ARIA digital anamorphosis: Digital transformation of health and care in airway diseases from research to practice
Funding information
MASK funding was obtained from EU grants (SPAL, POLLAR, Sunfrail, Rhinitis and Asthma TWINNING, DHE TWINNING on severe asthma), the Région Occitanie (France), unrestricted educational grants (Meda, Mylan, ALK, GSK, Novartis, Sanofi, Stallergènes and Uriach) and private donations. Euforea provided assistance for the ARIA website and the physician's questionnaire.
Abstract
Digital anamorphosis is used to define a distorted image of health and care that may be viewed correctly using digital tools and strategies. MASK digital anamorphosis represents the process used by MASK to develop the digital transformation of health and care in rhinitis. It strengthens the ARIA change management strategy in the prevention and management of airway disease. The MASK strategy is based on validated digital tools. Using the MASK digital tool and the CARAT online enhanced clinical framework, solutions for practical steps of digital enhancement of care are proposed.
Abbreviations
-
- AIRWAYS-ICPs
-
- integrated care pathways for airway diseases
-
- AIT
-
- allergen immunotherapy
-
- AR
-
- allergic rhinitis
-
- ARIA
-
- Allergic Rhinitis and its Impact on Asthma
-
- CARAT
-
- Control of Allergic Rhinitis and Asthma Test
-
- CDSS
-
- clinical decision support system
-
- DB-PC-RCT
-
- double-blind, placebo-controlled, randomized trial
-
- EFA
-
- European Federation of Allergy and Airways Diseases Patients' Association
-
- EIP on AHA
-
- European Innovation Partnership on Active and Healthy Ageing
-
- EIT
-
- European Institute for Innovation and Technology
-
- EQ5D
-
- EuroQuol
-
- EU
-
- European Union
-
- GA2LEN
-
- Global Allergy and Asthma European network
-
- GARD
-
- Global Alliance against Chronic Respiratory Diseases
-
- GRADE
-
- Grading of Recommendations Assessment, Development and Evaluation
-
- ICP
-
- integrated care pathway
-
- IT
-
- Internet technology
-
- JA-CHRODIS
-
- Joint Action on Chronic Diseases and Promoting Healthy Ageing across the Life Cycle
-
- MACVIA
-
- fighting chronic diseases for active and healthy ageing
-
- MASK
-
- Mobile Airways Sentinel NetworK
-
- MASK-air®
-
- (formerly Allergy Diary)
-
- MeDALL
-
- Mechanisms of the Development of Allergy
-
- POLLAR
-
- Impact of air POLLution on Asthma and Rhinitis
-
- QOL
-
- quality of life
-
- RCT
-
- randomized controlled trials
-
- RWD
-
- real-world data
-
- RWE
-
- real-world evidence
-
- SCIT
-
- subcutaneous immunotherapy
-
- SLIT
-
- sublingual immunotherapy
-
- SMS
-
- symptom-medication score
-
- TRL
-
- technology readiness level
-
- TWINNING
-
- Transfer of Innovation
-
- WHO
-
- World Health Organization
1 INTRODUCTION
Anamorphosis—from the Greek αναμόρφωση: transformation—is used in several fields to describe the transformation of a distorted object (e.g. painting, architecture, entomology, biology). Digital technology reveals the day-to-day experience of patients and provides a new type of information that—when properly collected and interpreted—will restore the real expression of the disease. In this paper, anamorphosis is used to define a distorted image of health and care that may be viewed correctly using digital tools and strategies.
The strategic overview (Table 1, Figure 1) and the vision of MASK include several considerations (Table 2). The disease burden and the healthcare costs for people with allergic and chronic respiratory diseases are increasing rapidly.1 Transformation of the healthcare system for integrated care through leveraging developments in digital health is urgently needed.2 The term “digital health” includes advanced medical technologies, disruptive innovations and digital communication tools aiming to provide best practice health care.3 Smart devices and internet-based applications are largely used in airway diseases and are likely to address certain unmet needs.4 However, these new tools need to be tested (a) for privacy rules, security and legislation of the Medical Device Regulation (May 2020); (b) for acceptability, usability and cost-effectiveness5; and (c) for validity. They should then be evaluated in the frame of the overall digital transformation of health and care, their impact on healthcare delivery as well as health outcomes. mHealth tools and strategies enabling the digital transformation of health and care, empowering citizens and building a healthier society represent a novel important step in health care. However, a practical integrated approach is required.
| Acronym | Name | Dates | |
|---|---|---|---|
| WHO-associated projects | |||
| ARIA | Allergic Rhinitis and its Impact on Asthma | 1999– | |
| WHO Collaborating Center for Rhinitis and Asthma | 2004–2014 | ||
| GARD | Global Alliance against Chronic Respiratory Diseases | 2003– | |
| EU grants and projects | |||
| GA2LEN | Global Allergy and Asthma European Network (FP6) | 2004– | |
| MeDALL | Mechanisms of the Development of Allergy (FP7) | 2009–2014 | |
| Sunfrail | |||
| EIP on AHA | European Innovation Partnership on Active and Healthy Ageing (DG Santé & CONNECT) | 2012–2020 | |
| TWINNING | Transfer of Innovation | 2017–2019 | |
| DHE TWINNING | Transfer of innovation in severe asthma (H2020) | 2019–2020 | |
| Vigour | 2019–2021 | ||
| POLLAR | Impact of Pollution on Asthma and Rhinitis (EIT health) | 2018–2019 | |
| Good Practice DG Santé on digital health (DG Santé) | 2018 | ||
- Abbreviations: ARIA, Allergic Rhinitis and its Impact on Asthma; CARAT, Control of Allergic Rhinitis and Asthma Test; EAACI, European Academy of Allergy and Clinical Immunology; e-CDSS, electronic clinical decision support system; GA2LEN, Global Allergy and Asthma European Network; GARD, Global Alliance against Chronic Respiratory Diseases; POLLAR, Impact of Pollution on Asthma and Rhinitis; WHO, World Health Organization.
