Furan fatty acid metabolite in newborns predicts risk of asthma
Abstract
Background
Intake of fish-oil and fatty fish during pregnancy has been shown to reduce the risk of childhood asthma but biomarkers of such intake are lacking.
Objective
To establish biomarkers of prenatal fish-oil exposure from newborn dry blood spot metabolomics profiles and assess their relevance for childhood asthma risk stratification.
Methods
The Danish COPSAC2010 mother–child cohort was utilized to investigate the effect of a double-blinded randomized controlled trial of fish-oil supplementation during pregnancy on dry blood spot liquid-chromatography mass spectrometry-based metabolomics profiles of 677 newborns. We thereafter investigated the association between fish-oil associated biomarkers in the newborn and development of asthma-related outcomes. Replication was sought in the independent observational COPSAC2000 cohort with 387 newborn metabolomics profiles.
Results
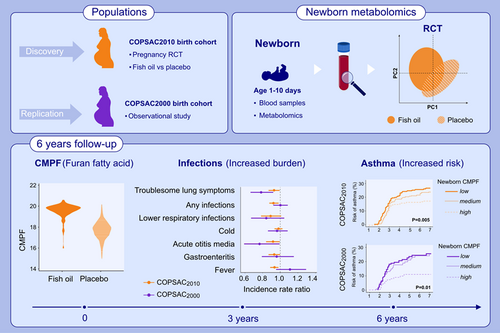
The newborn metabolomics profiles differed between children in the fish-oil vs. placebo group in COPSAC2010 (area under the receiver operator curve = 0.94 ± 0.03, p < .001). The fish-oil metabolomics profile and the top biomarker, 3-carboxy-4-methyl-5-propyl-2-furan propanoic acid (CMPF) were both associated with a decreased risk of asthma by age 6 years (HR = 0.89, p = .002 and HR = 0.67, p = .005, respectively). In COPSAC2000, newborn CMPF level was also inversely associated with asthma risk by age 6 years (HR = 0.69, p = .01). Troublesome lung symptoms and common infections in the first 3 years were also inversely associated with newborn CMPF levels in both cohorts.
Conclusions
Newborn children's blood levels of the furan fatty acid metabolite CMPF reflect fish-oil and fatty fish intake during pregnancy and are associated with a lower risk of asthma across two cohorts, which could aid newborn screening for childhood asthma.
Graphical Abstract
This study investigates the effect of fish-oil supplementation during pregnancy on newborn dry blood spot metabolomics profiles in the COPSAC birth cohorts. The furan fatty acid metabolite CMPF was identified as top biomarker of the fish-oil supplementation. Increasing newborn CMPF level was associated with decreased risk of infections and asthma in both cohorts. CMPF, 3-carboxy-4-methyl-5-propyl-2-furan propanoic acid; COPSAC, Copenhagen Prospective Studies of Asthma in Childhood; RTC, randomized controlled trial.Abbreviations: CMPF, 3-carboxy-4-methyl-5-propyl-2-furan propanoic acid; COPSAC, Copenhagen Prospective Studies of Asthma in Childhood; RTC, randomized controlled trial
1 INTRODUCTION
Increasing evidence suggests that maternal diet and micronutrient status during pregnancy play an important role in the development of childhood asthma.1 In line with this, we demonstrated that fish-oil derived n-3 long-chain polyunsaturated fatty acid (n-3 LCPUFA) supplementation during pregnancy reduced the offspring's risk of asthma and lower respiratory tract infections in a randomized controlled trial (RCT).2, 3
Unbiased assessment of dietary intake in dietary RCTs and observational studies for risk stratification is one of the major challenges within clinical nutrition research,4 posing a need for biomarkers of accurate intake. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are used as biomarkers of fish intake and fish-oil consumption, but blood levels vary due to subsequent metabolism and modification by genetic factors such as rs1535 FADS2 genotype5 and therefore may provide biased estimates of intake.6 Metabolic profiling has emerged as a powerful tool for biomarker discovery and for uncovering the underlying mechanisms leading to disease.7 A metabolomics study led to discovery of a furan fatty acid metabolite, CMPF (3-carboxy-4-methyl-5-propyl-2-furan propanoic acid), as a biomarker of fatty fish intake,8 but it is unknown if CMPF is influenced by FADS2 genotype and of relevance for asthma risk stratification.
