatant of PCIS were significantly greater following exposure to PE and elevated in response to FcɛRI-crosslinker in tissue sensitized with peanut allergic plasma. Unstimulated tissue or stimulation with an irrelevant allergen (OVA) did not result in similar tryptase levels, demonstrating the specificity of the response and linkage to degranulation (Figure 4C). PE stimulation in slices sensitized with peanut sensitized non-allergic plasma did not result in a significant change in tryptase concentration, reflecting their lack of reactivity to PE exposure (Figure 4C).
3.5 PCIS can be used to study anti-allergic drugs
Antihistamines were used to demonstrate the suitability of PCIS as a model of FA to assess specific mechanisms of action. Pre-treatment of the tissue with the antihistamines diphenhydramine hydrochloride, cetirizine dihydrochloride, and rupatadine resulted in significant suppression of contraction responses to histamine stimulation (Figure 4D).
3.6 Peanut OIT suppresses allergen-specific responses
As a promising treatment option for FA, the suppression of allergic responses following OIT was also investigated using the PCIS model. PCIS passively sensitized with plasma from a peanut allergic individual pre-peanut OIT, stimulated with PE resulted in a strong smooth muscle contraction response (Figure 5). Following completion of the OIT protocol, plasma from the same individual resulted in a diminished response after stimulation with PE, which is reflective of the clinical phenotype of the plasma donor. Co-incubation of pre- and post-OIT plasma also resulted in a reduced contraction response in comparison with the pre-OIT condition, reflecting the sensitivity of the model (Figure 5).
4 DISCUSSION
As the incidence and awareness of FA increases, there is a demand for appropriate models of FA for mechanistic studies and the development of therapeutics. This study aims to generate a human gut tissue-based model of acute, IgE-mediated FA. Using the PCIS system, allergic responses that occur within GI tissues may be characterized.
Cellular composition is key for accurate inferences on the nature of an investigated response. To ensure the representation of distinct cell subsets, sNuc-Seq was employed to define the cellular composition of the PCIS. The resulting map of cellular distribution confirmed that all relevant cell types were present in the samples, including stromal and epithelial cells as well as key immune cell populations that are commonly described in human colon tissue.39, 40 Not only is this dataset useful in this context, but also forms the framework for future functional assessments and adds to single cell libraries for healthy infant colon tissue, a relatively understudied group.
In addition to confirmation of cellular diversity, establishing tissue viability and metabolic activity in the tissue is essential to ensure accurate readouts. Using routine LDH and WST-1 assays, PCIS maintained consistent viability for at least 24 h in culture.41 This model was specifically designed for acute allergic responses, which did not warrant investigations beyond 24 h. Previous reports have shown that the viability and morphology of human PCIS can be maintained up to 48h21 and even 72 h.20, 42
Smooth muscle contraction was chosen as the readout for allergic responses in the PCIS model, as allergic reactions stemming from food exposure often result in symptoms centralized to the GI tract.43 Moreover, food allergen-induced muscle contraction in sensitized individuals has been reproducibly demonstrated in both animal and human models.44-46 The inflammatory mediators stored in mast cell granules, which includes histamine, serotonin, and tryptase, have all been shown to directly cause smooth muscle contraction in the gut.4, 47, 48 Similar studies using precision cut lung slices have also utilized bronchoconstriction to model allergic responses to aeroallergens.29, 49, 50 To measure the contraction response in PCIS, a novel analysis program was developed internally, as other commercial motion tracking software were unable to reflect the patterns observed in the videos. This program, designed to quantify the muscle contraction responses in PCIS, also has the potential to be applied beyond this system. Any model in which movement is a central aspect of response could utilize this program to quantify results. The configurations can also be set based on controls and adjusted to accommodate changes in lighting during filming.
PCIS generated from non-allergic donor tissue underwent passive sensitization to induce sensitivity to the allergen of the allergic plasma donor. Passive sensitization is a common experimental procedure used to induce sensitivity in non-allergic cells or tissue.28, 29, 51, 52 The use of this process not only reduces the need for relatively rare allergic tissue donors, but also extricates the humoral component of the allergic response. PCIS passively sensitized with clinically confirmed peanut allergic plasma stimulated with PE or positive controls displayed a strong contraction response that was significantly greater than the baseline movement due to enteric reflexes.53 This response was allergen-specific, as demonstrated by the lack of response to a clinically irrelevant allergen (OVA). In contrast, PCIS passively sensitized with plasma from a peanut-sensitized non-allergic donor did not display a strong response to PE stimulation, indicating that the PCIS model reflects the clinical phenotype of the donor. Although the overall patterns of response were clear between stimuli, the kinetics and magnitude of the muscle contraction responses may vary between individual PCIS. This was expected as the gut slices differ in terms of smooth muscle composition and mast cell distribution due to variability between tissue donors and along an individual GI tract.
Tryptase release from intestinal mast cells occurs immediately upon activation.54 Measurements of tryptase in the PCIS culture supernatant confirmed inflammatory mast cell-derived mediators were released following stimulation with a relevant allergen (peanut) or FcɛRI-crosslinker but not an irrelevant allergen (OVA), further highlighting the specificity of responses.