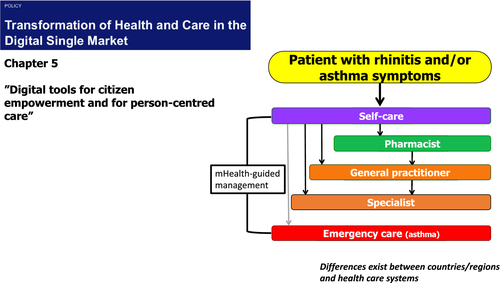
| 1. The burden of rhinitis and asthma (multimorbidity) and unmet medical needs are unacceptable and require a novel approach to tackle them |
| 2. Healthcare costs should be sustainable despite the increased prevalence of allergic diseases and the availability of new expensive treatments |
| 3. mHealth should be applied to rhinitis and asthma to reduce unmet medical needs and sustain health care costs |
| 4. A novel approach should embed medical knowledge, patients' needs and mHealth |
| 5. The ultimate goal is change management for rhinitis and asthma |
In 2014, on behalf of the European Innovation Partnership on Active and Healthy Ageing (EIP on AHA),6 AIRWAYS-ICPs (integrated care pathways for airway diseases) was initiated.7, 8 The objective was to launch a collaboration to develop multisectoral care pathways (ICPs) for chronic respiratory diseases in European countries and beyond as a Global Alliance against Chronic Respiratory Diseases (GARD) demonstration project (Figure 1). MASK (Mobile Airways Sentinel NetworK) is the mHealth strategy of AIRWAYS-ICPs and ARIA.9 It was based on the ARIA study group which exists in 92 countries. MASK is deployed in 26 countries and 18 languages. MASK, as a practical ICT integrated approach, was initially developed as an app (MASK-air®) and is now an e-platform for allergic diseases and asthma.
The Control of Allergic Rhinitis and Asthma Test (CARAT) is a patient-reported outcome that assesses the level of control of both asthma and AR using a single tool.10 It enables the implementation of the ARIA recommendations in the simultaneous assessment and management of both allergic rhinitis (AR) and asthma.11
This paper proposes the ARIA change management strategy in the prevention and management of airway disease.12 MASK digital anamorphosis represents the process used by MASK to develop the digital transformation of health and care in rhinitis. It also strengthens ARIA change management. Using the MASK digital tool and the CARAT online enhanced clinical framework, solutions for each practical step of digital enhancement of care are provided.
2 DIGITAL TRANSFORMATION OF HEALTH AND CARE IN RHINITIS AND ASTHMA MULTIMORBIDITY
2.1 The MASK e-platform
MASK, the Phase 3 ARIA (Allergic Rhinitis and its Impact on Asthma) initiative,11, 13 is a Good Practice of DG Santé for digitally-enabled, patient-centred care.14 It has been developed from the MASK-air® app and is a flexible e-platform for allergic diseases and asthma. It includes (a) a freely available app (MASK-air®, formerly the Allergy Diary, Android and iOS),13 (b) tools to support healthcare professionals in shared decision-making through an interoperable electronic decision support system (e-CDSS),15 (c) a web-based interoperable questionnaire for physicians,16 (d) a questionnaire on asthma and rhinitis (CARAT) for screening allergic diseases and assessing their control and (e) a sentinel network for air quality and pollen seasons. Other tools can be added when needed.
The maturity level of the MASK Good Practice is presented in Tables 1 and 3.
| MASK achievement | TRL | References |
|---|---|---|
| App for rhinitis and multimorbidity (MASK-air®): available in 26 countries, 18 languages, 35 000 users | 9 | [17, 18] |
| CARAT questionnaire for screening and control of rhinitis and asthma, available in 20 countries | 9 | [20, 21] |
| e-physician questionnaire for rhinitis (available on the Euforea website) deployed in 23 countries and 17 languages | 9 | [16] |
| Embedding air quality (outdoor air pollution) and pollen data in MASK-air® (POLLAR) | 9 | [23] |
| e-CDSS for shared decision-making in rhinitis | 7 | [15] |
| EAACI-ARIACARE-digital network | 8 | |
| Allergy score | 7 | [24] |
| Embedding artificial intelligence in MASK-air® | 3 |
Note
- Abbreviations: CARAT, Control of Allergic Rhinitis and Asthma Test; EAACI, European Academy of Allergy and Clinical Immunology; e-CDSS, electronic clinical decision support system; POLLAR, Impact of Pollution on Asthma and Rhinitis.
MASK is scaled up using the European Innovation Partnership on Active and Healthy Ageing (EIP on AHA) strategy.16, 25
2.2 MASK-air®
2.2.1 Characteristics
MASK-air® is an ICT (Information and Communication Technology) system centred around the patient.17 It is operational in 26 countries and 18 languages. It uses a treatment scroll list which includes all of the medications customized for each country. Furthermore, a visual analogue scale (VAS) assesses rhinitis control (global allergy impact, nose, eyes, asthma), sleep and work productivity.26, 27 MASK-air® is combined with prediction on allergen season and air quality (POLLAR: Impact of POLLution on Asthma and Rhinitis, EIT Health-funded project).23 MASK is available in 26 countries and 18 languages including some middle-income countries (Table 2). Patients' organizations and scientific societies are involved.