Newborn dried blood spot samples are routinely collected in Denmark to diagnose inborn errors of metabolism and provide a unique resource to investigate the influence of prenatal exposures.9, 10 Therefore, we measured the metabolic profile of newborn dried blood spots from the Copenhagen Prospective Studies on Asthma in Childhood (COPSAC2010) cohort to discover biomarkers of a RCT with fish-oil supplementation during pregnancy. Thereafter, we investigated the relevance of such biomarkers for asthma risk stratification in newborn children using COPSAC2010 for discovery and the observational COPSAC2000 birth cohort for replication.
2 METHODS
2.1 Study population and design
The COPSAC2010 is a population-based mother–child cohort including 736 pregnant women and their children,11 while the COPSAC2000 is a high-risk cohort including 411 children born to mothers with a history of asthma.12
The pregnant women from COPSAC2010 were enrolled in a RCT at 24 weeks of gestation,2 where they received either 2.4 g of fish-oil derived n-3 LCPUFA capsules (55% EPA and 37% DHA, Incromega TG33/22, Croda Health Care) or placebo olive oil capsules (72% n-9 oleic acid and 12% n-6 linoleic acid; Pharma-Tech A/S) (ClinicalTrials.gov: NCT00798226). The daily intake of the capsules continued until 1 week after delivery, where the number of capsules returned was recorded. The maternal habitual diet was obtained from a 360-item food frequency questionnaire at 24 weeks of gestation. The n-3 LCPUFA RCT was a 2 × 2 factorial design (illustrated in Figure S1) with a daily high-dose or standard dose of vitamin D (2800 vs. 400 IU/day).13 The 2 × 2 trial design allowed investigation of n-3 LPUFA intake independently of the vitamin D intervention. Maternal blood levels of EPA and DHA were measured at 24 weeks of gestation and 1 week after birth.14
The COPSAC2000 and COPSAC2010 studies are conducted according to the principles of the Declaration of Helsinki and are approved by the Ethics Committee of Copenhagen (KF 01-289/96, H-B-2008-093) and the Danish Data Protection Agency (2015-41-3696). Both parents gave oral and written informed consent before enrollment.
2.2 Primary endpoint
Persistent wheeze/asthma was diagnosed by the COPSAC pediatricians based on a validated, symptom-based algorithm using daily diary recordings of troublesome lung symptoms, medication use since birth, and objective assessments at acute care and scheduled visits to the COPSAC research unit.11 In brief, the diagnosis was based on (1) troublesome lung symptoms, that is, diary recordings of at least five episodes within 6 months, each lasting at least 3 days; (2) symptoms typical of asthma, for example, exercise-induced symptoms, prolonged nocturnal cough, persistent cough outside common cold; (3) rescue use of inhaled bronchodilator; and (4) response to a 3-month trial of inhaled corticosteroids and relapse after end of treatment.2, 13 The diagnosis was termed “persistent wheeze” before age 3 and “asthma” thereafter.
2.3 Secondary endpoints
2.3.1 Troublesome lung symptoms
An episode of troublesome lung symptoms was defined as diary recorded troublesome lung symptoms verified at the COPSAC research unit, lasting at least three consecutive days.
2.3.2 Infections
Any infections, lower respiratory tract infections, otitis media, cold, croup, fever, and gastroenteritis were captured in daily diary cards at age 0–3 years in COPSAC2010, while infections in COPSAC2000 were based on diagnosis from general practitioners and health registries. The number of infection episodes was used for data analysis.
2.3.3 Lung function
Forced expiratory volume (FEV1) and maximal mid-expiratory flow (MMEF) were measured with spirometry and specific airway resistance (sRaw) with whole-body plethysmography at age 6 years. FEV1, MMEF, and sRaw were calibrated for age, sex and height.15
2.3.4 Allergic sensitization
Skin prick test and specific-IgE levels against common aeroallergens were measured at age 6 years defining sensitization as a positive skin prick test and/or elevated specific-IgE.16
2.4 Newborn dried blood spot samples
Newborn dry blood spot samples were collected at 2–3 and 1–12 days after birth for neonatal screening for inborn errors of metabolism from COPSAC2010 during year 2008–2010 and from COPSAC2000 during year 1998–2001. The samples were stored at −20°C at the Danish National Biobank until analysis, that is, approx. 10- and 20-year storage time for the two cohorts, respectively. The dry blood spot samples were analyzed in separate batches to avoid potential bias due to storage time.