As observed in the PCIS model, histamine is a key mediator of the allergic response that affects smooth muscle contraction. Thus, antihistamines were the drug of choice to demonstrate the utility of this ex vivo model for testing anti-allergic therapeutics in development, as illustrated by the suppression of contraction responses. While anti-allergic drugs are commonly tested in animal models,44, 55, 56 the use of a human tissue-based model either in place of or in addition to animal testing would allow for more translatable and clinically relevant results. Of note, the PCIS model has often been used to study pharmacokinetics in the context of human gut tissue.15-17, 21, 22
Passive sensitization of PCIS with plasma from a peanut allergic donor pre-OIT resulted in a robust contraction response following stimulation with PE, while a comparatively diminished response was observed post-peanut OIT as well as in combination. OIT has become a standard treatment option for FA and has been broadly effective in treating children with established FA. During OIT, the levels of allergen-specific IgG, IgG4, and IgA increase, and are considered to act as protective antibodies, whereas levels of allergen-specific IgE decline over time.57, 58 These results demonstrate the use of PCIS as a model of FA in potentially assessing responses to therapy and immunomodulation.
In summary, a novel human gut tissue-based FA model has been developed. PCIS generated from non-allergic tissue, passively sensitized with plasma from allergic donors, displayed visible and quantifiable allergen-specific smooth muscle contractions upon allergen stimulation. This model has great potential as a valuable experimental tool in FA research as it can be used to differentiate sensitized allergic versus sensitized non-allergic individuals, test anti-allergic drugs within a relevant environment and observe the progression of allergen-specific immunotherapy. As mast cells are difficult to isolate in peripheral blood, and the complex interactions between immune cells and structural cells in the gut are not easily observed, the utility of this FA model addresses a relevant research need.
As with all models, there are several constraints. A significant limitation is the lack of tissue availability from appropriate sources. Stringent exclusion and inclusion criteria for donor tissue is required to limit the potential impact of underlying pathologies. Additionally, gut tissue is sensitive to culture conditions and has a limited viability. While this is appropriate for acute, short-term outcomes, this model cannot be used without modifications for long-term studies or for the study of delayed allergic responses. Additional considerations include the isolated nature of the PCIS model, which cannot replicate circulation or migration of cells from other tissue, as well as equal exposure to stimulants on both surfaces (apical/basal) which does not reflect the human system. Importantly, as the tissue is from non-atopic donors, it cannot reflect the pre-existing inflammation observed within the GI tract of allergic patients.
Despite these limitations, this human tissue-based model is directly translatable in contrast to individual cell culture models or animal models of disease which cannot replicate the human system. It contains all resident cell types interacting in a physiologically accurate environment. This allows for observation of cellular mechanisms in tissues that are not represented by peripheral blood samples and may also be adapted to investigate other GI-based diseases. The promising potential of PCIS as a model of FA will also expand to future studies including early changes in gene expression following allergen exposure.
AUTHOR CONTRIBUTION
TE designed and conceptualized the project. LH, AC, XY, KY, AB, AK, IS, GRS, KLB, MG, FAQ, and PPLC contributed to data acquisition and sample processing. LH, HO, KS, and TE contributed to experimental design. LH, AC, and TE contributed to analysis and data interpretation. LH, AC, AK, JEMU, KLB, and TE were involved in generation and critical revision of the manuscript. The final version of the manuscript was approved by all authors.
ACKNOWLEDGMENTS
This work was supported by The Canadian Institutes of Health Research Fredrick Banting and Charles Best Canada Graduate Scholarship and The Hospital for Sick Children (SickKids) grants: HSBC Catalyst Grant, POS Innovation Grant, and Restracomp Graduate Scholarship, as well as the SickKids Food Allergy and Anaphylaxis Program and start-up funds by SickKids Research Institute and the Department of Paediatrics.
CONFLICT OF INTEREST
LH, AC, XY, KY, AB, AK, HO, KLB, GRS, IS, MG, FAQ, KS, and PPLC have nothing to disclose. JEMU reports grants/research support from DBV Technologies, Regeneron, CIHR, ALK-Abelló, SickKids Food Allergy and Anaphylaxis Program, Advisory board for Pfizer, Kaleo, Bausch Health, Food Allergy Canada; in-kind drug donation from Novartis, other for Astra-Zeneca, all outside the current work. TE reports to act as local PI for company sponsored trials by DBV Therapeutics, Greer Stallergens, and sub-investigator for Regeneron and ALK-Abelló. He is Co-Investigator or scientific lead in three investigator-initiated oral immunotherapy trials supported by the SickKids Food Allergy and Anaphylaxis Program and serves as an associate editor for Allergy. He/his laboratory received unconditional/in-kind contributions from Macro Array Diagnostics and an unrestricted grant from ALK-Abelló. He holds advisory board roles for ALK-Abelló, VAMED, Nutricia/Danone and Aimmune. TE reports lecture fees from Novartis, ThermoFisher, Nutricia/Danone, Aimmune, ALK-Abelló.