2.2.2 Privacy, General Data Protection Regulation (GDPR) and Medical Device Regulation (MDR)
The General Data Protection Regulation (GDPR) regulates the processing of personal data in the European Union (EU).28, 29 MASK-air® follows the five main principles of personal data protection to be respected during the development of the app: purpose, proportionality and relevance, limited retention period, security and confidentiality, as well as the rights of the users regarding management of personal data (including withdrawal and modification).30 Moreover, MASK-air® uses k-anonymity for geolocation.31 A double encryption database has been set up.
MASK-air® is currently a Class 1 Medical Device but will be upgraded to Class 2A with the new MDR to be enforced in the EU in May 2021.32
2.2.3 Validation
- The MASK-air® questions have been validated by patients (studies by Madopa and STIMCO, unpublished) and are easily understood by patients in different countries.
- MASK-air® has followed the COSMIN (COnsensus-based Standards for the selection of health Measurement INstruments) guidelines.33
- The independence of data has been confirmed.24
- Translations have been validated using a back-translation.
- MASK-air® has been implemented in the different situations in which it is used.14, 15, 17, 18, 24, 30, 33-41
2.3 CARAT
CARAT is a validated questionnaire that can summarize the clinical status of asthma and rhinitis (multimorbidity) of the previous 4 weeks. It complements the frequent/daily self-assessment in the MASK-air app and the physician's clinical assessment.
2.3.1 Characteristics
The CARAT questionnaire has two domains - allergic rhinitis and asthma - and 10 items regarding symptoms, sleep, activities and drug use within the past 4 weeks.20 CARAT's minimal clinically important difference can detect change over time (high responsiveness).21 CARAT supports shared decisions between the patient and the physician as well as within the healthcare team. CARAT has been used in 19 countries globally including developing countries.22
CARAT can be used in a range of different aims: (a) screening of patients with rhinitis or asthma in different settings including pharmacies,42 (b) follow-up consultations together with lung function,43 (c) patient self-management 44 and (d) identifying patients with uncontrolled asthma at pharmacies.42 It should increase awareness of the level of disease control and strengthen the partnership between patients and doctors in the management of asthma and rhinitis by helping to define shared treatment goals.
CARAT has been used in epidemiology and clinical research45: it has been included in international multicentre studies, such as the technology transfer of innovative practices (TWINNING) project16 and the observational longitudinal multicentre prospective study, the “@IT2020” study.46 CARAT has been implemented as an mHealth tool in several smartphone applications including MASK-air,17 InspirerMundi,47 the Adolescent Adherence Patient Tool (ADAPT) app48 and Lung Manager.49
2.3.2 Validation
CARAT has been thoroughly studied in cross-sectional and prospective studies conducted at all levels of MASK Care Pathways. It meets all COSMIN criteria for patient-reported outcome measures.50
CARAT has been used in clinical studies and in clinical practice. It has enabled comparison between groups as well as evaluation of individual patients over time.10, 20 The questionnaire has been deployed in patient care and/or research. CARAT has been implemented in different settings (pharmacies, primary care, secondary care, epidemiology and clinical research) and technologies including mHealth tools,17, 42, 45, 47, 48, 51-53 but also in severe asthma by specialists.54
2.3.3 New functionalities
CARAT has the potential to evolve in order to further strengthen multimorbidity assessment and to focus on more severe patients. This change can be carried out simply by reassessing questions that were excluded during the initial developing process.10 In particular, eye symptoms should be included: within the asthma-rhinitis multimorbidity, they are associated with more severe phenotypes as demonstrated by the MASK-air app37 and confirmed by an epidemiologic study with full medical observation.22
2.4 Electronic clinical decision support system (eCDSS) for rhinitis
The interoperable electronic decision support system (eCDSS)15 is based on an algorithm designed by the ARIA expert group and validated using real-world evidence.56 This eCDSS is to be used on tablets by pharmacists and physicians.
2.5 Web-based physician's questionnaire for rhinitis and asthma
An interoperable questionnaire for physicians is available online on the Euforea website (https://www.euforea.eu). Around 1,000 patients have been enrolled in the rhinitis-TWINNING using the questionnaire. They are then followed up using the MASK-air® app.16
2.6 Sentinel network for air quality and pollen prediction
POLLAR has confirmed the interactions between air pollution, asthma and rhinitis in order to propose the prediction of these environmental factors in MASK-air®.23, 40 It uses the MASK-air® app combined with a new tool allowing queries on pollen and air quality, in geolocalized patients. Allergic symptoms of the MASK-air® app are integrated with the Symptom Forecasting Model developed within the PASYFO project of Copernicus Atmospheric Monitoring Service, which also supplies the meteorological, air quality and pollen information for Europe. Additional pollen and global air quality forecasts are generated by the SILAM model of the Finnish Meteorological Institute (FMI).57-59 Machine learning will be used to assess the relationship between air pollution, AR and asthma to further refine the prediction.
3 PATIENTS' VIEWS
Many patients do not understand the needs and benefits of mHealth and may worry about data privacy (Table 3). Thus, the uptake of mHealth is slow. On the other hand, too many patients rely on internet-based information and on untested mHealth solutions. This attitude may have dangerous implications since patients may receive an incorrect diagnosis or management strategy.