2.5 Metabolomics profiling
Metabolic profiles of the dry blood spot samples from COPSAC2010 (n = 682) and COPSAC2000 (n = 388) were acquired using a Q-Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific) coupled with a Dionex Ultimate 3000 ultra-pressure liquid chromatography (UPLC, Thermo Fisher Scientific; see details in Text S1). The data preprocessing of full-scan spectra and data-dependent tandem spectra were performed using XCMS and MZmine. Preprocessing of full-scan spectra resulted in a feature table, which was used for statistical analysis after filtering and batch correction (Text S2). Tandem spectra were submitted to the Global Natural Products Social Molecular Networking Platform (GNPS),17 and the MolNetEnhancer workflow18 was used for compound annotation (Text S3).
2.6 Statistical analysis
Multivariate data analysis was performed in the PLS_Toolbox (version 6.5, Eigenvector Research, Inc., MA, USA) built in MATLAB R2018b, and statistical analyses were conducted using R version 3.61. The COPSAC2010 metabolic profiles were subjected to double cross-validated19 partial least square-discriminant analysis (PLS-DA) to discriminate children in the n-3 LCPUFA vs. placebo group.20 The combined effect of key metabolites reflecting n-3 LCPUFA supplementation was described by principal component analysis (PCA) scores. The key metabolites were log-transformed, and the effect size of the n-3 LCPUFA RCT was calculated by Cohen's d (standardized mean difference).
Cox regression survival analysis was applied using the survival R package to assess the association between PCA scores and single key metabolites and the risk of developing asthma during childhood, visualized using Kaplan–Meier curves. Thereafter, we conducted a causal mediation analysis (R package “mediation”) to investigate whether the reduced risk of asthma by the n-3 LCPUFA intervention was mediated through the PCA scores. Statistics of average causal mediation effect (ACME) was investigated for assessment of mediation.
Quasi-poisson regression was applied to estimate the effect of key metabolite levels on relative incidence rate of common infections and troublesome lung symptoms.
As an additional analysis, a Weighted Gene Correlation Network Analysis (WGCNA) was performed using R package WGCNA (version 1.70-3) to define modules consisting of highly correlated metabolites. Modules correlated with the n-3 LCPUFA RCT were identified using Student asymptotic p-value (see details in Text S4).
3 RESULTS
The total number of dried blood spot samples included in this analysis was 671 (1 were excluded due to low analytical quality) for COPSAC2010 and 387 for COPSAC2000. The number of mass spectral features detected was 1922 and 2313 for COPSAC2010 and COPSAC2000, respectively. For simplicity, we denominate a mass spectral feature as a metabolite throughout the text.
3.1 Newborn metabolic profile after pregnancy n-3 LCPUFA supplementation
The PLS-DA models performed well in terms of discriminating the offspring's metabolome according to n-3 LCPUFA vs. placebo supplementation during pregnancy (area under the receiver operator curve [AUC] = 0.94 ± 0.03, p < .001). The number of metabolites selected by at least 80% of the PLS-DA models with different test/training pairs was 47. These 47 metabolites were thereafter subjected to a PCA, which showed significant separation by n-3 LCPUFA supplementation group in PC2 (β = 1.94, 95% CI [1.62–2.27]). Figure 1 shows PC1 vs. PC2 scores and loadings plots and the 47 metabolites contributing to the separation by the supplementation groups.
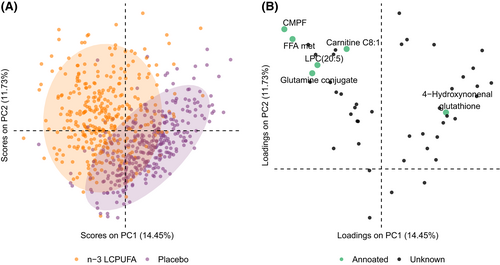
Among the annotated metabolites, the furan fatty acid metabolite CMPF and lysophosphatidylcholine (LPC) (20:5) were significantly associated with n-3 LCPUFA supplementation in univariate tests (FDR adjusted p < .001). Of those, CMPF showed the strongest association with n-3 LCPUFA supplementation (Cohen's d = 2.68, 95% CI [2.47–2.89]) (Figure 2A). Further, the mothers of the newborns with lower levels of CMPF returned larger numbers of supplement capsules within the n-3 LCPUFA group (Figure 2B). In both the n-3 LCPUFA and the placebo group, CMPF was associated with the mother's reported average fish intake during pregnancy (Figure 2C).