3.1 Features required to satisfy patients
A qualitative study was carried out by MADOPA in 2016 for MASK to better understand the patients' needs and expectations (Table 4).
| A. Problems patients encounter using an app |
|---|
| Fear of using an app (particularly in elderly patients) |
| Customer loyalty problems (young adult patients) |
| Not willing to use one app regularly |
| Changing the app frequently |
| Not understanding how to fill in the app |
| Not understanding or caring about what must be done (e.g. seeing a physician), despite clear results/instructions provided by the app |
| Not feeling ill (usually males) |
| Feeling too ill and filling in the app too much (females, some males also) |
| B. Patients' expectations | |||
|---|---|---|---|
| Patients' expectations | Existing feature in MASK | To be added to MASK | |
| Feature | Expected | ||
| Advice to modify the treatment | Simple advice exists in line with the GDPR | ||
| More sophisticated advice will be ready with Medical Device Regulation (MDR) Class 2A | 06-2021a | ||
| Pollen and pollution | POLLAR | 06-2020 | |
| Visualization of control and medications | Existing but poorly found by patients and physicians | More user friendly and better information | 06-2020 |
| Help science to better understand the disease in order to get future benefits | Existing | ||
- Abbreviations: GDPR, General Data Protection Regulation; MASK, Mobile Airway Sentinel NetworK; POLLAR, Impact of Pollution on Asthma and Rhinitis.
- a Due to new regulation not yet published.
3.2 Implementation and communication strategy for patients
Without a communication strategy, the app will not be largely used. However, the communication plan will only be put in place in 2020 once the POLLAR module has been added. Documents are available in 18 languages and can be downloaded from the MASK website (https://www.mask-air.com). They include leaflets for patients, physicians and pharmacists as well as other documents. In Mexico, this strategy was found to be effective. It will be deployed to other countries.
- Better understanding the symptoms.
- Sentinel network linking aerobiology data and control.
- Improved adherence.
- Self-management.
- Patient empowerment.
4 MASK ACHIEVEMENTS IN DIGITAL ANAMORPHOSIS
4.1 Anamorphosis steps based on digital learning and Real-World Data
MASK-air® has been in use for 5 years and has evolved since its first inception. Major RWD results of the MASK strategy (MASK-air®, POLLAR and CARAT) are presented in Table 5.
| A* | Areas of innovation | Novel findings using RWD | Solutions for digital health | References |
|---|---|---|---|---|
| Innovation in phenotypes | ||||
| 1 | Allergic phenotypes (based on epidemiologic evidence) |
MASK
These findings were confirmed in classical epidemiologic studies |
A novel approach of multimorbidity is needed to select and stratify patients using artificial intelligence |
[37] |
| Innovation in diagnosis | ||||
| 2 | Diagnosis |
Using the CARAT questionnaire:
|
The CARAT questionnaire is in MASK-air® and can be used in the physician's waiting room to help in the diagnosis of allergic diseases and to initiate the stratification of patients | Submitted |
| Innovation in management | ||||
| 3 | Adherence to treatment |
|
Poor adherence of patients to treatment indicating that RCTs carried out in adherent patients do not reflect real life and that change management is needed with a new registration of medications (prn) Need to change practice and medication registration |
[37, 39] |
| 4 | Novel approach for efficacy assessment |
|
Guidelines assume that patients follow the doctor's orders. Adherence to medication is turned to partnership using novel models of education (IT) |
[64] |
| 5 | The same tool is used for RCTs, RWD, chamber studies and clinical practice | A symptom-medication score (SMS) based on MASK has been set up and can be used for all purposes |
|
|
| Health outcomes | ||||
| 6 | Health outcomes and impact |
Daily VAS work can be used for economic studies |
|
[34-36, 38] |
| Next-generation care pathways | ||||
| 7 | Next-generation care pathways |
|
Next-generation care pathways are needed
|
[41] |
| 8 | Air pollution |
|
|
[23, 40] |
| Centres of excellence in digital health | ||||
| 9 | Centres of Excellence | ARIACARE digital is a novel network with the aim to implement the digital transformation of health and care in airway diseases |
|
|
| Transfer of innovation | ||||
| 10 | Rhinitis-TWINNING | Completed (but still ongoing) TWINNING in rhinitis and asthma |
|
[16, 69] |
| 11 | Asthma-TWINNING | DHE TWINNING in severe asthma |
|
|
| Digital transformation of health and care to sustain planetary health | ||||
| 12 | POLLAR |
|
|
[23, 40] |
| 13 | Finland's EU Presidency meeting, December 3-4, 2019 |
|
|
Bousquet et al., in preparation [104] |
Note
- A*: anamorphosis.
- Abbreviations: CARAT: Control of Allergic Rhinitis and Asthma Test, DHE: DigitalHealthEurope, EQ5D: EuroQuol, MASK: Mobile Airway Sentinel NetworK, RCT: randomized control trial, RWD: real-world data, TWINNING: Transfer of Innovation, VAS: visual analogue scale.
4.2 Health outcomes
In AR and asthma, a relevant outcome providing information on the cost-effectiveness of interventions is needed. EQ-5D (EuroQol), a standardized and validated non-disease-specific instrument used to describe and value health-related quality of life, has been used in allergic rhinitis 36, 70-75 but it cannot be used for daily assessment. EQ-5D is one of the MASK-air® questionnaires.36 In MASK, VAS work correlates with other MASK outcomes (VAS global, nose, eye and asthma)24, 34 and should be considered as a potentially useful allergic rhinitis outcome in intervention studies.
RWD make health technology assessment possible.
4.3 Use of real-world data to develop next-generation care pathways for chronic respiratory diseases
Care pathways are structured multi-disciplinary care plans detailing the key steps of patient care.7 They promote the translation of guideline recommendations into local protocols and their application to clinical practice. ICPs have been proposed with a focus on mHealth technologies that should enhance self-management and adherence to guidelines and ICPs.
Next-generation care pathways for airway diseases follow the 2014 AIRWAYS integrated care pathways (ICPs) concept (Figures 1 and 2).56 As a proof of concept for chronic disease care, RWD obtained from MASK provide a framework for real-life ICPs centred around the patient with rhinitis, using the mHealth monitoring of environmental exposure. This is implemented in collaboration with professional and patient organizations.
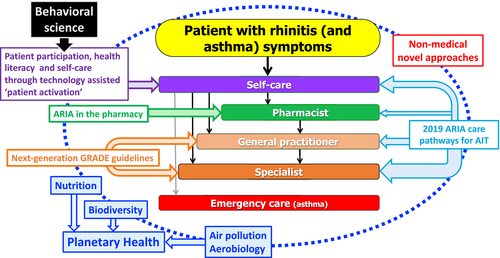
ARIA is constantly evolving and its most recent advance was determined following a meeting of experts/stakeholders in Paris in December 201876, 77 (Table 6). Three aspects of care pathways were developed during this meeting: (a) patient participation, health literacy and self-care through technology-assisted “patient activation”, (b) implementation of care pathways by pharmacists66 and (c) next-generation guidelines assessing the recommendations of GRADE (Grading of Recommendations, Assessment, Development and Evaluation) guidelines in rhinitis and asthma using RWE 56 and AIT.67 Next-generation guidelines for the pharmacologic treatment of allergic rhinitis were developed using existing GRADE-based guidelines,78-80 RWD provided by mHealth Apps19, 37, 39 and additive studies (allergen chamber studies64) to refine the MACVIA algorithm.
| Title | Journal | Publication | |
|---|---|---|---|
| 1 | From ARIA guidelines to the digital transformation of health in rhinitis and asthma multimorbidity | Eur Respir J | [9] |
| 2 | Mobile technology in allergic rhinitis: evolution in management or revolution in health and care? | JACI Practice | [5] |
| 3 | Next-generation ARIA care pathways for rhinitis and asthma: a model for multimorbid chronic diseases | CTA | [81] |
| 4 |
2018 Good Practice: ARIA digitally-enabled, integrated, person-centred care for rhinitis and asthma Practice presented during the Steering Group on Promotion and Prevention marketplace workshop on "digitally-enabled, integrated, person-centred care" best practices on 12-13 December 2018 in the premises of the Joint Research Centre in Ispra, Italy |
CTA | [14] |
| 5 | Next-generation care pathways for allergic rhinitis and asthma multimorbidity: a model for multimorbid non-communicable diseases (Meeting Report. Part 1) | J Thorac Dis | [76] |
| 6 | Next-generation care pathways for allergic rhinitis and asthma multimorbidity: a model for multimorbid non-communicable diseases (Meeting Report. Part 2) | J Thorac Dis | [77] |
| 7 | ARIA pharmacy 2018: “Allergic rhinitis care pathways for community pharmacy” | Allergy | [66] |
| 9 | ARIA Care pathways for allergen-specific immunotherapy following the ARIA recommendations to fill gaps in knowledge82 | Allergy | [67] |
| 10 | ARIA-EAACI Care pathways for allergen-specific immunotherapy Pocket Guide | ||
| 11 | Next-generation ARIA guidelines for allergic rhinitis based on GRADE and real-world evidence, validating the management algorithm, following GRADE recommendations78, 79, 83 and chamber studies64, 84 | JACI | [81] |
| 12 | Digital transformation of health and care in asthma | Allergy | |
| 13 | 2020 DHE TWINNING on severe asthma | ||
| 14 | Uniform stratification of severe chronic diseases in adults using mobile technology: App-MM | ||
| 15 | ARIA Phase 4 (2018): Change management in allergic rhinitis and asthma multimorbidity using mobile technology | JACI | [12] |
Note
- App-MM: App for multimorbidity, ARIA: Allergic Rhinitis and its Impact on Asthma, CARAT: Control of Allergic Rhinitis and Asthma Test, CTA: Clinical and Translational Allergy, DHE: DigitalHealthEurope, EAACI: European Academy of Allergy and Clinical Immunology, JACI: Journal of Allergy and Clinical Immunology, TWINNING: Transfer of Innovation
4.4 Network of centres of excellence in digital health
ARIA was established 20 years ago and includes more than 600 members in over 80 countries. In ARIA Phase 4 (change management for airways diseases), a network of centres of excellence has been organized. GA2LEN ARIACARE is one of the GA2LEN centres of excellence85 and includes urticaria care (UCARE)86 and atopic dermatitis care (ADCARE). Accreditation follows the UCARE proposals.
ARIACARE digital is a novel network with the aim to implement the digital transformation of health and care in airway diseases. Both members of MASK and others can join the network. ARIACARE-Digital has links with GA2LEN but is a separate entity.
4.5 Transfer of innovation (TWINNING)
4.5.1 Rhinitis-Asthma TWINNING
A transfer of innovative practices (TWINNING)16, 69 was performed with the aim to transfer and implement MASK-air®. The “Organization transferring the innovative practice” (originator organization) had the experience and know-how developed in rhinitis and asthma IT solutions. The “Organization adopting the innovative practice” (receiving/adopter organization) received the innovative practice and implemented it in its territory. The rhinitis TWINNING was deployed from MASK to 22 countries. Around 1,000 patients were enrolled in the study. The phenotypic characteristics of rhinitis and asthma multimorbidity in adults and the elderly were compared using validated information and communication technology (ICT) tools (i.e. MASK-air®, CARAT and a physician's questionnaire developed for the TWINNING). This improved the understanding, assessment of burden, diagnosis and management of rhinitis in the elderly by comparison with an adult population. The TWINNING was selected as a success story.
4.5.2 DigitalHealthEurope (DHE) Severe Asthma TWINNING
- To form a European network for severe asthma in old age people globally (this does not currently exist);
- To better understand the phenotype and treatment of severe asthma with possible differences between countries, age and gender;
- To include the results into the MASK Good Practice for disease stratification and personalized health care with a vision to optimizing the prescription of expensive treatments (biologics) and following up the patients using RWD;
- To be the basis for a further deployment beyond the funding, including a network of centres of excellence on severe asthma (ARIACARE and ARIACARE digital).