Maternal EPA and DHA 1-week postpartum were previously used to assess adherence to the n-3 LCPUFA RCT.2 Here, in the newborn blood metabolome, EPA is incorporated into lysophospholipid and observed as LPC(20:5), which reflected n-3 LCPUFA supplementation group although the effect size was less than for CMPF (Cohen's d = 0.63 [0.47–0.78]). Furthermore, both EPA, DHA, LPC(20:5), and an unknown FFA metabolite provided less strong associations with number of returned capsules and maternal average fish intake compared to CMPF (Figure S2).
We previously demonstrated that maternal EPA and DHA levels are influenced by FADS2 genotype (rs1535) with lower levels in mothers carrying the G minor allele.2 In contrast, CMPF was not associated with maternal FADS2 genotype (β = −0.102 [−0.39; 0.19], p = .149). These findings support previous studies suggesting CMPF as an unbiased biomarker of fatty fish intake and n-3 LCPUFA supplementation.8
In addition, 4-Hydroxynonenal glutathione, a well-known marker of n-6 LCPUFA-derived lipid peroxidation, was lower in the n-3 LCPUFA strata (FDR adjusted p = .016).
Furthermore, WGCNA highlighted the association between similar metabolites and n-3 LCPUFA supplementation. A total of 17 modules containing metabolites with similar intensities were identified (Figure S3). Based on p < .05 and Pearson's correlation >0.3 cutoffs, a total of 33 metabolites were significantly correlated with fish-oil intake across modules. Of those metabolites, 31 belonged to one of the 17 modules which included CMPF, showing a strong correlation of 0.79 and p < 10−10.
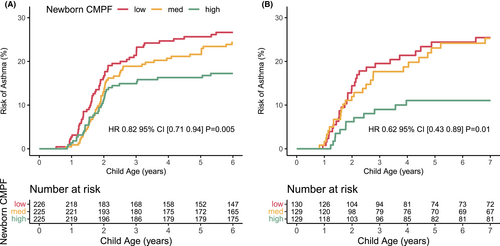
3.2 CMPF and risk of childhood asthma
We have demonstrated that n-3 LCPUFA supplementation during pregnancy reduced the offspring's risk of developing asthma.2 Thus, using CMPF as a biomarker of the intervention, increasing CMPF levels were associated with a decreased risk of asthma in COPSAC2010 (Figure 3A). We further investigated whether the observed association between CMPF and risk of childhood asthma replicated in the observational COPSAC2000, which showed that newborns in COPSAC2000 with higher CMPF levels had lower risk of asthma (Figure 3B). Adjustment for confounders, that is, smoking, gestational age, and season of birth, did not change the findings (aHR 0.62; CI 95% [0.43–0.89]; p = .01).
Previously, we also demonstrated the influence of the FADS2 genotype on the preventive effect of n-3 LCPUFA supplementation on asthma development.2 Performing similar analysis using newborn CMPF and maternal postpartum EPA and DHA levels, a lower risk of asthma was only present for higher CMPF and DHA levels among mothers carrying the minor allele of rs1535, which was most pronounced for CMPF (Figure S4).
3.3 Fish-oil metabolite score and childhood asthma
We further investigated whether the newborn blood metabolic profile of the n-3 LCPUFA supplementation described by PC2 scores (Figure 1A), that is, so-called n-3 LCPUFA metabolite score, explained the underlying biochemical mechanisms leading to protection against childhood asthma. Indeed, newborns with an increasing n-3 LCPUFA metabolite score had a lower risk of asthma by age 6 (HR = 0.89 [0.83–0.96]; p = 0.002). Furthermore, the effect of the n-3 LCPUFA supplementation on childhood asthma was significantly mediated through the n-3 LCPUFA metabolite score (ACME β = −1678.6; 95% CI [−4273.9; −370.4]; p = 0.02), which explained 41% of the effect of the fish-oil intervention on asthma. To avoid potential bias due to inclusion of a marker of the n-3 LCPUFA trial, that is, CMPF, we excluded CMPF and re-calculated the PC scores, yet the associations were still significant (ACME β = −2212.9 [−5776.3; −489.8]; p = 0.03). Overall, our findings suggest that the preventive effect of n-3 LCPUFA supplementation in pregnancy against childhood asthma is partially driven through metabolic changes in the newborn child.