The DHE TWINNING on SA (Project acronym: H2020, DigitalHealthEurope Grant Agreement Number: 826 353, Project full title: Support to a Digital Health and Care Innovation initiative in the context of Digital Single Market strategy,Call identifier: SC1-HCC-05-2018) was accepted on 16 September 2019.
5 ONGOING AND FUTURE MASK ACTIONS
5.1 Advance capabilities: The same IT tool from epidemiologic studies to clinical trials and clinical practice
Symptom-medication scores (SMSs) are needed to investigate the effect of AR treatments, in particular allergen immunotherapy.87 Several scores have been proposed and the European Academy of Allergy and Clinical Immunology has designed one.88 However, a recent MASK analysis 24 has found that this commonly used SMS is not very well correlated with VAS work used as an end point. When considering MASK data,19 it is possible that some patients with very high levels of VAS global (and work) may not be able to be controlled with current pharmacologic treatments, and a new SMS has been proposed. This SMS for rhinitis has been validated with MASK-air® data. Other artificial intelligence analyses are being carried out to obtain an optimal score.
Real-world evidence (RWE) combines results of double-blind, placebo-controlled, randomized trials (DB-PC-RCT) and RWD. However, observational studies provide clinically relevant information in addition to DB-PC-RCT.19, 37, 39 RWD can provide new insights into disease patterns and help improve the safety and effectiveness of health interventions. The same SMS will allow the comparison of the results of DB-PC-RCTs and RWD in population studies or for the individual patient.81 This will provide complementary information to DB-PC-RCTs and a real-life approach. Since patients are using the app and the same system, it will be possible—using machine learning—to target the efficacy of AIT at the individual level and to propose automatic advice to the physician for the indication of AIT as well as an early stopping rule in clinical practice.67
Patient stratification is an important step for expensive treatments such as allergen immunotherapy in allergic diseases or biologics in severe asthma. There are currently no validated genetic or blood biomarkers for predicting or monitoring the efficacy of treatments at an individual patient level in allergic diseases.89 mHealth biomarkers (SMS)67 and eCDSS15 may change the scope of AIT in allergic diseases or biologics in asthma or chronic rhinosinusitis.
5.2 Towards severe asthma
The lessons learnt by MASK will be used to build MASK-asthma which will include (a) a standardized assessment of severity and control, (b) the development of an upgraded e-platform for severe asthma including screening, assessment by physicians and follow-up, (c) the analysis of MASK-air® data on file for asthma, (d) a pan-European IT-based alert system for exacerbations, (e) MASK-asthma IT tools for registries and databases, (f) transfer of innovation, (g) a digital network of centres of excellence (ARIACARE-Digital) and (h) the development of next-generation care pathways for severe asthma.
5.3 United perspective for chronic diseases to sustain planetary health
Planetary health refers to “the health of human civilization and the state of the natural systems on which it depends”.90 Most risk factors for noncommunicable diseases (NCDs) are associated with planetary health.
Digital tools can also empower patients in the context of the UN sustainable development goals and in particular regarding those related to sustainability and natural resources.91 Future apps in AR could consider providing information to promote behavioural changes that could reduce the planetary impacts of human activity.
During a conference entitled “Europe That Protects: Safeguarding Our Planet, Safeguarding Our Health” - co-organized by the Finnish Institute for Health and Welfare, the Finnish Environment Institute and the European Commission under the auspices of Finland's Presidency of the EU in 2019 - a symposium was held to better understand the digital transformation of health and care to sustain planetary health in airway diseases. The Finnish Allergy Programme is a proof of concept of planetary health, and MASK (Mobile Airways Sentinel NetworK), a Good Practice of DG Santé on digitally-enabled, patient-centred care pathways, is in line with the objectives of this programme.
Lessons learnt in rhinitis and asthma multimorbidity17 can be deployed to other NCDs for change management in health care. A uniform approach can be used12 for the development of next-generation care pathways in chronic diseases embedding the risk factors involved in planetary health.
This perspective is global since planetary health needs to be tackled in all countries. The World Health Organization and the International Telecommunication Union recognize the importance of mHealth globally, and particularly in developing countries.5
5.4 Value-added medicines: The example of the combination of intra-nasal antihistamine and corticosteroid used as needed
Value-added medicines represent the concept of drug repurposing.92 They are medicines based on known molecules that address healthcare needs and deliver relevant improvement for patients, healthcare professionals and/or payers. MASK is a proof of concept of drug repurposing as it suggests the importance of as-needed treatment for AR. Value-added medicines are medicines based on known molecules that address healthcare needs,8, 13, 17 and deliver relevant improvement for patients,18, 37, 64, 93 healthcare professionals18, 37 and payers.34-36, 38 They contribute to addressing unmet patient needs, moving from a tailored and patient's specific approach. By answering patients' unmet needs, they represent a new horizon for those who are currently looking forward to a better quality of life with their treatment.
6 CONTRIBUTION OF MASK TO THE EU DIGITAL SINGLE MARKET
The Digital Single Market (https://ec.europa.eu/digital-single-market/en), part of the Digital Agenda for Europe 2020 programme of the EU, includes three “pillars”: (a) access to online products and services, (b) conditions for digital networks and services and (c) growth of the European digital economy. MASK is involved in this strategy by (a) the management of care process, (b) digital networks (ARIACARE-digital network), (c) innovation to market (I2M) to foster the cross-border adoption of digitally-driven marketable solutions, (d) the political, organizational, technological and financial readiness, (e) the contribution to European co-operation and transferability, (f) and the contribution to the European Digital Transformation of Health and Care (Bousquet et al., submitted).