3.4 CMPF and secondary endpoints
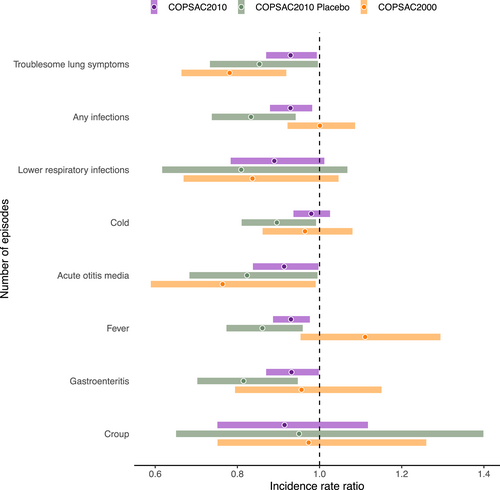
In both COPSAC cohorts, increasing newborn CMPF level was significantly associated with a reduced rate of troublesome lung symptoms (p = .03 for COPSAC2010 and p = .003 for COPSAC2000) and acute otitis media episodes (p = .04 for both cohorts) in the first 3 years of life (Figure 4). In COPSAC2010, increasing CMPF was also associated with fewer episodes of gastroenteritis, fever, and any infections. Notably, the significant effect of newborn CMPF level on troublesome lung symptoms and common infections in COPSAC2010 was not solely dependent on higher levels due to the n-3 LCPUFA intervention since the association was also significant in the placebo strata (Figure 4), while CMPF was not associated with any of the secondary endpoints within the fish-oil strata. CMPF was not associated with lung function measures or allergic sensitization in any of the cohorts (Table S1).
4 DISCUSSION
4.1 Primary findings
We utilized a RCT of fish-oil derived n-3 LCPUFA supplementation during pregnancy in the COPSAC2010 cohort to identify biomarkers of fish-oil intake by metabolomics profiling of dry blood spots from the newborn children collected as part of a national screening program for inborn errors of metabolism. We discovered that increasing levels of the furan fatty acid metabolite CMPF in the newborn's blood were strongly associated with prenatal n-3 LCPUFA exposure and associated with a reduced risk of developing asthma before age 6 years. These findings were replicated in the independent, observational COPSAC2000 cohort, suggesting that newborn CMPF level may be utilized as an asthma-relevant biomarker of prenatal n-3 LCPUFA exposure for risk stratification. Further, in both cohorts CMPF levels in newborn children were also inversely associated with number of episodes with troublesome lung symptoms and common infections in the first 3 years of life but not with lung function or allergy, suggesting that CMPF depicts the very common non-atopic childhood asthma endotype characterized by early debut and high load of infections.
4.2 Strengths and limitations
It is a strength of this analysis that it is based on a RCT with n-3 LCPUFA supplementation in pregnancy, which is particularly informative for discovery of intake biomarkers due to a better control of intake and less proneness to variability.7 The detailed information on the number of fish-oil capsules returned, food frequency questionnaire-based estimation of fish intake and FADS2 genotype enabled further validation of CMPF as a marker of fish-oil and fatty fish consumption. Further, the association between CMPF and childhood asthma risk was replicated in the observational COPSAC2000 cohort, suggesting that the association is not restricted to populations with pregnancy fish-oil supplements.
Investigation of the secondary endpoints revealed that incidence of common infections and troublesome lung symptoms in the first 3 years of life were lower in children with higher newborn CMPF levels, while CMPF was not associated with neither lung function measures nor allergic sensitization. Therefore, maternal fish-oil and fatty fish intake is likely to be preventive against non-atopic asthma through protection against infections, which is in contrast to previous studies suggesting an effect of fish-oil intake in pregnancy against allergic diseases.21, 22
The associations between CMPF and the secondary endpoints were stronger in the placebo strata of COPSAC2010 compared to COPSAC2000. A possible explanation might be the higher quality of reporting for the secondary endpoints in COPSAC2010, that is, diaries reviewed by pediatricians, compared to registry-based evaluation in COPSAC2000.
Unfortunately, only a limited number of metabolites reflecting n-3 LCPUFA supplementation were annotated, and acquisition was only done in positive ESI. Therefore, additional annotations and acquisition in negative ESI may shed light on other underlying metabolic effects of prenatal fish-oil exposure, particularly EPA and DHA in phospholipid form. Furthermore, newborn CMPF levels were only semi-quantified in this study, and therefore, the absolute CMPF level required to decrease the risk of childhood asthma cannot be predicted by our data. For future studies, absolute quantification of blood CMPF levels should be performed.23
Another limitation is the need for invasive blood samples for measurement of CMPF. However, in many countries, including Denmark, dry blood spots are collected as part of newborn screening for inborn errors of metabolism, and therefore, our findings could readily be implemented in clinical practice.
Finally, the different length of storage time of the dry blood spots in the two cohorts of approx. 10 and 20 years is a limitation as we lack data on stability of CMPF over this length of time. However, the dry blood spot samples were analyzed in separate batches to avoid bias due to storage time, and the findings were similar across cohorts in relation to the clinical endpoints.
4.3 Interpretation
3-Carboxy-4-methyl-5-propyl-2-furan propanoic acid is a furan fatty acid metabolite that has been proposed as a biomarker of fish-oil and fatty fish intake,8 which in some studies have been utilized to investigate the association between fatty fish intake and diseases such as diabetes.24 Furan fatty acids originate from plants and algae and accumulate in fish and crustaceans. Humans do not produce furan fatty acids de novo but acquire it through nutritional intake. CMPF formation has been proposed to occur through β-oxidation and ⍵-oxidation of furan fatty acids in the human liver, but the metabolic conversion from furan fatty acid to CMPF is not yet fully elucidated.25
A previous randomized cross-over study of 6 days consumption of EPA, DPA, DHA, or olive oil among 10 healthy women showed that intake of DPA and DHA but not EPA or olive oil was positively correlated with plasma CMPF levels and therefore suggested that different n-3 LCPUFAs have different impact on CMPF.26 Unfortunately, we do not have data on DPA content in the fish-oil capsules. FADS2 genotype influences EPA and DHA, which are detected at lower levels in subjects carrying minor alleles of the rs1535 SNP in the FADS2 gene.5 However, the newborn's CMPF level was not associated with maternal FADS2 genotype suggesting that CMPF is not likely to be downstream product of EPA and DHA. Further studies are needed to elucidate the biochemical mechanism leading to CMPF formation.26 In this study, we speculate that the observed increase in CMPF could be related to furan fatty acid impurities in the n-3 LCPUFA supplement.
The independence of CMPF from enzymatic desaturase activity supports its potential use over EPA and DHA as an unbiased representation of fish-oil and fatty fish intake. In terms of compliance assessment, newborn CMPF level performed better or equally good as compared to maternal one-week postpartum levels of EPA and DHA. This is supported by our previous study also demonstrating that CMPF can be transferred from mother to offspring.27 Indeed, hydroxy-CMPF, possibly a product of CYP450 metabolism of CMPF, was associated with maternal n-3 LCPUFA supplementation at the age of 6 months in the infants from the same COPSAC2010 cohort.28 Together, this suggests that CMPF is either preserved in the infant for longer periods or stored in the mother and transferred to the infant through breastfeeding. Thus, CPMF seems to reflect the cumulative intake, while the metabolically more unstable fatty acids EPA and DHA merely reflect time since last intake.
In both COPSAC cohorts, a higher exposure to n-3 LCPUFA during pregnancy through either fish-oil or habitual fatty fish intake led to increased newborn blood levels of CMPF and decreased the risk of developing childhood asthma, although the average intake differed between the two studies. In COPSAC2010, a high-dose fish-oil supplementation through the RCT led to an intake much above the average intake of fatty fish in Denmark,29 while the consumption for the majority of the pregnant women in COPSAC2000 was presumably closer to the average habitual fish consumption. A recent meta-analysis30 including the COPSAC2010 RCT suggested that higher doses of n-3 LCPUFAs (>2000 mg/day) are required for prevention of childhood asthma. In the current study, we found that only the COPSAC2000 newborns within the highest CMPF tertile were protected against developing asthma, but the intake in the highest tertile is likely to be much lower than 2000 mg/day. However, COPSAC2000 is a high-risk cohort including children born to mothers with asthma, where an above average intake of n-3 LCPUFAs during pregnancy might have a preventive potential compared to an unselected population, where a higher intake during pregnancy might be necessary.