The digital transformation of health and care can improve the quality of health services and ultimately people's health and well-being as well as the economy, in line with EIT Health. In the context of implementing communication on the digital transformation of health and care, DG SANTE, in collaboration with the EU Commission Expert Group “Steering Group on Health Promotion, Disease Prevention and Management of Non-Communicable Diseases” (https://ec.europa.eu/transparency/regexpert/index.cfm?do=groupDetail.groupDetail&groupID=3622), scaled up good practices in the field of digitally-enabled, integrated, patient-centred care. MASK was one of the nine Good Practices selected, along with chronic disease and Parkinson's disease.14
7 POLITICAL AGENDA
In the severe Asthma TWINNING, the engagement through the Salerno local health agency of ProMIS@Campania network69 will ensure that adoption is progressively achieved through a multicentric scale-up pilot. The good practice will then be scaled up to other Italian regions through the National ProMIS network.94
The EU political agenda is of great importance in supporting the digital transformation of health and care for chronic respiratory diseases. The Polish Presidency of the EU Council (2011) prioritized the early diagnosis, prevention and control of chronic respiratory diseases in children.95 AIRWAYS-ICPs (integrated care pathways for airway diseases),7 initiated in 2014 by the EIP on AHA,6, 8 launched a collaboration to develop multisectoral ICPs. It was a GARD96 demonstration project.97
Euforea (European Forum for Research and Education in Allergy and Airway Diseases) proposed a yearly stepwise strategy at the EU or ministerial levels.98-100 Euforea organized an EU Summit in Vilnius, Lithuania (March 2018) to propose multisectoral ICPs embedding guided self-management, mHealth and air pollution in chronic respiratory diseases.101
POLLAR (Impact of air POLLution on Asthma and Rhinitis, EIT Health) is focussing on the impact of allergens and air pollution on airway diseases to propose novel ICPs integrating pollution, sleep and patient literacy.23 AQuAS, the Catalonia Health Agency, is involved in POLLAR.
8 CHANGE MANAGEMENT
ARIA phase 4 focusses on change management with the aim of providing an active and healthy life to rhinitis sufferers and to those with asthma multimorbidity across the life cycle—whatever their gender or socio-economic status—in order to reduce health and social inequities incurred by the disease. ARIA has followed the 8-step model of Kotter102 to assess and implement the impact of rhinitis on asthma multimorbidity and to propose multimorbid guidelines.12 A second change management strategy is proposed by ARIA Phase 4 on the digital transformation of health and care.
9 CONCLUSION: TOWARDS A REVOLUTION IN RHINITIS AND ASTHMA MANAGEMENT
The MASK strategy represents a proof of concept for other chronic diseases, as asthma-rhinitis multimorbidity plays a key role in understanding asthma and can be used as a general model of multimorbidity. Moreover, asthma and rhinitis have a life-course approach, whereas most chronic diseases start early in life but are only clinically evident in adulthood. The lessons learnt by the MASK strategy are therefore transposable to other chronic diseases.
Anamorphosis is a metaphor for reimagining and expanding on appearances and overcoming otherness. MASK digital anamorphosis makes it possible to look at data from a different angle. The data then appear to be different to their familiar, expected and/or generally accepted form. Anamorphosis may be associated with fear as phenomenological otherness often accompanies new technology. Education for a better appraisal of mHealth by all stakeholders is therefore essential. Metaphorical language can facilitate communication and shape of thought, thus providing key challenges and opportunities for future research.
mHealth has the potential to profoundly impact health care.103 mHealth apps now represent an important evolution of health and care for allergic rhinitis and asthma multimorbidity. The digital revolution is underway for rhinitis and asthma.5 Innovative health strategies and services will change management6 and create a new kind of partnership between the patients, the healthcare providers and the health system.
CONFLICT OF INTEREST
MA reports personal fees from POCI-01-0145-FEDER-029130 mINSPIRERS—mHealth to measure and improve adherence to medication in chronic obstructive respiratory diseases—generalization and evaluation of gamification, peer support and advanced image processing technologies from ERDF (European Regional Development Fund) funded by the COMPETE2020 and by National Funds through FCT (Fundação para a Ciência e a Tecnologia). EB reports personal fees from Novartis, Menarini, ALK, Sanofi Regeneron, Boehringer Ingelheim, AstraZeneca, Sanofi Genzyme, Orion, and is a member of the Science Committee and Board of the Global Initiative for Asthma (GINA). PB reports personal fees and other from Roche, Boehringer and Novartis, personal fees from AstraZeneca and TEVA, and other from Chiesi and Stallergenes. LPB reports research grants for participation to multicentre studies from AstraZeneca, Boston Scientific, GlaxoSmithKline, Hoffman La Roche, Novartis, Ono Pharma, Sanofi and Takeda, support for research projects introduced by the investigator from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck and Takeda, fee for consulting and advisory boards from Astra Zeneca, Novartis and Methapharm, nonprofit grants for production of educational materials from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck and Novartis, conference fees from AstraZeneca, GlaxoSmithKline, Merck and Novartis, and support for participation in conferences and meetings from Novartis and Takeda. JB reports personal fees from Chiesi, Cipla, Hikma, Menarini, Mundipharma, Mylan, Novartis, Purina, Sanofi Aventis, Takeda, Teva, and Uriach, and other from KYomed INNOV. RB reports personal fees from AstraZeneca, Chiesi, Cipla, Sanofi and Teva, grants and personal fees from Boehringer Ingelheim, Novartis and Roche, and grants from GlaxoSmithKline, all outside the submitted work. VC reports personal fees from ALK, Allergopharma, Allergy Therapeutics, Diater, LETI, Thermo Fisher and Stallergenes. JCS reports other from Boehringer Ingelheim, GSK, personal fees, nonfinancial support and other from AstraZeneca, personal fees and other from Mundipharma. AC reports personal fees from Novartis, Regeneron, Thermo Fisher Scientific, Philips and Sanofi. ME reports personal fees from DBV Technologies and Mylan. JF reports being a partner in a company developing mobile technologies for monitoring airways diseases. EH reports personal fees from AstraZeneca, Novartis, GSK, Sanofi Genzyme, Teva, Circassia and Nestlè Purina. GI is consultant for Amicus Therapeutics and received a research grant from Amicus therapeutics. PK reports personal fees from Aflofarm, Fresenius, Lek-AM, Novartis, Polpharma and Sandoz, grants from European Union, European Commission. LK reports personal fees from Allergopharma, HAL Allergie, ALK-Abelló, LETI Pharma, Allergy Therapeutics, Stallergenes, Quintiles, AstraZeneca, GSK, ASIT biotech and Lofarma, and grants and personal fees from MEDA/Mylan and Sanofi. DLL reports personal fees from Armstrong, Astrazeneca, Boehringer Ingelheim, Chiesi, DBV Technologies, Grunenthal, GSK, MEDA, Menarini, MSD, Novartis, Pfizer, Novartis, Sanofi, Siegfried and UCB, grants from Sanofi, Astrazeneca, Novartis, UCB, GSK, TEVA, Boehringer Ingelheim and Chiesi. RL reports grants and personal fees from GSK, from AZ and Novartis and grants from Chiesi. Dr Loureiro reports personal fees from AstraZeneca, Novartis, GSK, Sanofi, TEVA and Menarini. JM reports personal fees and nonfinancial support from Novartis, Sanofi, AstraZeneca and Immunotek. MM reports grants and personal fees from Aralez, AstraZeneca, FAES, Genentech, Novartis, MSD, Roche, Sanofi, UCB and Uriach. JM reports personal fees from ALK-Abelló, Sanofi Genzyme & Regeneron, Menarini Group, MSD, Mitsubishi-Tanabe, Novartis, UCB Pharma, and GENENTECH—Roche, grants and personal fees from URIACH Group, MYLAN-MEDA Pharma. AM reports personal fees from Aimmune, DVB, Nestlè Health Institute and Nestlè Purina. BN reports other from Co-founded AsthmaTuner, eHealth system for asthma. YO reports personal fees from Kyowa Co., Ltd, Eizai Co., Ltd, Shionogi Co., Ltd., Torii Co., Ltd., GSK and MSD, grants and personal fees from Kyorin Co., Ltd., and Tiho Co., Ltd., grants from Yakuruto Co., Ltd., and Yamada Bee Farm. NP reports personal fees from Novartis, Nutricia, HAL, MENARINI/FAES FARMA, Sanofi, Mylan/Meda, BIOMAY, AstraZeneca, GSK, MSD, ASIT BIOTECH and Boehringer Ingelheim, and grants from Gerolymatos International SA and Capricare. JLP reports grants and personal fees from Air Liquide Foundation, Agiradom, AstraZeneca, Philips and ResMed, grants from Fisher and Paykel, Mutualia and Vitalaire, personal fees from Boehringer Ingelheim, Jazz pharmaceutical, Night Balance and Sefam. DP reports personal fees, nonfinancial support and other from Revenio, grants and personal fees from GlaxoSmithKline, personal fees from Merck and Sandoz, other from Boehringer Ingelheim, Novartis, MSD and Chiesi, nonfinancial support from Menarini, nonfinancial support from Pharmas, and personal fees and nonfinancial support from Salveo. DP reports personal fees from Amgen, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Cipla, GlaxoSmithKline, Kyorin and Thermo Fisher, grants and personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia and Mylan, grants from Respiratory Effectiveness Group, Sanofi Genzyme, Teva and Theravance, grants from UK National Health Service, nonfinancial support from Efficacy and Evaluation Mechanism Programme, Health Technology Assessment and stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals, and owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore). FP has been scientific consultant, researcher and speaker supported by the following commercial companies: Menarini, Alk-Abello, Almirall, Allergy Therapeutics, Anallergo, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, GSK, Hal Allergy, Lab. Guidotti, Lofarma, Malesci, MSD, Mundipharma, Novartis, Roche, Sanofi, Stallergenes and Valea. Dr Sastre is consultant for Thermo Fisher, Hycor, Novartis, Sanofi, Leti, Mundipharma, ALK and GSK, paid conferences from Novartis, GSK, Circassia, Sanofi, LETI and FAES FARMA, and research grants from Thermo Fisher, Mundipharma, ALK, Sanofi. GS reports personal fees from ALK, Mylan and ALK and other from Rhinology & Laryngology Research Fund, BSACI and EAACI. MS reports fees from ASIT Biotech.sa, ALK and Allergopharma. AMTB reports grants and personal fees from Novartis, Sanofi, Mundipharma, GSK (GlaxoSmithKline) and Teva Pharma, personal fees from AstraZeneca, grants from Boehringer Ingelheim, outside the submitted work. IT reports personal fees from Honoraria for educational activities, speaking engagements, advisory boards from Boehringer Ingelheim, Astra Zeneca, GSK and Novartis and grants from GSK Hellas and Elpen. MW reports personal fees from ALK-Abello, AstraZeneca, Bencard Allergie, HAL Allergy, Leti Pharma, Meda Pharma, Novartis, Sanofi Aventis, Stallergenes and Teva. SW reports personal fees and other from CSL Behring, Shire, AstraZeneca, Teva, Meda, Merck, GSK and Novartis, personal fees from Pediapharm, Aralez, Sanofi and Stallergenes. The other authors have no conflict of interest to declare.