It has been demonstrated that children experiencing an increased burden of common infections and troublesome lung symptoms during early life are more likely to develop childhood asthma.31 In our study, increasing newborn CMPF level was associated with a decreased rate of common infections and troublesome lung symptoms within the first 3 years of life, whereas there was no association with lung function or allergy endpoints at school age, which is also in line with the results of the RCT.2 These observations suggest that newborn CMPF level denotes the risk of a particular endotype of childhood asthma with high symptom burden and common infections early in life, but not allergic asthma at age 6.
It is speculated that n-3 LCPUFAs may have a direct anti-inflammatory property through displacement of arachidonic acid from cell membranes, which reduces the amount of proinflammatory eicosanoids that are key in asthmatic airway inflammation and pathophysiology.32 Furthermore, EPA and DHA-derived pro-resolving mediators play an important role in healthy resolution of inflammatory responses in the lungs and defects in pro-resolving mediator generation is related to infection proneness and asthma development.33 However, it is unknown whether CMPF by itself has a pathophysiological role in asthma or is just an asthma-relevant biomarker of n-3 LCPUFA exposure during pregnancy. Moderate levels of CMPF in mice have been demonstrated to have a protective effect on metabolic homeostasis and hepatic steatosis via enhancement of fatty acid utilization.34 Further studies are needed to investigate whether CMPF is actively involved in metabolic regulations of the immune system or lung function development during early life.
5 CONCLUSION
This study showed that newborn children's blood level of the furan fatty acid metabolite CMPF measured in dry blood spots collected for routine screening for inborn errors of metabolism reflects fish-oil and/or fatty fish intake during pregnancy and was associated with a lower risk of developing asthma across two independent birth cohorts. CMPF level in the newborn depicted risk of the very common non-atopic childhood asthma endotype characterized by a high load of infections and troublesome lung symptoms early in childhood, suggesting that CMPF has a potential for precision risk stratification.
AUTHOR CONTRIBUTIONS
The guarantor of the study is BC, who is responsible for the integrity of the work, from conception and design to conduct of the study and acquisition of data, analysis and interpretation of data and writing of the manuscript. BC had full access to all the data in the study and takes the responsibility for the integrity of the data and the accuracy of the data analysis. GG was responsible for data analysis and wrote the first draft of the manuscript. GG, MK, NB, JS, KB, DH, AC, and BC contributed to the design of the study, interpretation of data, and writing of the manuscript. ME, FR, and MAR contributed to the data analysis. All co-authors have contributed substantially to the analyses and/or interpretation of the data and have provided important intellectual input and approval of the final version of the manuscript.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
ACKNOWLEDGMENTS
We gratefully express our gratitude to the children and families of the COPSAC2000 and COPSAC2010 cohort studies for all their support and commitment. We acknowledge and appreciate the efforts of the COPSAC research team. This research has been conducted using the Danish National Biobank resource, supported by the Novo Nordisk Foundation.
FUNDING INFORMATION
COPSAC is funded by private and public research funds all listed on www.copsac.com. The Lundbeck Foundation; Danish State Budget; Danish Council for Strategic Research; Danish Council for Independent Research and The Capital Region Research Foundation have provided core support for COPSAC. This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement No. 946228).
No pharmaceutical company was involved in the study. The funding organizations did not have any role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
CONFLICT OF INTEREST
All the authors declare that (1) no authors have support from any medical company for the submitted work; (2) no authors have any relationship with companies that might have an interest in the submitted work in the previous 3 years; (3) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) no authors have any non-financial interests that may be relevant to the submitted work.
GOVERNANCE
We are aware of and comply with recognized codes of good research practice, including the Danish Code of Conduct for Research Integrity. We comply with national and international rules on the safety and rights of patients and healthy subjects, including Good Clinical Practice (GCP) as defined in the EU's Directive on Good Clinical Practice, the International Conference on Harmonisation's (ICH) good clinical practice guidelines and the Helsinki Declaration. Privacy is important to us which is why we follow national and international legislation on General Data Protection Regulation (GDPR), the Danish Act on Processing of Personal Data and the practice of the Danish Data Inspectorate.
This article has an online data supplement, which is accessible from this issue's table of content online at www.atsjournals.org.